COMPARISON OF AUTOTROPHIC AND MIXOTROPHIC BIOFILTERS FOR
H
2S
REMOVAL
By Ying-Chien Chung; Chihpin Huang/
Jill
Ruhsing Pan/ and Ching-Ping Tseng
4 ABSTRACT: We demonstrate that the facultative chemoautotrophThiobacillus novellusCH 3 removes hydrogensulfide (H2S) gas in continuous reactors under nutrient-limiting conditions. Extensive tests including removal
characteristics, metabolic products, and removal efficiencies of H2S by T. novellus CH 3 were conducted in
autotrophic and mixotrophic environments. The optimal pH value and temperature required to remove hydrogen
sulfide are found to be pH 7 and 26°C. The biofilter had an H2S removal efficiency greater than 99.5% under
the mixotrophic condition after 10-day operation. The results show that the maximum removal rate and saturation
constant were 1.9 g SId per kg bead and 69.2 ppm, respectively. The main metabolic product of H2S oxidation
was determined to be sulfate, but the conversion ratio was dependent on the growth environment. These results
suggest that the mixotrophic potential ofT. novellus CH 3 biofilter provides a significant advantage in H2S
removal over autotrophic biofilters.
INTRODUCTION
Hydrogen sulfide (H2S) is a colorless and corrosive air
pol-lutant that is extremely toxic (Roth 1993).Itoccurs widely in
nature and is released by industrial processes, such as petro-chemical refining, wastewater treatment, food preparation, pa-per and pulp manufacturing, and in the treatment of fuels
(Eikum and Storhang 1986; Yang and Allen 1994). Excess H2S
must be removed for,reasons of health and safety, because it has a great potential to irritate eyes and injure the human cen-tral nervous system (Vanhoorne et al. 1995). Conventional treatment of waste gases, wastewater, and ground water
con-taining H2S use some common technologies. These
technolo-gies include activated carbon adsorption, ozone oxidation, in-cineration, air stripping, and microfiltration (Eby and Wilson 1969; Barth et al. 1984; Mannebeck 1986; Thompson et al. 1995). However, conventional treatment and disposal costs are high and secondary-pollutant issues may arise. The contin-uing demand for improved process economy and efficiency has led to investigations into microbiological alternatives to
conventionally physicaVchemical methods (Bohn 1992).
Biofilters decontaminate waste gas by passing it through a damp medium that supports a vigorous culture of microorgan-isms. The biofiltration process can serve as a most effective means when applied to dilute, easily biodegradable waste gases under appropriate sets of conditions (Leson and Winer
1991). Thus, H2S is an excellent candidate for removal by
biofiltration.
A wide range of biofilter bed materials as carriers have been studied (Rands et al. 1981; Lee and Shoda 1989; Leson and Winer 1991). Originally, biofilters are developed with soils as carriers; however, soils are limited in effectiveness because they are prone to short-circuiting and clogging (Carlson and Leisner 1966). Compost is inexpensive and purifies waste gases well, but it suffers from aging effects that create short-circuiting of the biofilter and further decrease the effectiveness 'PhD, Nat. Sci. Council, Taipei, Taiwan, RO.C.; formerly, Inst. of Envir. Engrg., Nat. Chiao Tung Univ., Hsinchu, Taiwan, RO.C. 30039.
'Prof., Inst. of Envir. Engrg., Nat. Chiao Tung Univ., Hsinchu, Taiwan, RO.C. 30039. Correspondence should be addressed to Chihpin Huang.
'Res., Inst. of Envir. Engrg., Nat. Chiao Tung Univ., Hsinchu, Taiwan, R.O.C. 30039.
'Assoc. Prof., Inst. of BioI. Sci. and Techno!., Nat. Chiao Tung Univ., Hsinchu, Taiwan, R.O.C. 30039.
Note. Associate Editor: Oliver J. Hao. Discussion open until Septem-ber I, 1998. To extend the closing date one month, a written request must be filed with the ASCE Manager of Journals. The manuscript for this paper was submitted for review and possible publication on October II, 1996. This paper is part of theJournal of Environmental Engineering,
Vol. 124, No.4, April, 1998. ©ASCE, ISSN 0733-9372/98/0004-0362-0367/$4.00+$.50 per page. Paper No. 14304.
of the biofilter (Langenhove et al. 1992). Activated carbons also perform well in removing waste gases, but they are too expensive to justify the efficiency difference (Medina et al. 1995). Fibrous peat, used as a packing material, has been shown to perform better than soil, compost, or activated carbon (Leson and Winer 1991); however, a larger space is required when a biofilter packed with microorganism-laden peat is used to treat large quantities of hydrogen sulfide at low concentrations «20 ppm) (Tanji et al. 1989). Ca-alginate beads have advantages as a biofilter medium in comparison with other commonly used biofilters. Recent work has shown that Ca-alginate beads can have a high microorganism content and prevent microorganism losses (Kokufuta et al. 1982).
