Infrared Spectra of the 1
‑Pyridinium (C
5
H
5
NH
+
) Cation and Pyridinyl
(C
5
H
5
NH and 4
‑C
5
H
6
N) Radicals Isolated in Solid
para-Hydrogen
Barbara Golec,
†Prasanta Das,
†Mohammed Bahou,
†and Yuan-Pern Lee*
,†,‡†Department of Applied Chemistry and Institute of Molecular Science, National Chiao Tung University, Hsinchu 30010, Taiwan ‡Institute of Atomic and Molecular Sciences, Academia Sinica, Taipei 10617, Taiwan
*
S Supporting InformationABSTRACT: Protonated pyridine and its neutral counterparts (C5H6N) are important intermediates in organic and biological reactions and in the atmosphere. We have recorded the IR absorption spectra of the 1-pyridinium (C5H5NH+)
cation, 1-pyridinyl (C5H5NH), and 4-pyridinyl (4-C5H6N) produced on electron
bombardment during matrix deposition of a mixture of pyridine (C5H5N) and p-H2 at 3.2 K; all spectra were previously unreported. The IR features of C5H5NH+
diminished in intensity after the matrix was maintained in darkness for 15 h, whereas those of C5H5NH and 4-C5H6N radicals increased. Irradiation of this
matrix with light at 365 nm diminished lines of C5H5NH+ and C
5H5NH but
enhanced lines of 4-C5H6N slightly, whereas irradiation at 405 nm diminished lines
of 4-C5H6N significantly. Observed wavenumbers and relative intensities of these
species agree satisfactorily with the anharmonic vibrational wavenumbers and IR intensities predicted with the B3LYP/6-31+ +G(d,p) method. Assignments of C5H5NH and 4-C5H6N radicals were further supported by the observation of similar spectra when a Cl2/C5H5N/p-H2matrix was irradiatedfirst at 365 nm and then with IR light to generate H atoms to induce the H +
C5H5N reaction.
1. INTRODUCTION
Pyridine (C5H5N) is an important solvent and reagent in
organic synthesis and also a precursor widely used for agrochemical and pharmaceutical compounds. The reaction of pyridine with acids leads to formation of the pyridinium cation (C5H6N+).
1
Because pyridine is emitted into the atmosphere as the result of coal combustion and other industrial activities, the pyridinium cation and its derivatives have been observed in a significant proportion in the lower troposphere.2−4 The pyridinium ion also plays an important role in Friedel−Crafts acylation; when pyridine is employed in the reaction, the pyridinium ion forms a complex with the electrophilic acylium ion, rendering it more reactive.5 The photoinduced proton transfer in a pyridine-based polymer gel, a candidate for light-sensitive memory devices and optical switches, is responsible for its structural changes.6 The neutralization of pyridinium cations results in formation of pyridinyl radicals (C5H6N). Both
pyridinium cation and pyridinyl radical were suggested as intermediates in the hydrogenation of pyridine on metal surfaces.7,8
Pyridinium cations have four isomeric forms, shown in Figure 1, with the proton attached to the N atom of pyridine (1-C5H6N+, designated C5H5NH+) or to the carbon atom at
ortho, meta, or para positions of the pyridine ring, designated 2-C5H6N+, 3-C
5H6N+, or 4-C5H6N+, respectively. The
proto-nation of pyridine was a subject of numerous experimental investigations,1,7−10 but only C5H5NH+ was positively
identi-fied. This observation is consistent with theoretical calculations that predicted C5H5NH+as the most stable isomer; the 2-, 3-,
and 4-C5H6N+ isomers were predicted to be greater in energy
than C5H5NH+ by 266, 238, and 287 kJ mol−1, respectively,
with the MP2/6-311G(2d,p) method.1 The experimentally determined enthalpy of formation of C5H5NH+ is 746 kJ
mol−1.11 The experimentally determined proton affinity of pyridine at 298 K is 930 kJ mol−1,12 consistent with values 921−937 kJ mol−1predicted using various methods.1,13,14
The ultraviolet (UV) spectra of C5H6N+ in an aqueous
solution show absorption bands near 201, 251, 256, and 261 nm.15The reported infrared (IR) absorption bands of C5H6N+
are derived mostly from the pyridinium salts in their solid state,16in solutions,17−20 in zeolites,9,21or on a Pt surface;7,8 these spectra might be strongly perturbed by nearby molecules or ions. Nguyen and Tureček predicted vibrational wave-numbers of various isomers of C5H6N+,1
but no IR spectrum of isolated pyridinium cations, either in the gaseous phase or in matrices, has been reported.
The pyridinyl radicals, C5H6N, have four isomers with the hydrogen atom attached to the nitrogen atom (1-C5H6N,
designated C5H5NH in this paper) or to one carbon atom at
ortho, meta, or para positions of the pyridine ring, designated 2-C5H6N, 3-C5H6N, or 4-C5H6N, respectively, as shown in Figure 2. At 0 K, the energies of 2-C5H6N and 3-C5H6N Special Issue: Terry A. Miller Festschrift
Received: August 1, 2013 Revised: September 10, 2013 Published: September 11, 2013
radicals were predicted to be∼30 and 25 kJ mol−1greater than that of C5H5NH at the MP2/6-311G(2d,p) level of theory.1
Cercek and Ebert employed pulsed radiolysis to estimate the molar absorption coefficient at 275 nm for C5H5NH in an
aqueous solution to be 1800 dm3mol−1cm−1.22
The electron paramagnetic resonance (EPR) spectrum of pyridine at pH = 12.1 indicates that the structure of the pyridinyl radical is C5H5NH.23 Nguyen and Tureček predicted vibrational
wave-numbers of C5H5NH, 2-C5H6N, and 3-C5H6N,1 but no IR spectrum of pyridinyl radical in any isomeric form has been reported.
The use of solid para-hydrogen (p-H2) as a matrix host has
generated considerable interest in recent years because of the unique properties of this quantum solid.24−26 We have demonstrated that the diminished matrix cage effect makes feasible production of free radicals in solid p-H2via photolysis
in situ of precursors or via photoinduced bimolecular reactions.27−29 We extended this method to use electron bombardment during matrix deposition to produce protonated aromatic compounds and their neutral counterparts. We demonstrated several advantages of this method with the investigations of protonated benzene (C6H7+) and the
cyclohexadienyl radical (c-C6H7),
30
and protonated naphtha-lene (1-C10H9+and 2-C10H9+) and their neutral counterparts;31
the method is clean (with negligible fragmentation), sensitive, and enables high resolution and a wide spectral coverage as compared with other methods for spectral investigations of protonated aromatics. In this paper we report the IR absorption spectra of 1-pyridinium cations (C5H5NH+) and pyridinyl
radicals (C5H5NH and 4-C5H6N) produced on electron
bombardment of a mixture of C5H5N/p-H2 during matrix
deposition.
2. EXPERIMENTS
The experimental setup has been described previously.28,30 A gold-plated copper substrate cooled to 3.2 K served also as a mirror to reflect the incident IR beam to the detector. IR absorption spectra were recorded with a Fourier-transform infrared (FTIR) spectrometer equipped with a KBr beamsplit-ter and Hg−Cd−Te detector (cooled to 77 K) to cover the spectral range 450−5000 cm−1. Nine hundred scans at resolution 0.25 cm−1were generally recorded at each stage of the experiment.