The presence of microorganisms is necessary for effective
removal of H2S gas. Although activated sludges (mixed
cul-ture) have been used in operating biofilters, acclimation times of at least 1-3 weeks are required (Ottengraf and Van Den Oever 1983). Recently, the use of pure cultures is gaining a lot of attention because it shortens start-up time and enhances removal efficiencies and capacities. Both autotrophic and het-erotrophic microorganisms have been employed in pure cul-ture studies, and there are inherent differences in their nutri-tional requirements and abilities to catalyze specific reactions.
Some autotrophic bacteria, such as members of the
Thioba-eillusspecies have been seeded into bioreactors and used to
metabolize H2S. The products of H2S oxidation are dependent
on the strain ofThiobaeillus sp. employed (Sublette and
Syl-vester 1987; Cho et al. 1991a; Chung et al. 1996a). Among
heterotrophic bacteria, only Xanthomonas sp. and
Pseudo-monas putida have been reported to oxidize H2S in biofilter systems (Cho et al. 1991b; Chung et al. 1996b). Autotrophic
biofilters have shown high affinity for H2S, but failed to
re-move reliably low concentrations of H2S through sustained
experiments. However, heterotrophic biofilters have shown op-posite tendencies (Huang et al. 1996). Moreover, facultative
chemoautotrophs such as Thiobacillus novellus possess the
unique potential for autotrophic as well as heterotrophic growth. Hence, these bacteria are apparently adaptable to dif-ferent environments, i.e., autotrophic, heterotrophic, or mixo-trophic conditions (Matin 1978). Studies have shown adaptive
physiologic/metabolic response ofT. novellus to mixotrophic
environments (Leefeldt and Matin 1980; Perez and Matin 1980), but little engineering information, such as desired con-trol mechanisms, and proper design and maintenance of
bio-filters for H2S removal is available.
In this study, we used Ca-alginate to immobilizeT. novellus
CH 3 and studied an H2S-fed biofilter operated under
auto-trophic and mixoauto-trophic conditions.
(1) MATERIALS AND METHODS
Organism Cultivation and Buffer Preparation
The bacteria used in this study were isolated from piggery wastewater. The wastewater was mixed with a pH 7 mineral salts medium containing KH2P04, 2 gIL; K2HP04, 2 giL; NH4Cl, 0.6 gIL; MgCI2' 6H20, 0.3 gIL; and FeS04 . 7H20, 0.02 gIL. As the only source of energy, hydrogen sulfide generated by feeding solution of Na2S and HCl was supplied from 5 to 150 ppm into the Erlenmeyer flasks. Erlenmeyer flasks were closed with rubber stoppers containing inlet and outlet pores and the inlet and outlet gas concentrations were measured reg-ularly. When the outlet gas concentration was nearly zero, the inlet gas concentration was increased to a desired higher level. This process was repeated until a constant level of outlet gas concentration was detectable. At this stage, the process of ac-climating microbes was assumed to be completed. One milli-liter of bacterial solution was transferred repeatedly to fresh solid medium by spread plate method. The dominant colonies were reserved and further purified until appearance of single colony. The isolated strain was identified asT. novel/usby the Food Industry Research and Development Institute in Taiwan. During continuous-treatment experiments, the autotrophic in-flow medium included KH2P04, 1.2 gIL; K2HP04, 1.2 giL;
N~Cl,0.4 gIL; MgCl2. 6H20, 0.2 gIL; and FeS04 . 7H20, 0.01 gIL. The mixotrophic inflow medium was obtained by supple-menting the autotrophic inflow medium with 0.2 g glucose. The final pH of the culture was adjusted to 7 using 2 N NaOH or HC!. In batch experiments, for measurement of the pH ef-fect on sulfide removal byT. novel/usCH 3 under autotrophic and mixotrophic conditions, the T. novel/us CH 3 were sus-pended in phosphate buffers in the pH range of 5.5-8.0 at 30°C. The removal rates were measured at sulfide concentra-tions between 0 and 10mM. For each pH value, the values of the saturation constantK, and the maximum removal rate Vm were calculated using Michaelis-Menten equation via the lin-ear regression method.
Preparation of Immobilized Cells
T. novel/usCH 3, grown in lOO-mL nutrient broth, was har-vested by centrifugation (7,500 X g for 10 min). The organ-isms were washed three times with sterile distilled water, fol-lowed by immersing in a sterile 4% Na-alginate solution (105 cells/mL) and then mixing with a 4% CaCh solution. Upon mixing, 3-mm-diameter immobilized beads were fonned im-mediately. These gel beads then were activated by flushing with sterile buffer solution for 5 h. The activated beads exhib-ited excellent mechanical strength in the continuous experi-ments.
Apparatus and H2S Removal for Continuous
Operation
A description of the laboratory-scale experimental biofilter was described previously by Chung et a!. (1996c). Glass col-umns (60 mm <l> X 40 cm of working height) were packed with celI-laden Ca-alginate beads. In each column, 0.5 kg dry beads were packed in a column of 1.44L. Initial cell numbers in each column were counted to be approximately 101 colony-fonning units (cfu) per 1 g of dry bead. In the continuous experiment, H2S gas at different concentrations (10, 20, and 60 ppm) was introduced to the biofilter at a flow rate of 36 or 72 Lih. The H2S(g) was supplied from a gas cylinder, diluted with compressed air, forced through a filter unit (0.45 ILm), and then passed through the humidification bottle into the bot-tom of biofilter. The effect of temperature on the volumetric oxidation rates and removal efficiencies of the biofilters was studied in the range of 10-30°C controlled by refrigerated
circulator circulating water via heat exchanger and while the flow rates were from 18 to 185 Lih. The products from the metabolization of H2S by T. novel/usCH 3 also were analyzed during the continuous experiment.
Kinetic Analysis
The H2S removal rate in the immobilized-cell biofilter was calculated using the following equation derived from the Mi-chaelis-Menten equation (Hirai et a!. 1990):
1 K, 1 1
= X +
-R Vm C1n Vm
whereR (g Sid per kg dry bead)= removal rate; C1n(ppm)= logarithmic mean concentration of H2S at the inlet and outlet of the biofilter; Vm (g Sid per kg dry bead) = maximum re-moval rate; and K,(ppm)= half-saturation constant. With the use of the linear relationship between l/Cln and lIR, Vm and
K,were calculated from the slope and intercept.
Criteria for Design of Scale-Up Biofilter
The target concentrations of H2S at the biofilter outlet were presumed as 0.1 and 1 ppm. The maximum inlet concentra-tions and critical H2S loads needed to satisfy this effluent con-centration (0.1 or 1 ppm) were obtained at various space ve-locity (SV) according to the following equation (Tiwaree et a!. 1992):
sv =
<X X VmX C1n(2)
(Co - C,) (K,
+
Cln )where SV (lid) = F (l/S,J.); F = gas flow rate (m3/d); Sa =
column cross section (m2); L
=
packing height (m); Co=
inlet concentration (ppm); C, = outlet concentration (ppm); and <X=conversion coefficient (kg dry bead/g S). LetC,be O. I or 1 ppm in (2), and the maximum Cocan be estimated at various SVs. The critical loads (g Sid per kg bead) of the biofilters can be obtained using (3)
SV X Co
critical load
=
(3)<X
Analytical Methods
Inlet and outlet H2S gas concentrations in the biofilter were measured continuously using a single point monitor (MDA Scientific) in the range of 50-1,500 ppb or periodically mea-sured by gas detector tubes (GASTEC, Tokyo, Japan) in the range of 1-60 ppm. During continuous experiments, the var-iation of H2S concentration at steady state was found to be within ::!:5%. The H2S outlet concentration was reported as average values from 12 assays. Five grams (wet-weight) of cell-laden beads was dissolved in 95 mL of 0.1 M sodium citrate solution and the sulfur compounds and their amounts in the solution were detennined. Sulfate ion concentrations in the solution were measured by ion chromatography (Dionex 4500i). Sulfite was detennined by titration using a standard potassium iodide-iodate titrant and a starch indicator [Ameri-can Public Health Association (APHA) 1992]. Sulfide was de-tennined using an ion-specific electrode (SCHOTT, Gennany).
RESULTS AND ANALYSIS
Effect of pH on Sulfide Degradation
At six different pH values ranging from 5.5 to 8, the kinetics of H2S removal were measured at concentrations between 0 and 10 mM in autotrophic and mixotrophic batch cultures. The pH effects on the sulfide oxidation kinetics ofT. novellusCH 3 are presented in Table I. For each pH value, the saturation constantK,and the maximum removal rateVmwere calculated
JOURNAL OF ENVIRONMENTAL ENGINEERING / APRIL 1998/363
2 3 4 5 6 7 8 9 10 11 12 13 14 15
Effect of Residence Time on H2S Removal H2S Removal Rate In Continuous Operation
···~_···---··~T
...1•
Autotrophic GrowthI.