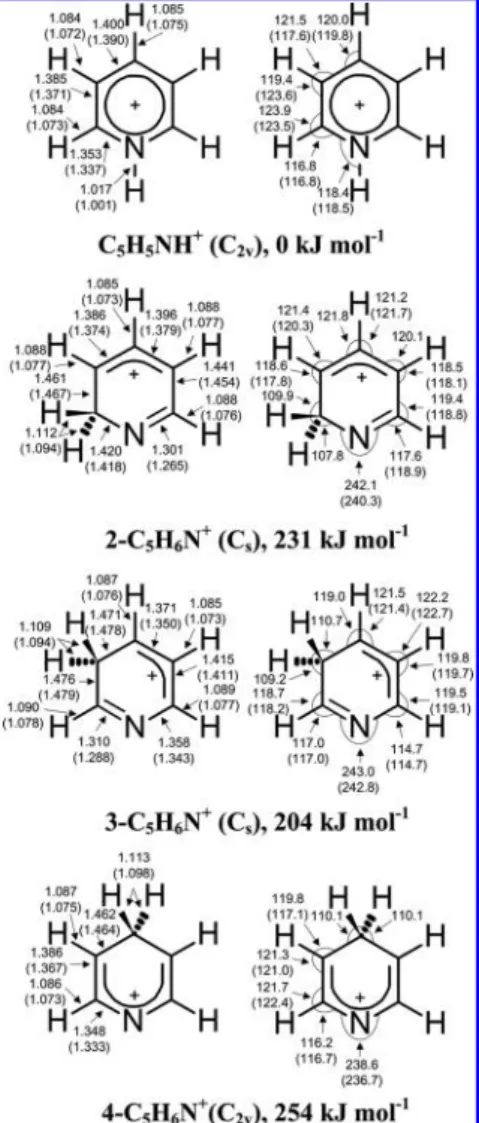
Figure 1.Geometries and relative energies (corrected for zero-point vibrational energy) of isomers of pyridinium (C5H6N+) cations calculated with the B3LYP/6-31++G(d,p) method. Bond distances are in Å and bond angles in degree. Literature values predicted with the RHF/6-31G(d,p) method1are listed parenthetically.
Figure 2.Geometries and relative energies (corrected for zero-point vibrational energy) of isomers of pyridinyl (C5H6N) radicals calculated with the B3LYP/6-31++G(d,p) method. Bond distances are in Å and bond angles in degree. Literature values predicted with the UHF/6-31G(d,p) method1are listed parenthetically.
The protonated pyridine and its neutral counterpart were produced by electron bombardment during deposition of a gaseous sample of p-H2 containing a small proportion of
C5H5N. Typically, a mixture of C5H5N/p-H2 (1/2500−1/
3500) was deposited at a rate of 13 mmol h−1over a period of 4−8 h. An electron gun was used to generate an electron beam at 250 V and 55−65 μA to aim at the cold substrate during deposition. To differentiate various products produced under electron bombardment of C5H5N/p-H2, we typically main-tained the matrix in darkness for∼15 h or performed secondary photolysis using light at 365 ± 10 nm from a light-emitting diode (375 mW) and at 405 nm from a diode laser (120 mW). The C5H6N radicals were produced also on irradiation of a Cl2/C5H5N/p-H2 matrix with ultraviolet (UV) light for 3 h followed by IR light for 0.5 h. The SiC source from the FTIR spectrometer served as a source of IR light. We have shown that these experimental procedures produce H atoms30 because irradiation of Cl2 molecules with light at 365 nm produces
isolated Cl atoms32and subsequent IR irradiation of the matrix produces vibrationally excited H2that reacts with Cl to produce H atom and HCl. The H atoms thus produced subsequently react with pyridine to form C5H6N radicals. Typically, a gaseous
mixture of C5H5N/p-H2 (1/3000−1/3500) and Cl2 were
codeposited with aflow rate ∼12 mmol h−1over a period of 10 h. The ratio of Cl2/C5H5N/p-H2 was approximately 1.8/1/
3000. To differentiate further the absorption of various products, secondary photolysis was undertaken with light at 365 nm for 30 min. During UV photolysis and during acquisition of spectral data, an IRfilter with cutoff wavelength at 2.4μm was used to avoid the reaction between Cl and p-H2. The efficiency for conversion to p-H2was controlled by the
temperature of the catalyst, typically ∼13 K, which gives a proportion of o-H2 less than 100 ppm according to the
Boltzmann distribution. Pyridine (C5H5N, Sigma-Aldrich, 99.8%) was vacuum distilled over KOH and degassed for a few minutes before use. Cl2 (99.99%, AGA Specialty Gases) was used without further purification.
3. QUANTUM-CHEMICAL CALCULATIONS
Geometry optimizations and calculations of vibrational wave-numbers were performed using B3LYP hybrid functionals33,34 and the 6-31++G(d,p) basis set. Algebraicfirst derivatives were utilized in the geometry optimization, and harmonic vibrational wavenumbers were calculated analytically at each stationary point. The anharmonic effects were calculated with a second-order perturbation approach using an effective finite-difference Table 1. Comparison of Observed Vibrational Wavenumbers (cm−1) and Relative IR Intensities of Lines in Group A+ with
Harmonic and Anharmonic Vibrational Wavenumbers and Relative IR Intensities of C5H5NH+Predicted with the
B3LYP/6-31++G(d,p) Method
calculations experiments
mode sym harmonica anharmonic p-H
2a saltsb ν1 a1 3566 (100) 3407 3381.9 (100) 2375−3300 ν2 a1 3252 (0) 3122 ν3 a1 3240 (7) 3072 2929.3 (10) 3040−3150 ν4 a1 3222 (0) 3086 ν5 a1 1672 (22) 1634 1634.7 (7) 1631−1640 ν6 a1 1520 (13) 1489 1490.2 (14) 1478−1490 ν7 a1 1227 (1) 1211 1180−1207 ν8 a1 1080 (2) 1062 ν9 a1 1046 (0) 1029 ν10 a1 1020 (2) 1004 ν11 a1 620 (0) 613 ν12 a2 1002 (0) 993 ν13 a2 880 (0) 870 ν14 a2 401 (0) 397 ν15 b1 1042 (0) 1041 ν16 b1 985 (1) 976 ν17 b1 850 (5) 832 ν18 b1 743 (58) 736 735.1 (33) 738−755 ν19 b1 669 (54) 671 666.5 (31) 662−681 ν20 b1 389 (0) 385 ν21 b2 3250 (13) 3119 2965.4 (44) 3040−3150 ν22 b2 3238 (1) 3104 ν23 b2 1652 (21) 1608 1607.9 (13) 1600−1613 ν24 b2 1577 (28) 1543 1541.8 (22) 1525−1542 ν25 b2 1414 (2) 1388 1358−1378 ν26 b2 1371 (6) 1339 1330.1 (6) 1318−1336 ν27 b2 1297 (2) 1277 1235−1255 ν28 b2 1198 (2) 1186 ν29 b2 1084 (3) 1055 ν30 b2 644 (0) 638
aIR intensities as percent of that of most intense line near 3500 cm−1are listed in parentheses. The IR intensity of the line at 3566 cm−1is predicted to be 160.9 km mol−1. Additional lines at 2938.3, 2911.8, and 2880.0 cm−1are assigned to the nonfundamental such as combination and/or overtone bands of (2ν19+ν23), (ν23+ν26), and 2ν6/(2ν18+ν26), respectively.bSummary of results from various pyridinium salts.16
evaluation of the third and semidiagonal fourth derivatives. Some calculations were performed also with (1) the B3PW91 method that uses Becke’s three-parameter hybrid exchange functionals and a correlation functional of Perdew and Wang35 with a 6-311++G(2d,2p) basis set and (2) the MP2 (Møller− Plesset second-order perturbation) method36 with an aug-cc-pVDZ basis set.37 All calculations employed the Gaussian 09 programs.38
3.1. Pyridinium (C5H6N+) Cations. Important structural
parameters and relative energies predicted with the B3LYP/6-31++G(d,p) method for various isomers of C5H6N+ are
presented in Figure 1. A list of parameters of all isomers of C5H6N+ is given in Table S1 (Supporting Information); a
comparison of structural parameters of C5H5NH+ predicted
with various quantum-chemical methods is given in Table S2 (Supporting Information). The N-protonated form, 1-pyridi-nium (C5H5NH+) cation, is predicted to be the most stable.
The energies of 2-, 3-, and 4-C5H6N+ relative to that of
C5H5NH+, corrected with ZPE (zero-point vibrational energy),
are predicted to be 231, 204, and 254 kJ mol−1 with the B3LYP/6-31++G(d,p) method; these values are slightly smaller than values 266, 238, and 287 kJ mol−1 predicted with the MP2/6-311G(2d,p) method.1
The harmonic and anharmonic vibrational wavenumbers and IR intensities of C5H5NH+ predicted with the B3LYP/6-31+
+G(d,p) method are listed in Table 1. Those of 2-, 3-, and 4-C5H6N+ are listed in Table S3 (Supporting Information). A
comparison of harmonic vibrational wavenumbers and IR intensities of C5H5NH+ predicted with various computational
methods is presented in Table S4 (Supporting Information). 3.2. Pyridinyl Radicals (C5H6N). Important structural
parameters and relative energies of C5H6N radicals predicted with the B3LYP/6-31++G(d,p) method are presented in Figure 2. An extended list of parameters of these pyridinyl radicals is given in Table S5 (Supporting Information). The 1-pyridinyl (C5H5NH) radical is predicted to be the most stable, with
enthalpy of reactionΔH0=−128.8 kJ mol−1from H + C 5H5N.
The ZPE-corrected energies of 2-, 3-, and 4-C5H6N relative to
that of C5H5NH are predicted to be 30, 28, and 32 kJ mol−1 with the B3LYP/6-31++G(d,p) method. These values are similar to values 30 and 25 kJ mol−1 predicted for 2- and 3-C5H6N with the MP2/6-311G(2d,p) method; the energy of
4-C5H6N was previously uninvestigated.1
The harmonic and anharmonic vibrational wavenumbers and IR intensities of C5H5NH and 4-C5H6N according to the
B3LYP/6-31++G(d,p) method are listed in Tables 2 and 3, Table 2. Comparison of Observed Vibrational Wavenumbers (cm−1) and Relative IR Intensities of Lines in Group A with Harmonic and Anharmonic Vibrational Wavenumbers and Relative IR Intensities of C5H5NH Predicted with the B3LYP/6-31+
+G(d,p) Method
calculations experiments
mode sym harmonica anharmonic (e-impact)p-H2 a (H rx)p-H2a
ν1 a′ 3660 (64) 3567 3493.1 (77) 3493.1 (84) ν2 a′ 3238 (0) 3106 ν3 a′ 3228 (14) 3088 ν4 a′ 3196 (1) 3075 ν5 a′ 1666 (3) 1614 ν6 a′ 1462 (2) 1430 ν7 a′ 1211 (0) 1189 ν8 a′ 1036 (1) 1018 ν9 a′ 989 (16) 968 972.9 (20) 972.9 (20) ν10 a′ 961 (57) 949 952.8 (54) 952.9 (53) ν11 a′ 923 (2) 878 ν12 a′ 690 (48) 662 641.8 (33) 641.7 (32) ν13 a′ 618 (100) 606 616.2 (100) 616.0 (100) ν14 a′ 615 (69) 593 605.4 (57) 605.5 (51) ν15 a′ 577 (3) 582 ν16 a′ 272 (112) ∼218?b ν17 a′ 196 (56) ∼408?b ν18 a″ 3234 (16) 3089 ν19 a″ 3198 (23) 3060 ν20 a″ 1566 (0) 1527 ν21 a″ 1467 (36) 1438 1447.9 (37) 1447.9 (37) ν22 a″ 1367 (5) 1339 1346.2 (3) 1346.2 (4) ν23 a″ 1352 (76) 1310 1311.6 (64) 1311.4 (60) ν24 a″ 1220 (1) 1203 ν25 a″ 1080 (4) 1058 ν26 a″ 1011 (3) 969 ν27 a″ 929 (0) 894 ν28 a″ 711 (1) 684 ν29 a″ 638 (1) 635 ν30 a″ 434 (0) 423
aIR intensities as percent of that of the intense line near 610 cm−1(ν
13) are listed in parentheses. The IR intensity of the line near 618 cm−1is predicted to be 73.4 km mol−1.bUnreliable anharmonicity due toflat potential energy surfaces.
respectively. Those of 2-, 3-, and 4-C5H6N are compared in Table S6 (Supporting Information).
The molecular electrostatic potential map and Mulliken atomic charges (in units of e, listed in parentheses) for individual atoms of C5H5N computed with the B3PW91/6-311++G(2d,2p) method are shown in Figure 3. The predicted atomic charges on N, C1, C2, C3, and C4 atoms in C5H5N are −0.257e, −0.129e, +0.012e, and −0.145e, respectively.
4. EXPERIMENTAL RESULTS
4.1. Electron Bombardment on C5H5N/p-H2 Mixtures.
The IR spectrum of a C5H5N/p-H2 matrix exhibited intense
lines at 3084.6, 3039.0, 3007.0, 1598.5, 1583.0, 1579.5, 1483.2, 1440.9, 1217.7, 1031.1, 991.3, 744.3, 701.7, and 601.9 cm−1, as shown in Figure 4a of ref 39. The wavenumbers of these lines, listed in Table 1 of ref 39, agree with the reported spectra of gaseous C5H5N (ref 40) and C5H5N isolated in N2(refs 41 and 42) and Ar matrices.43 When the C5H5N/p-H2 mixture was
bombarded with an e-gun during deposition, many new features appeared. The difference spectrum obtained by subtraction of the spectrum of a C5H5N/p-H2(1/3000) matrix from the spectrum of the electron bombarded C5H5N/p-H2
(1/3000) mixture is presented in Figure 4a. To differentiate the absorption lines of positively charged species from those of the
Table 3. Comparison of Observed Vibrational Wavenumbers (cm−1) and Relative IR Intensities of Lines in Group B with Harmonic and Anharmonic Vibrational Wavenumbers and Relative IR Intensities of 4-C5H6N Predicted with the B3LYP/6-31+
+G(d,p) Method
calculations experiments
mode sym harmonica anharmonic p-H
2(e-impact)a p-H2(H rx)a ν1 a1 3195 (2) 3043 ν2 a1 3167 (25) 3045 ν3 a1 2936 (83) 2823 2782.7 (34) 2782.8 (35) ν4 a1 1599 (17) 1548 1553.2 (18) 1553.2 (21) ν5 a1 1459 (4) 1428 1428.2 (13) 1428.2 (10) ν6 a1 1422 (32) 1394 1376.5 (44) 1376.5 (46) ν7 a1 1222 (2) 1197 ν8 a1 1043 (6) 1015 ν9 a1 978 (45) 960 961.9 (59) 961.8 (61) ν10 a1 892 (10) 879 ν11 a1 565 (7) 558 ν12 a2 1162 (0) 1125 ν13 a2 989 (0) 968 ν14 a2 747 (0) 734 ν15 a2 361 (0) 352 ν16 b1 2928 (33) 2746 2737.3 (13) 2737.3 (20) ν17 b1 973 (1) 949 ν18 b1 912 (29) 890 885.9 (33) 885.7 (35) ν19 b1 700 (98) 688 685.9 (94) 685.9 (90) ν20 b1 532 (100) 520 518.8 (100) 518.8 (100) ν21 b1 194 (0) 190 ν22 b2 3194 (146) 3064 ν23 b2 3165 (76) 3035 ν24 b2 1490 (89) 1452 1452.6 (96) 1452.5 (87) ν25 b2 1421 (9) 1388 1390.8 (6) 1390.8 (9) ν26 b2 1355 (7) 1327 1331.9? (7) ν27 b2 1201 (21) 1176 ν28 b2 1131 (6) 1112 ν29 b2 979 (4) 964 ν30 b2 637 (18) 630 623.5 (12) 623.5 (12)
aIR intensities as percent of that of the intense line near 532 cm−1are listed in parentheses. The IR intensity of the line near 532 cm−1is predicted to be 32.6 km mol−1.