A.···I ..
r·~··l...'1,
"1···1··
I.. .
~
.
···1··1..
I-..;
~
.
.I..
l
.
100 ,-.. ?f- 99 ' - 'G
98 c u '(j 97!E
u 96 <ij > 95 0!
94 93the basis of experimental results using linear regression anal-ysis from Fig. I and the values were
9
=
1.023 under autotrophic conditions9
=
1.025 under mixotrophic conditionsThere was no significant difference in 6 between the auto-trophic and the mixoauto-trophic biofilter at the temperature be-tween 10 and 26°C.
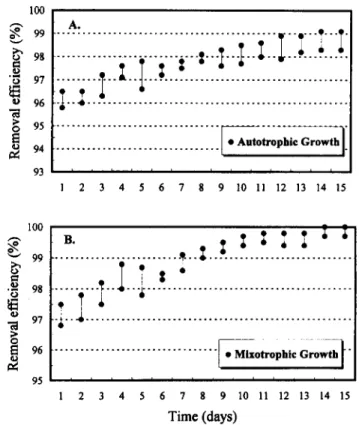
The H2S removal efficiency as a function of residence time
at inlet concentration of 60 ppm is shown in Fig. 3. The T.
novellus CH 3 biofilter achieved a steady-state condition in 72
Fluctuations in inlet H2S concentrations were examined in
the 10-60 ppm range at flow rates of 36 and 72 Lih. The H2S
removal efficiency was calculated from the difference in inlet
and outlet concentrations. The H2S removal efficiencies of the
immobilized T. novellus CH 3 biofilters in autotrophic and
mixotrophic environments at 36 L/h are shown in Figs. 2(a
and b), respectively. The variation ranges in the figures were between maximum and minimum removal efficiency for dif-ferent inlet concentrations. These results demonstrated that the mixotrophic biofilter achieved a steady-state condition (10 days) earlier than did the autotrophic biofilter (13 days). The mixotrophic biofilter showed excellent operational efficiency (>99.5%) regardless of whether the flow rate was 36 or 72 LI
h (data not shown). By contrast, a slightly lower H2S removal
efficiency (>98.3%) for the autotrophic biofilter was observed.
The higher H2S removal efficiency achieved by the
mixo-trophic biofilter appears to be the contribution of enzymatic affinities. The data suggest that the mixotrophic potential of theT. novellus CH 3 biofilter could provide significant
advan-tages over the autotrophic biofilter.
(4) Autotrophic Environments Mixotrophic Environments
K. Vm K. Vm
pH (ppm) (gSid per kg bead) (ppm) (g Sidper kg bead)
(1 ) (2) (3) (4) (5) 5.5 185.6 0.76 152.3 0.89 6 138.3 1.08 111.3 1.26 6.5 86.8 1.79 77.8 1.74 7 84.3 1.85 74.5 1.81 7.5 89.3 1.73 80.4 1.68 8 139.5 1.13 100.5 1.34
Effect of Temperature on H2S Removal
Fig. I shows the H2S oxidation rate ofT. novellus CH 3 in
autotrophic and mixotrophic environments ranging in temper-ature from 10 to 30°C. When the biofilters were supplied with
60 ppm H2S at a flow rate of 36 L/h, the H2S oxidation rate
increased with the temperature, reaching its maximum at
ap-proximately 26°C. The effect of temperature on H2S oxidation
rate was almost the same whether T. novellus CH 3 was in an
autotrophic or mixotrophic environment. In general, the rela-tionship between reaction rate and temperature can be approx-imated using the Arrineus equation
from (I) using linear regression. Trends of pH versusKs
pro-files indicate that Ks decreases with the increase of pH. The
optimal pH value for sulfide removal was 7 regardless of au-totrophic or mixotrophic growth conditions. From an opera-tional standpoint, the control of pH is an important parameter in sulfide treatment, and this study suggests the optimal pH value is approximately 7. The saturation constants under mix-otrophic conditions were lower than those under autmix-otrophic
conditions. Thus, in mixotrophic environments,T.novellus CH
3 exhibited higher enzymatic affinity for sulfide than it did in autotrophic environments. Our results are in agreement with
previous studies, which have shown thatT.novellus had higher
cell-growth rates and glucose-transport activity in mixotrophic environments than in autotrophic environments (Matin et aI. 1980; Perez and Matin 1982).