Figure 3.Molecular electrostatic potential of C5H5N mapped onto 0.0004 e Å−3 isosurface of the electron density predicted with the B3PW91/6-311++G(2d,2p) method. Individual Mulliken atomic charges (in units of e) on N, C, and H atoms are listed in parentheses.
neutral ones, we maintained the matrix in darkness for 15 h to allow diffusion of trapped electrons to react with the cations in solid p-H2. The resultant difference spectrum is shown in
Figure 4b; lines pointing upward indicate production, whereas those pointing downward indicate destruction. Subsequently, we irradiated the matrix at 365 nm for∼40 min to release the trapped electrons to neutralize the cations in the p-H2matrix;
photolysis of various species might also take place. The observed difference spectrum is shown in Figure 4c. Some experiments with secondary photolysis were also performed. In Figure 4d we show the difference spectrum of the matrix upon further irradiation at 405 nm for 30 min.
As shown in Figure 4b, lines at 3381.9, 2965.4, 2938.3, 2929.3, 2911.8, 2880.0, 1634.7, 1607.9, 1541.8, 1490.2, 1330.1, 735.1, and 666.5 cm−1decreased in intensity after maintaining the matrix in darkness for a prolonged period and upon further irradiation at 365 and 405 nm. These features, designated as
group A+, demonstrate a correlated change in intensity at
various stages of experiments and in separate experiments. They are assigned to the 1-pyridinium (C5H5NH+) cation, to
be discussed in section 5.1. Observed wavenumbers and relative IR intensities of lines in group A+are compared with predicted values in Table 1. As the region 3000−3100 cm−1is severely interfered with by absorption of C5H5N, no line in this region
can be positively identified and characterized.
The features pointing upward in Figure 4b are separated into two groups according to their behavior upon secondary photolysis. Lines at 3493.1, 1447.9, 1346.2, 1311.6, 972.9, 952.8, 641.8, 616.2, and 605.4 cm−1 decreased in intensity significantly upon irradiation at 365 nm and only slightly upon irradiation at 405 nm; they are designated as group A and assigned to the 1-pyridinyl (C5H5NH) radical, to be discussed in section 5.2. Observed wavenumbers and relative IR intensities of lines in group A are compared with predicted values in Table 2.
The intensities of lines at 2782.7, 2737.3, 1553.2, 1452.6, 1428.2, 1390.8, 1376.5, 961.9, 885.9, 685.9, 623.5, and 518.8 cm−1that increased slightly upon irradiation of the sample at 365 nm but decreased significantly upon irradiation at 405 nm, are designated as group B and assigned to the 4-C5H6N radical,
to be discussed in section 5.2. Observed wavenumbers and relative IR intensities of lines in group B are compared with predicted values in Table 3.
The mixing ratios of the precursor and observed products were estimated using the method described by Ruzi et al.44 Using the predicted IR intensities of several intense lines at 1490.2, 1541.8, and 3381.9 cm−1for C5H5NH+, 616.2, 1447.9,
and 3493.1 cm−1 for C5H5NH, and 518.8, 685.9, 961.9, and 1452.6 cm−1for 4-C5H6N, we estimated the mixing ratios after
deposition to be [C5H5NH+] = 2.0± 0.4 ppm, [C5H5NH] =
2.6 ± 0.6 ppm, and [4-C5H6N] = 0.85 ± 0.12 ppm. The variations in mixing ratio as a result of maintaining the sample in darkness for 15 h (Figure 4b) areΔ[C5H5NH+] =−0.8 ±
0.2 ppm,Δ[C5H5NH] = 1.9 ± 0.5 ppm, and Δ[4-C5H6N] =
1.6± 0.3 ppm. Upon irradiation of the sample at 365 nm for 40 min (Figure 4c), Δ[C5H5NH+] = −0.31 ± 0.04 ppm,
Δ[C5H5NH] =−2.5 ± 0.7 ppm, and Δ[4-C5H6N] = 0.37 ±
0.05 ppm. Upon irradiation of the sample at 405 nm for 30 min (Figure 4d),Δ[C5H5NH+] =−0.34 ± 0.02 ppm, Δ[C5H5NH]
=−1.9 ± 0.5 ppm, Δ[4-C5H6N] =−1.6 ± 0.3 ppm.
4.2. Photolysis of Cl2/C5H5N/p-H2 matrices. After
codeposition of Cl2 and C5H5N in p-H2 we observed, in addition to lines of C5H5N, new features, intense at 3091.4,
3047.2, 1591.8, 1446.7, 1212.7, 1070.3, 1003.7, 746.2, 699.0, 616.2, and 458.4 cm−1that have been assigned to theσ-bonded C5H5N−Cl2complex.
39
Upon irradiation of the sample at 365 nm for 3 h, the intensities of lines of C5H5N increased slightly and those of C5H5N−Cl2diminished and a set of new lines at
1449.7, 1200.6, and 688.7 cm−1due to C5H5NCl appeared; as reported previously, because C5H5NCl was also dissociated by
light at 365 nm, these lines are weak.39Figure 5a shows a partial spectrum of the UV-irradiated Cl2/C5H5N/p-H2(1.8/1/3000) sample; lines of C5H5N, C5H5N−Cl2, and C5H5N−HCl are
marked with“o”, “*”, and “Δ”, respectively. Broad and intense features at 1052.1 and 904.5 cm−1are due to the C5H5N−HCl
complex.39,45 The matrix was subsequently irradiated with IR light for 30 min. The difference spectrum obtained on subtracting the spectrum in Figure 5a from the spectrum recorded after irradiation with IR light is presented in Figure 5b. New lines similar to those in groups A and B discussed in
Figure 4.Difference spectra of (a) the C5H5N/p-H2(1/3000) mixture upon electron bombardment during deposition at 3.2 K for 6 h (lines of C5H5N are stripped according to the spectrum of a C5H5N/p-H2 (1/3000) matrix), (b) the sample after being maintained in darkness at 3.2 K for 15 h, (c) the sample upon irradiation at 365 nm for 40 min, and (d) the sample upon further irradiation at 405 nm for 30 min. The assignments of lines in each group are A+, C5H5NH+, A, C5H5NH, and B, 4-C5H6N. Spectral regions suffering from severe interference from absorption of C5H5N are marked with light gray.
the preceding sections were observed. Upon secondary photolysis at 365 nm, intensities of lines in group A decreased, whereas those of lines in group B increased, as shown in Figure 5c. Lines of group A are indicated in Figure 5b,c and those of group B are indicated in Figure 5c.
Similarly to the description in the preceding section, we estimated the variation in mixing ratios after IR irradiation (Figure 5b) to be Δ[C5H5NH] = 2.4 ± 0.4 ppm and
Δ[4-C5H6N] = 3.8± 0.7 ppm. The variation in mixing ratios after
secondary photolysis at 365 nm (Figure 5c) was estimated to beΔ[C5H5NH] =−0.7 ± 0.1 ppm and Δ[4-C5H6N] = 1.0 ±
0.2 ppm.
Upon photolysis of the Cl2/C5H5N/p-H2samples at 365 nm, weak lines near 2894, 2836, and 2873 cm−1 appeared; these lines are due to HCl, (HCl)2, and (HCl)n, respectively.46Their
intensities increased significantly when the matrix was irradiated with IR light because of the reaction between Cl and H2.
5. DISCUSSION
5.1. Assignment of Lines in Group A+to C
5H5NH+. The
observation of the decay of the lines assigned in group A+
indicates that the most likely carrier of these lines is one of the four isomeric forms of pyridinium cations because the neutralization took place when the matrix sample was maintained in darkness for a prolonged period or irradiated with light at 365 nm. In Figure 6a we show the inverted spectrum of Figure 4b so that lines in group A+ are pointing upward; regions of interference due to absorption of C5H5N are
shaded with light gray. In Figure 6b−e, we plot the simulated IR spectra of four isomers of pyridinium cations: C5H5NH+and
2-, 3-, and 4-C5H6N+, respectively. These spectra were
simulated according to the anharmonic vibrational
wave-Figure 5.(a) Spectrum of the Cl2/C5H5N/p-H2(1.8/1/3000) sample, deposited at 3.2 K for 10 h and irradiated at 365 nm for 3 h. Lines due to C5H5N, C5H5N−Cl2, and C5H5N−HCl complex are marked with “o”, “*”, and “Δ”, respectively. Difference spectra of (b) the sample after subsequent irradiation with IR light for 30 min, and (c) the sample after further irradiation at 365 nm for 30 min. The assignments of lines in each group are A, C5H5NH, and B, 4-C5H6N. Spectral regions suffering from severe interference from absorption of C5H5N are marked with light gray.
Figure 6.Comparison of experimental spectra with simulated spectra. (a) Inverted spectrum of Figure 4b, the difference spectrum of the electron-bombarded C5H5N/p-H2 (1/3000) sample after being maintained in darkness at 3.2 K for 15 h. IR stick spectra of (b) C5H5NH+, (c) 2-C5H6N+, (d) 3-C5H6N+, and (e) 4-C5H6N+simulated according to anharmonic vibrational wavenumbers and IR intensities predicted with the B3LYP/6-31++(d,p) method. Lines in group A+are assigned to C5H5NH+. Spectral regions that suffer from severe interference from absorption of C5H5N are marked with light gray.
numbers and IR intensities predicted with the B3LYP/6-31+ +G(d,p) method, listed in Table 1 and Table S3 (Supporting Information), and a spectral width of 0.5 cm−1. All four spectra show distinct spectral patterns because the additional N−H or C−H bonding at various sites has distinct effects on the bonding of the aromatic ring.
The observed wavenumbers and relative IR intensities of lines agree satisfactorily with those calculated for the C5H5NH+
cation, but not with any of the 2-, 3-, and 4-C5H6N+cations, as
illustrated in Figure 6. Observed line positions and relative intensities of lines in group A+are compared with harmonic and
anharmonic vibrational wavenumbers and relative IR intensities predicted for C5H5NH+ with the B3LYP/6-31++G(d,p)
method in Table 1. The calculated harmonic wavenumbers and relative IR intensities of C5H5NH+ obtained with various
methods1,7,47 are compared in Table S4 (Supporting Information). The six most intense lines were observed at 3381.9, 1637.8, 1607.9, 1541.8, 735.1, and 666.5 cm−1, near the anharmonic vibrational wavenumbers predicted at 3407 (NH stretch), 1634 (CC stretch), 1608 (CC and CN stretch), 1543 (CH in-plane bend), 736 (CH/NH out-of-plane bend mixed with ring out-of-plane deformation), and 671 (CH/NH out-of-plane bend mixed with out-of-plane ring deformation) cm−1; the NH-stretching mode is characteristic of the structure of this cation. Assignments of lines at 2965.8 and 2929.2 cm−1 in the CH-stretching region are tentative because the predicted anharmonic vibrational wavenumbers, 3119 and 3072 cm−1, are slightly greater; the possibility that these two lines are due to some overtone or combination bands and these two CH-stretching lines are buried in the 3000−3100 cm−1region with which there is severe interference from absorption of pyridine cannot be positively excluded. The deviations between the calculated anharmonic vibrational wavenumbers and the observed wavenumbers are smaller than 0.7%, except lines tentatively assigned to the symmetric and antisymmetric CH-stretching (ν3andν21) modes, which show deviations ∼5%.
Most observed vibrational wavenumbers are similar to those reported for pyridinium salts C5H5NH+X−, in which X−is the
counteranion;16the ranges of observed wavenumbers in various salts are listed in Table 1 for comparison. We observed a line at 3381.9 cm−1for the NH-stretching mode. This line is to the blue of the range 2375−3300 cm−1 reported for this mode, consistent with the expectation that the N−H moiety of isolated C5H5NH+ is free whereas that of the pyridinium salt
interacts with the anion.
Considering the observed photolytic and chemical behavior, the agreement of wavenumber and relative IR intensity between lines observed in group A+ and those predicted for the
C5H5NH+ radical, the absence of some unique features
expected for the 2-, 3-, and 4-C5H6N+ radicals, and the
calculated thermochemistry, we assign these new features in group A+ to the 1-pyridinium (C5H5NH+) cation, the most
stable isomer.
5.2. Assignment of Lines in Groups A and B to the C5H5NH and 4-C5H6N Radicals. The intensities of lines in
group A increased after the e-impacted C5H5N/p-H2 matrix
was maintained in darkness for 15 h and decreased significantly upon irradiation at 365 nm, indicating that the carrier might be a neutral species that dissociates at 365 nm. The intensities of lines in group B increased after the sample was maintained in darkness and upon irradiation at 365 nm but decreased significantly upon irradiation at 405 nm, indicating that the carrier might be a neutral species that dissociates at 405 nm.
Both these features were observed also in experiments of Cl2/ C5H5N/p-H2 irradiated with UV and IR light, in which
reactions of H with C5H5N are expected and no ion can be produced. We hence expect that the carriers of lines in groups A and B are likely the isomers of C5H6N radicals.