55 ...- - - ,
TABLE 1. Effect of pH on Saturation Constant and Maximum Removal Rate of Thiobacillus novellus CH 3 for Sulfide Degra-dation In Autotrophic and Mlxotrophlc Environments at T= 26°C
where KT
=
reaction rate at TOC (g S/m3 per day); K
20
=
re-action rate at 20°C (g S/m3
per day); 6=
temperature-depen-dency coefficient; andT=temperature caC). We obtained 6 on
~ 50 ~
':§
45 VJ I b.O ~ 40 1;j...
§ 35 .~ ~ 30 ly=0:6949x+30.417! Ii \.....
Q ~ ••••••• I •Autotrophic Growth o MiIotrophic Growth 100 ,-.. ;;;R ~ 99G
.§
98 (,)!6
u 97ca
>0e
96 ~ 95 B. : : : : : • •.
., ..1
.
... ·1·
.1.
··1···~···~···
, T
~ .~ . ...·r-:-~~:otroPhicGro~~l'
2 3 4 5 6 7 8 9 10 II 12 13 14 15 Time (days)FIG. 2. H.S Removal Efficiencies by Thlobacillus novel/us CH 3 Blofllters: (a) In Autotrophic; (b) In Mlxotrophlc Environments at 36 Uh with Inlet Concentrations of 10-60 ppm at pH 7
30 25
15 20
Temperature
COC)
FIG. 1. Effect of Temperature on H.S Oxidation Rate of
Thio-bacillus novellus CH 3 Bloflltersln Autotrophic (Solid Line) and
Mixotrophic (Dash Line) Environments
0.004 (0.4%) 0.005 (2.5%) 0.003 (0.3%) 0.001 (0.5%) 58 288 58 288 2 Residence
time H2S removed SO; produced SO. produced 5- produced (s) (g S1kg bead) (g S1kg bead) (g S1kg bead) (g S1kg bead)
(1 ) (2) (3) (4) (5)
Kinetic Analysis
the optimal residence time shouldbe held at either 288 or 72
s, depending on which biofilter condition is present.
TABLE 2. Sulfur Mass Balances In Blofllters In Mlxotrophlc and Autotrophic Environments at 60 ppm H S at pH 7 .
300 o 250 • MixotrophicGrowth 100
• •
'0'-.r:. 99 '-"C
c:: 98 u '0IE
u•
«l 97 ~e
~ 96 95 0 50 100 150 200Residence time (sec)
FIG. 3. H2S Removal Efficiency as Function of Residence
Time at Inlet Concentration of 60 ppm
0.25 0.2 0.15 0.1 0.05 OL...._---...- - _... ... ..._ - J
o
10 , - - - -... 9 8 7 6 ~...
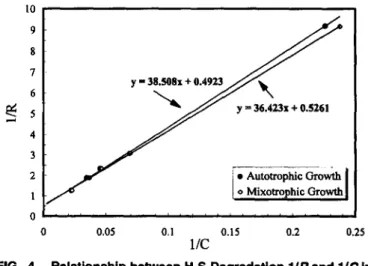
5 4 3 2As the foregoing studies show, immobilizedT. novellus CH
3 possessed an excellent ability to degrade H2S, especially in
mixotrophic environments. Kinetic analysis was performed to
d~terminethe enzymatic affinities for H2S in the experimental blOfilters. Results obtained using regression methods are s?own in Fig. 4, along with corresponding regression
equa-tIOns. The H2S half-saturation constants of autotrophic and
mixotrophic biofilters were 78.2 and 69.2 ppm, respectively.
The maximum removal capacities were 2.0 and 1.9 g SId per
kg bead. Note that the half-saturation constant (69.2 ppm) of the mixotrophic biofilter was smaller than that of the
auto-trophic biofilter (78.2 ppm). Ifwe infer a physical meaning
for Ksanalogous to enzymatic kinetics, a decrease in Ks
sug-gests an enhancement in biomass affinity for H2S. Hence, the
T. novellus CH 3 biofilter operation in the mixotrophic
envi-ronment enhanced H2S removal over autotrophic operations.