In Figure 7a we reproduce the spectrum of Figure 4b, the difference spectrum recorded on maintaining the e-impacted
C5H5N/p-H2 matrix in darkness for 15 h. In Figure 7b we
reproduce the spectrum of Figure 5c, the difference spectrum upon secondary irradiation at 365 nm of the Cl2/C5H5N/p-H2
matrix that was previously irradiated with UV and IR light. Lines in group A (pointing upward in Figure 7a and pointing
Figure 7.Comparison of experimental spectra with simulated spectra. (a) Spectrum reproduced from Figure 4b and (b) spectrum reproduced from Figure 5c. IR stick spectrum of (c) C5H5NH, (d) 2-C5H6N, (e) 3-C5H6N, and (f) 4-C5H6N simulated according to anharmonic vibrational wavenumbers and IR intensities predicted with the B3LYP/6-31++(d,p) method. Lines in groups A and B are assigned to C5H5NH and 4-C5H6N, respectively. Spectral regions that suffer from severe interference from absorption of C5H5N are marked with light gray.
downward in Figure 7b) and B (pointing upward in Figure 7a,b) are marked; regions of interference due to absorption of C5H5N are marked with light gray.
In Figure 7c−f, we plot simulated IR spectra of four possible isomers of pyridinyl radicals: C5H5NH, 2-, 3-, and 4-C5H6N,
respectively; the spectra were simulated according to the anharmonic vibrational wavenumbers and IR intensities predicted with the B3LYP/6-31++(d,p) method, listed in Table 2 and Table S6 (Supporting Information), and a spectral width of 0.5 cm−1. Similar to the case of C5H6N+, the spectral
patterns of all four spectra are distinct because the additional N−H or C−H bonding at various sites has varied effects on the bonding of the aromatic ring.
The observed wavenumbers and relative IR intensities of lines in group A agree satisfactorily with those calculated for the C5H5NH radical, but not with any of the 2-, 3-, and 4-C5H6N
radicals, as illustrated in Figure 7. The observed positions and relative intensities of lines in group A are compared with harmonic and anharmonic vibrational wavenumbers and relative IR intensities predicted for C5H5NH with the B3LYP/6-31++G(d,p) method in Table 2. The five most intense lines were observed at 3493.1, 1311.6, 952.8, 616.2, and 605.4 cm−1, near the anharmonic vibrational wavenumbers predicted at 3567 (NH-stretch), 1310 (CCC and CNC asymmetric stretch), 949 (CNC symmetric stretch), 606 (CH out-of-plane bend mixed with ring out-of-plane deformation), and 593 (CH out-of-plane bend) cm−1. The NH-stretching mode near 3490 cm−1is characteristic of C5H5NH. It is slightly
greater than the value 3381.9 cm−1 observed for C5H5NH+,
indicating a slightly stronger N−H bonding, consistent with a predicted N−H bond length (1.008 Å) smaller than that (1.017 Å) of C5H5NH+. Assignments in the CH-stretching region are
difficult because of severe overlap with C5H5N. The observed deviations between calculated anharmonic wavenumbers and observed line positions are less than 0.7% for most observed lines, with the largest deviation of 3.0% forν12.
The observed wavenumbers and relative IR intensities of lines in group B agree satisfactorily with those calculated for the 4-C5H6N radical, but not with any of the C5H5NH, 2-C5H6N, and 3-C5H6N radicals, as illustrated in Figure 7. Thefive most intense lines were observed at 2782.4, 1452.6, 961.9, 685.9, and 518.8 cm−1, near the anharmonic vibrational wavenumbers predicted at 2823 (CH2symmetric stretch), 1452 (out-of-phase
CC stretch), 960 (out-of-phase CNC and CCC bends), 688 (CH out-of-plane bend), and 520 (CH2rock mixed with CNC out-of-plane deform) cm−1. The small (2782.4 and 2737.3 cm−1) CH-stretching frequencies and the intense CH2rocking mode (518.8 cm−1) are characteristic of theσ-bonded C5H6N
having a CH2moiety. Assignments in the CH-stretching region
are difficult because of severe overlap with C5H5N; we hence
are unable to identify the two intense lines near 3064 and 3035 cm−1 predicted by theory. The observed deviations between calculated anharmonic wavenumbers and observed line positions are less than 0.4% for most observed lines, with the largest deviations of∼1.4% for the CH2-stretching modes.
Although the H atoms produced in experiments of both types are expected to be small and the reaction of C5H6N with
the second H is less likely, for completeness we predicted the vibrational wavenumbers of dihydrogenated pyridines (C5H7N) for comparison. In Table S7 (Supporting Information) we list the vibrational wavenumbers and relative IR intensities calculated for 1,2-C5H7N, 1,3-C5H7N, and 1,4-C5H7N. These
predicted spectra of isomers of C5H7N agree with those observed for lines in neither group A nor B.
Considering the observed photolytic and chemical behavior, the agreement of wavenumber and relative IR intensity between lines observed in group A and predicted for the C5H5NH radical, the absence of some unique features predicted for 2-, 3-, and 4-C5H6N, and the calculated thermochemistry, we assign the observed new features in group A to the 1-pyridinyl (C5H5NH) radical, the most stable isomer. We analogously assign the observed new features in group B to the 4-pyridinyl (4-C5H6N) radical.
5.3. Formation Mechanism inp-H2. Ionization of H2by
electron impact produces H2+; subsequent rapid exothermic
proton transfer,
+ → +
+ +
H2 H2 H3 H (1)
produces H and H3+.48
The H3+ thus produced can readily
transfer a proton to C5H5N to form C5H6N+,
+ → +
+ +
H3 C H N5 5 C H N5 6 H2 (2)
We believe that these reactions took place mainly on the surface of p-H2because electron bombardment after deposition
produced protonated species in much less amount.
The enthalpies of reaction for the formation of C5H5NH+
and 2-, 3-, and 4-C5H6N+ at 0 K are predicted to be −534,
−290, −316, and −265 kJ mol−1, respectively, with the B3LYP/
6-31++G(d,p) method. Our observation of protonated pyridine as C5H5NH+, but not 2-, 3-, or 4-C5H6N+, agrees with the
prediction that C5H5NH+ is much more stable than the
C-protonated isomers. The barrier from the most stable isomer C5H5NH+to form 2-C
5H6N+is predicted to be∼276 kJ mol−1,
smaller than the exothermicity of the proton transfer in the reaction H3++ C
5H5N→ C5H5NH++ H2. We observed no line
of 2-C5H6N+ or 3-C5H6N+ or 4-C5H6N+, presumably because
the excess energy in C5H5NH+upon proton transfer from H 3+
to C5H5N was rapidly quenched.
The pyridinyl radicals, C5H6N, are expected to be formed in neutralization of pyridinium cations or in reactions between the H atom and pyridine:
+ →
+ −
C H NH5 5 e C H NH5 5 (3)
+ →
C H N5 5 H C H N5 6 (4)
The enthalpies of reaction 4 at 298 K for the formation of C5H5NH and 2-, 3-, and 4-C5H6N are predicted to be−129,
−100, −102, and −98 kJ mol−1with the B3LYP/6-31++G(d,p)
method; the corresponding barriers were predicted to be 7.5, 17.2, 16.1, and 18.0 kJ mol−1, respectively, similar to those predicted with the B3LYP/6-311+G(d,p)//B3LYP/6-31G(d) method.49
The observation of the C5H5NH radical in experiments of
electron bombardment of C5H5N/p-H2matrices is consistent with a theoretical prediction that C5H5NH is the most stable
isomer of pyridinyl radicals; the neutralization of the only observed isomer of protonated pyridine, C5H5NH+, leads to
only C5H5NH. In experiments with UV/IR irradiation of Cl2/ C5H5N/p-H2matrices, the observation of the C5H5NH radical
is consistent with a theoretical prediction that the formation of C5H5NH via reaction 4 has the least barrier; considering the
errors in predicting this small barrier (8± 4 kJ mol−1) and the excess energy that the H atom might have after the reaction Cl + H2(v = 1)→ HCl + H in solid p-H2at 3.2 K, the formation of C5H5NH via reaction 4 is likely to occur.
The reason that among all C-monohydrogenated pyridines only 4-C5H6N, not 2- or 3-C5H6N, was preferentially formed
from both electron bombardment and H-reaction experiments is unclear. The three isomers 2-, 3-, and 4-C5H6N were
predicted to have similar energies and similar barrier heights for formation from H + C5H5N, with those for formation of
4-C5H6N being the largest. One would expect that all three isomers would be formed from reaction 4. One possible reason is that the charge density of C4 predicted with the B3PW91/6-311++G(2d,2p) method is more negative than that of C3 and C2, as shown in Figure 3, so that the electrophilic H atom might prefer to attack the C4 atom to form 4-C5H6N. However,
this tendency should be reflected in the reaction barrier. More sophisticated theoretical calculations on the reaction H + C5H5N, the interconversion among isomers of C5H6N, and reactions of H + C5H6N are needed to explain our
observations.
6. CONCLUSION
Electron bombardment was applied during the deposition of a mixture of C5H5N and excess p-H2 at 3.2 K to generate
C5H5NH+, C
5H5NH, and 4-C5H6N in the p-H2 matrix. The
intensities of lines of C5H5NH and 4-C5H6N radicals increased
upon maintaining the matrix in darkness for a prolonged period, whereas those of C5H5NH+decreased. The formation
of C5H5NH and 4-C5H6N radicals was observed also in experiments in which a Cl2/C5H5N/p-H2matrix was irradiated
with light at 365 nm and in the IR region to generate H atoms for reaction with C5H5N. Secondary photolysis at 365 nm at
the initial stage diminished C5H5NH but enhanced 4-C5H6N, whereas photolysis at 405 nm diminished 4-C5H6N signi
fi-cantly.
The spectra were assigned according to the expected chemistry, the predicted exothermicity and energy barriers for possible reactions, and comparison with the anharmonic vibrational wavenumbers and IR intensities predicted with the B3LYP/6-31++G(d,p) method. The formation of C5H5NH+
and C5H5NH is consistent with theoretical predictions indicating that they are the most stable among all isomers, whereas the formation of 4-C5H6N but not 2-C5H6N or 3-C5H6N is inexplicable with the current theoretically predicted
barriers and enthalpies of formation of possible reactions but might be explained by the charge density over the C4 atom that is more negative than those of the C2 and C3 atoms so that the H atom might attack C4 more favorably. More sophisticated computations are needed to explain the observed selectivity.
■
ASSOCIATED CONTENT*
S Supporting InformationGeometric parameters of all isomers of C5H6N+optimized with
the B3LYP/6-31++G(d,p) method, comparison of parameters of C5H5NH+ predicted with various methods, harmonic and
anharmonic vibrational wavenumbers and IR intensities of 2-, 3-, 4-C5H6N+ cations, comparison of harmonic vibrational
wavenumbers and IR intensities of C5H5NH+ predicted with
various methods, geometric parameters of all isomers of C5H6N, harmonic and anharmonic vibrational wavenumbers and IR intensities of 2-, 3-, 4-C5H6N radicals, harmonic and
anharmonic vibrational wavenumbers and IR intensities of various isomers of C5H7N radicals (Tables S1−S7). This
material is available free of charge via the Internet at http:// pubs.acs.org.”
■
AUTHOR INFORMATIONCorresponding Author
*Y.-P. Lee: e-mail, yplee@mail.nctu.edu.tw. Notes
The authors declare no competingfinancial interest.
■
ACKNOWLEDGMENTSThe National Science Council of Taiwan (Grant No. NSC101-2745-M009-001-ASP) and the Ministry of Education, Taiwan (“Aim for the Top University Plan” of National Chiao Tung University) supported this work. The National Center for High-performance Computing provided computer time.
■
REFERENCES(1) Nguyen, V. Q.; Tureček, F. Gas-phase Protonation of Pyridine. A Variable-time Neutralization-Reionization and ab initio study of Pyridinium Radicals. J. Mass. Spectrom. 1997, 32, 55−63.
(2) Eisele, F. L.; McDaniel, E. W. Mass Spectrometry Study of Tropospheric Ions in the Northeastern and Southwestern United States. J. Geophys. Res., [Atmos.] 1986, 91, 5183−5188.
(3) Eisele, F. L. Natural and Transmission Line Produced Positive Ions. J. Geophys. Res., [Atmos.] 1989, 94, 6309−6318.
(4) Tanner, D. J.; Eisele, F. L. Ions in Oceanic and Continental Air Masses. J. Geophys. Res., [Atmos.] 1991, 96, 1023−1031.
(5) Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Blackwell Publishing: Chichester, U.K., 2010.
(6) Vaganova, E.; Wachtel, E.; Leitus, G.; Danovich, D.; Lesnichin, S.; Shenderovich, I. G.; Limbach, H.; Yitzchaik, S. Photoinduced Proton Transfer in a Pyridine Based Polymer Gel. J. Phys. Chem. B 2010, 114, 10728−10733.
(7) Lee, I. C.; Masel, R. I. Evidence for Pyridinium Cation Formation during Coadsorption of Pyridine and Hydrogen on (2× 1) Pt(110). J. Phys. Chem. B 2002, 106, 368−373.
(8) Bratile, K. M.; Komvopoulos, K.; Somorjai, G. A. Sum Frequency Generation Vibrational Spectroscopy of Pyridine Hydrogenation on Platinum Nanoparticles. J. Phys. Chem. C 2008, 112, 11865−11868.
(9) Parry, E. P. An Infrared Study of Pyridine Adsorbed on Acidic Solids−Characterization of surface acidity. J. Catal. 1963, 2, 371−379. (10) Paukshtis, E. A.; Karakchiev, L. G.; Kotsarenko, N. S. IR-spectroscopic Study of Mechanism of Pyridine Protomation on the Surface of HNaY Zeolite. React. Kinet. Catal. Lett. 1977, 6, 147−152. (11) Lias, S. G.; Liebman, J. F.; Levin, R. D. Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules. J. Phys. Chem. Ref. Data 1984, 13, 695−809.
(12) Hunter, E. P. L.; Lias, S. G. Evaluated Gas Phase Basicities and Proton Affinities of Molecules: An Update. J. Phys. Chem. Ref. Data. 1998, 27, 413−657.
(13) Hillebrand, C.; Klessinger, M.; Eckert-Maksić, M.; Maksić, Z. B. Theoretical Model Calculations of the Proton Affinities of Amino-alkanes, Aniline, and Pyridine. J. Phys. Chem. 1996, 100, 9698−9702. (14) Ebrahimi, A.; Habibi-Khorasani, S. M.; Johantab, M. Additivity of Substituent Effects on the Proton Affinity and Gas-phase Basicity of Pyridines. Comput. Theor. Chem. 2011, 966, 31−37.
(15) Spinner, E. The Electronic Spectra of Some Monosubstituted Pyridines and Pyridinium Ions. J. Chem. Soc. 1963, 3855−3859.
(16) Cook, D. Vibrational Spectra of Pyridinium Salts. Can. J. Chem. 1961, 39, 2009−2024.
(17) Foglizzo, R.; Novak, A. Spectres de Vibration de Quelques Halogenures de Pyridinium. J. Chim. Phys. 1969, 66, 1539−1550.