In addition, the enzymatic affinity in the continuous trials (i.e., half-saturation constants are 69.2 and 78.2 ppm, respectively) were higher than those in the batch experiments (i.e., half-saturation constants are 74.5 and 84.3 ppm, respectively), re-gardless of whether the biofilter was operated under
auto-tr0t:>hic ~r mix.otrophic co?ditions. From a microbial
physio-logical vlewpomt, the studies brought into focus differences in
~eregulation of bacterial enzyme expression in different
en-vironments. This observation is in accordance with Leefeldt
and Matin (1980) who showed that the enzymes inT.novellus
may be repressed under nutrient-excess conditions of batch
culture and activated under the nutrient-limited conditions of continuous culture.
lIC
FIG. 4. Relationship between H2S Degradation 1/R and 1/C In
Blofllters
JOURNAL OF ENVIRONMENTAL ENGINEERING / APRIL 1998/365
Identification of H2S Removal Products
h. The removal efficiency of the autotrophic biofilter decreased
significantly with decreases in H2S residence time. By contrast,
the mixotrophic biofilter exhibited a different removal pattern.
The removal efficiency increased with H2S residence time in
the range of 28-58 s (equivalent to flow rates from 185 to 90 Uh). When the residence time was longer than 58 s, the
re-moval efficiency decreased with further increases in the H2S
residence time. Under all operating conditions except for res-idence time of 288 s, the mixotrophic biofilter achieved higher removal efficiencies than the autotrophic biofilter. The cells obtained energy derived from hydrogen sulfide and glucose oxidation in mixotrophic environments. Based on our results and previous studies, it appears that glucose served as an
ad-ditional carbon source besides CO2 during mixotrophic
growth .. Leefeldt and Matin (1980) showed that at long
resi-dencet~mes some 30-50% more glucose was used in energy
generation byT. novellus; hence, less H2S was utilized by the
oxidation to provide energy. By contrast, during short resi-dence times less glucose (approximately 15%) was used in
energy generation suggesting that T. novellus would oxidize
more H2S to provide energy for survival (e.g., 58 s) (Leefeldt
and Matin 1980).
To understand H2Sremoval by T. novellus CH 3 in various
physiological situations, the sulfate, sulfite, and sulfide con-centrations in the middle layer of the biofilter were monitored after 72 h of operation. Elemental sulfur also was measured but it was always below detection limits (0.1 mg/L). Table 2 shows the mass balance of sulfur in the biofilter after the
biofilter was operated continuously with the inlet
~f
60 ppm~2S at residence times of 58 or 288 s for 3 days. When the
blOfi!ter was operated in mixotrophic environments, the pre-dommant metabolic product was sulfate, which thus accounted
for 97.6% of the total H2S conversion at a residence time of
58 s. At a residence time of 288 s, sulfate accounted for only
7~.511"0.When the biofilter was operated under autotrophic en-Vironments, the same metabolic products were found;
how-ever, H2S almost was converted to sulfate regardless of the
residence time, e.g., 99.3% at 58 sand 98.0% at 288 s. The
bio~onversion of aerobic sulfide removal pathway by
Thio-baCIllus sp. was suggested by Buisman et al. (1990)
S·-7SO -7SO;-7SO;
Itis, therefore, indicated that the nearly complete oxidation of
sulfide under autotrophic conditions at all residence times was an arti~actofs~lfidebeing the only source of energy. Because
the reSidence time strongly influences H2S removal efficiency,
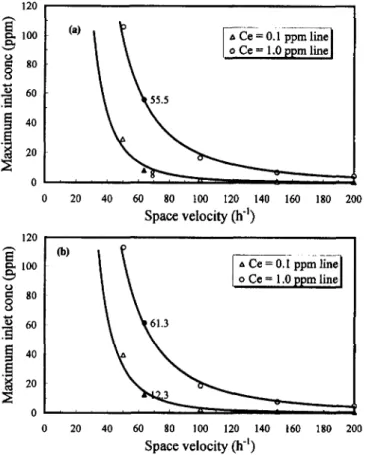
TABLE 3. Maximum Inlet Concentrations and Critical Loads In Mlxotrophic and Autotrophic Blofllters at Target Emission Con-centrations of 0.1 or 1 ppm at 64 h-'
The optimal condition of H2S removal by T. novel/us CH 3
biofilter was found at pH 7 and 26°C. In continuous trials, the mixotrophic biofilter achieved steady-state condition in a
shorter time and exhibited higher efficiency in removing H2S
than the autotrophic biofilter. The mixotrophic biofilter
achieved greater than 99.5% removal efficiency after 10-day
operation even at H2S concentration as low as 10 ppm. The
main product of H2S oxidation by T. novel/us CH 3 was
iden-tified as sulfate. The formation ratio of sulfate to sulfide in the
mixotrophic biofilter depended on the residence time ofHzS.