(18) Glazunov, V. P.; Odinokov, S. E. Infrared Spectra of Pyridinium Salts in Solution-II. Fermi Resonance and Structure of νNH Bands. Spectrochim. Acta A 1982, 38, 409−415.
(19) Gabes, W.; Stufkens, D. J.; Gerding, H. Structures, Raman, Infrared and Electronic Adsorption Spectra of Pyridinium Trihalides. J. Mol. Struct. 1973, 17, 329−340.
(20) Kotrla, J.; Florián, J.; Kubelková, L.; Fraissard, J. Pyridinium Ions-New Probe for Basic of Solid Acids. Collect. Czech. Chem. Commun. 1995, 60, 393−402.
(21) Farmer, V. C.; Mortland, M. M. An Infrared Study of the Co-ordination of Pyridine and Water to Exchangeable Cations in Montmorillonite and Saponite. J. Chem. Soc. A 1966, 344−351.
(22) Cercek, B.; Ebert, M. Pulse Radiolysis Studies of the Reaction of H and OH Radicals and Hydrated Electrons with Pyridine. Trans. Faraday Soc. 1967, 63, 1687−1698.
(23) Fessenden, R. W.; Neta, P. ESR Spectra of Radicals Produced by Reduction of Pyridine and Pyrazine. Chem. Phys. Lett. 1973, 18, 14− 17.
(24) Momose, T.; Shida, T. Matrix-Isolation Spectroscopy Using Solid Parahydrogen as Matrix: Application to High-Resolution Spectroscopy, Photochemistry, and Cryochemisty. Bull. Chem. Soc. Jpn. 1998, 71, 1−15.
(25) Yoshioka, T.; Raston, P. L.; Anderson, D. T. Infrared Spectroscopy of Chemically Doped Solid Parahydrogen. Int. Rev. Phys. Chem. 2006, 25, 469−496.
(26) Lee, Y.-P.; Wu, Y.-J.; Lees, R. M.; Xu, L.-H.; Hougen, J. T. Internal Rotation and Spin Conversion of CH3OH in Solid Para-Hydrogen. Science 2006, 311, 365−368.
(27) Bahou, M.; Lee, Y.-P. Diminished Cage Effect in Solid p-H2: Infrared Absorption of CH3S Observed from Photolysis in situ of CH3SH, CH3SCH3, or CH3SSCH3Isolated in p-H2Matrices. J. Chem. Phys. 2010, 133, 164316/1−164316/10.
(28) Amicangelo, J.; Lee, Y.-P. Site-Selective Reaction of Cl+ Propene in Solid Para-Hydrogen: Formation of 2-Chloropropyl Radicals. J. Phys. Chem. Lett. 2010, 1, 2956−2961.
(29) Lee, Y.-F.; Lee, Y.-P. Infrared Absorption of CH3SO2Observed Upon Irradiation of a p-H2Matrix Containing CH3I and SO2. J. Chem. Phys. 2011, 134, 124314/1−124314/8.
(30) Bahou, M.; Wu, Y.-J.; Lee, Y.-P. A New Method for Investigating Infrared Spectra of Protonated Benzene (C6H7+) and Cyclohexadienyl Radical (c-C6H7) Using Para-Hydrogen. J. Chem. Phys. 2012, 136, 154304/1−154304/8.
(31) Bahou, M.; Wu, Y.-J.; Lee, Y.-P. Formation and Infrared Absorption of Protonated Naphthalenes (1-C10H9+and 2-C10H9+) and Their Neutral Counterparts in Solid Para-Hydrogen. Phys. Chem. Chem. Phys. 2013, 15, 1907−1917.
(32) Raston, P. L.; Anderson, D. T. Infrared-induced Reaction of Cl Atoms Trapped in Solid Parahydrogen. Phys. Chem. Chem. Phys. 2006, 8, 3124−3129.
(33) Becke, A. D. Densityfunctional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648−5652.
(34) Lee, A.; Yang, W.; Parr, R. G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785−789.
(35) Perdew, J. P.; Burke, K.; Wang, Y. Generalized Gradient Approximation for the Exchange-correlation Hole of a Many-electron System. Phys. Rev. B 1996, 54, 16533−16539.
(36) Møller, C.; Plesset, M. S. Note on an Approximation for Many-Electron Systems. Phys. Rev. 1934, 46, 618−622.
(37) Dunning, T. H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007−1023.
(38) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; et al. GAUSSIAN 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009.
(39) Das, P.; Bahou, M.; Lee, Y.-P. Reactions between Atomic Chlorine and Pyridine in Solid Para-Hydrogen: Infrared Spectrum of the 1-Chloropyridiny (C5H5N−Cl) radical. J. Chem. Phys. 2013, 138, 054307/1−054307/10.
(40) Wong, K. N.; Colson, S. D. The FT-IR Spectra of Pyridine and Pyridine-d5. J. Mol. Spectrosc. 1984, 104, 129−151.
(41) Castellucci, E.; Sbrana, G.; Verderame, F. D. Infrared Spectra of Crystalline and Matrix Isolated Pyridine and Pyridine-d5. J. Chem. Phys. 1969, 51, 3762−3770.
(42) Morris, V. R.; Bhatia, S. C.; Stelson, A. W.; Hall, J. H., Jr. Matrix-Isolation Study of the Thermal Decomposition of Pyridine. Energy Fuels 1991, 5, 126−133.
(43) Destexhe, A.; Smets, J.; Adamowicz, L.; Maes, G. Matrix Isolation FT-IR Studies and ab Initio Calculations of Hydrogen-Bonded Complex of Molecules Modeling Cytosine or Isocytosine Tautomers. 1. Pyridine and Pyrimidine Complexes with H2O in Ar Matrices. J. Phys. Chem. 1994, 98, 1506−1514.
(44) Ruzi, M.; Anderson, D. T. Photodissociation of N-methylformamide Isolated in Solid Parahydrogen. J. Chem. Phys. 2012, 137, 194313/1−194313/11.
(45) Szczepaniak, K.; Chabrier, P.; Person, W. B.; Del Bene, J. E. Experimental Infrared Spectra of Matrix Isolated Complexes of HCl with 4-Substituted Pyridines. Evaluation of Anharmonicity and Matrix Effects Using Data from ab Initio Calculations. J. Mol. Struct. 2000, 520, 1−18.
(46) Anderson, D. T.; Hinde, R. J.; Tam, S.; Fajardo, M. E. High-resolution Spectroscopy of HCl and DCl Isolated is Solid Para-hydrogen: Direct, Induced, and Cooperative Infrared Transitions in a Molecular Quantum Solid. J. Chem. Phys. 2002, 116, 594−607.
(47) Szczepaniak, K.; Chabrier, P.; Person, W. B.; Del Bene, J. E. Ab Initio Theoretical and Matrix Isolation Experimental Studies of Hydrogen Bonding IV. The HBr:Pyridine Complex. J. Mol. Struct. 1997, 436−437, 367−386.
(48) Chan, M.-C.; Okumura, M.; Oka, T. Infrared Spectrum of p-Hydrogen Crystals Ionized by 3 MeV Electrons: Cluster Ions of Hydrogen in Condensed Phase. J. Phys. Chem. A 2000, 104, 3775− 3779.
(49) Barckholtz, C.; Barckholtz, T. A.; Hadad, C. M. A Mechanistic Study of the Reactions of H, O(3P), and OH with Monocyclic Aromatic Hydrocarbons by Density Functional Theory. J. Phys. Chem. A 2001, 105, 140−152.