Longer residence time benefited the H2S removal in the
au-totrophic biofilter, but the optimal residence time was found to be approximately 58 s in the mixotrophic biofilter. The ef-ficiency of sulfide oxidation and the products formed suggest that different physiological regulatory mechanisms affect en-zyme synthesis and expression in the mixotrophic biofilter
un-der various H2S flow rates. When a biofilter concurrently
re-ceived glucose and H2S, it exhibited a higher H2S affinity than
a biofilter receiving H2S only. From a design standpoint, the
mixotrophic biofilter possesses a removal capacity similar to that of the autotrophic biofilter when high emission concen-tration is permitted. However, the mixotrophic biofilter is su-perior to the autotrophic biofilter, when a stringent emission limit is required.
This work was supported by the National Science Council, Republic of China (NSC No. 87-2218-E-009-011).
ACKNOWLEDGMENT
concentration «0.1 ppm) was required. Therefore, the mixo-trophic biofilter operations were superior to the automixo-trophic biofilter operations when strict emission concentration con-straints were imposed.
CONCLUSIONS
APPENDIX I. REFERENCES
American Public Health Association (APHA). (1992). Standard method:
examination of water and wastewater, 18th Ed., American Public
Health Association, New York, N.Y.
Barth, C. L., Elliott, F. L., and Melvin, S. W. (1984). " Jsing odour control technology to support animal agriculture." Trans. ASAE, 27(8), 859-864.
Bohn, H. (1992). "Consider biofiltration for decontaminating gases."
Chem. Eng. Progress., 88, 35-40.
Huisman, C. J. N., Geraats, B. G., Ijspeert, P., and Lettinga, G. (1990). "Optimization of sulfur production in a biotechnological sulfide-re-moving reactor." Biotechnol. Bioengrg., 35, 50-56.
Carlson, D. A., and Leisner, C. P. (1966). "Soil beds for the control of sewage odors." J. Water Pollution Control Fed., 38(11), 829-834. Cho, K. S., Hirai, M., and Shoda, M. (1991a). "Degradation
character-istics of hydrogen sulfide, methanethiol, dimethyl sulfide and dimethyl disulfide by Thiobacillus thioparus DW44 isolated from peat biofilter." J. Ferment. Bioengrg., 71(6), 384-389.
Cho, K. S., Kuniyoshi,I.,Hirai, M., and Shoda, M. (199Ib). "A newly isolated heterotrophic bacterium, Xanthomonas sp. DY 44, to oxidize hydrogen sulfide to polysulfide." Biotechnol. Lett., 13(12),923-928. Chung, Y. C., Huang,c., and Tseng, C. P. (1996a). "Operation
optimi-zation of Thiobacillus thioparus CHll biofilter for hydrogen sulfide removal."J. Biotechnol., 52, 31-38.
Chung, Y. C., Huang, C., and Tseng, C. P. (1996b). "Microbial oxidation of hydrogen sulfide with biofilter." J. Envir. Sci. and Health, A31(6), 1263-1278.
Chung, Y. C., Huang, c., and Tseng, C. P. (1996c). "Biodegradation of hydrogen sulfide by a laboratory-scale immobilized Pseudomonas
pu-tida CHII biofilter." Biotechnol. Prog., 12,773-778.
Eby, H. J., and Wilson, G. B. (1969). "Poultry house dust, odour and their mechanical removal." Agric. Waste Mgmt. Proc., Cornell Univ.
Conf. on Agric. Waste Mgmt., Syracuse, N.Y., 303-308.
EiKum, A. S., and Storhang, R. (1986). "Odour problems related to waste water and sludge treatment." Odour prevention and control of organic
sludge and livestock farming,V. C.Neilsen, J. H. Voorburg, and P.L.
0.045 0.313
Critical load (g SId per kg bead)
(3) 8.0 55.5 Maximum inlet concentration (ppm) (2) 20 40 60 80 100 120 140 160 180 200 Space velocity (h-I) 0.1 1 Emission concentration (ppm) (1 ) 120 . - - - , ' [ 100 (a)
.,e,
~
80 l) 60:5
§
40I
20o
L",,--",,"-,-2===="':::::::::::=d
o
Criteria for Design of Scale-Up Blofilters
Complete H2S removal can be achieved only at less than
critical inlet load. If this critical inlet load is exceeded, H2S is
detected continuously at the outlet of the biofilter. The system load is defined as the amount of inlet gas per unit of time per weight of packing material (g SId per kg bead). Thus, inlet gas concentrations play an important role in the design of a scale-up biofilter if the weight of the packing material and the gas-flow rate are constant. Finding the maximum inlet con-centration and the optimal inlet load therefore becomes im-portant for the operation of a biofilter. The relationship be-tween the maximum inlet concentration and space velocity for
H2S removal is shown in Figs. 5(a and b). The values shown
in Fig. 5 also are listed in Table 3. At a space velocity of 64
h-I
(i.e., flow rate, 72 L/h), the mixotrophic biofilter tolerated higher loads than autotrophic biofilter. Although mixotrophic
biofilter removed more H2S than autotrophic biofilter, the
for-mer seems to be superior to the later only when a low emission
120 . - - - . . . ,
e
(b)5
100~
80 l) 60:5
§
40I
20o
L"'--_~:::::::lI:::c::==:::::==d
o 20 40 60 80 100 120 140 160 180 200Space velocity (hoi)
FIG. 5. Relationship between Maximum Inlet Concentration and Space Velocity for H.S Removal by ThlobBc/llus novel/us CH 3 Blofilters: (a) in Autotrophic; (b) In Mlxotrophlc Environ-ments
(a) Mixotrophic
Biofi~l_te_r---0.1 12.3
I
0.070I 61.3 0.346
_ _ _ _ _ _ _ _ _ _ _ _ _ _ L -_ _----:---:... _
(b) AutotrophicBiofi:.:.lt:.:.e~r _
I
Hennite. eds.• Elsevier Applied Science Publishers. London. England. 12-18.
Hirai. M.• Ohtake. M .• and Shoda. M. (1990). "Removal kinetic of hy-drogen sulfide. methanethiol and dimethyl sulfide by peat biofilters."
J. Ferment. Bioengrg.• 70(5). 334-339.
Huang. C.• Chung. Y. C.• and Hsu. B. M. (1996). "Hydrogen sulfide removal by immobilized autotrophic and heterotrophic bacterial in the bioreacter."Biotechnol. Tech.• 10(8).595-600.
Kokufuta. E.• Matsumoto. W.• and Nakamura.I.(1982). "Immobilization ofNitrosomonas eruopaea cells with polyelectrolyte complex." Bio-technol. Bioengrg.• 24.1591-1603.
Langenhove. H. V.• Bendinger. B.• Oberthur. R.• and Schamp. N. (1992). "Organic sulfur compounds: persistent odorants in biological treatment of complex waste gases." Biotechniques for air pollution abatement and odour control policies. A. J. Dragt and J.V.Ham. eds.• Elsevier Press. Amsterdam. The Netherlands. 177-182.
Lee. S. K.. and Shoda. M. (1989). "Biological deodorization using acti-vated carbon fabric as a carrier of microorganisms." J. Ferment. Bioengrg. 68(6).437-442.
Leefeldt. R. H.• and Matin. A. (1980). "Growth and physiology of Thio-bacillus novellus under nutrient-limited mixed conditions."J. Bacter-iol.• 142(2).645-650.
Leson. G.• and Winer. A. M. (1991). "Biofiltration: an innovative air pollution control technology for VOC emission." J. Air and Waste Mgmt. Assoc.• 41(8). 1045-1054.
Mannebeck. H. (1986). "Covering manure storing tanks to control odour." Odour prevention and control of organic sludge and livestock farming. V. C. Neilsen. J. H. Voorburg. and P.L.Hermite. eds.• Elsevier Applied Science Publishers. London. England. 188-192.
Matin. A. (1978). "Organic nutrition of chemolithotrophic bacteria."
Annu. Rev. Microbiol.. 32. 433-468.
Matin. A.. Schleiss. M .• and Perez. R. C. (1980). "Regulation of glucose transport and metabolism in Thiobacillus novellus." J. Bacteriol.•
142(2). 639-644.
Medina. V. F.. Webster. T.• and Devinny. J. S. (1995). "Treatment of gasoline residuals by granular activated carbon based biological filtra-tion."J. Envir. Sci. and Health. A30(2). 407 -422.
Ottengraf. S. P. P.• and Van Den Oever. A. H. C. (1983). "Kinetics of organic compound removal from waste gases with a biological filter."
Biotechnol. Bioengrg.• 25. 3089-3102.
Perez. R. C .• and Matin. A. (1980). "Growth ofThiobacillus novellus on
mixed substrates (mixotrophic growth)." J. Bacteriol.. 142(2).
633-638.
Perez. R. C .• and Matin. A. (1982). "Carbon dioxide assimilation by
Thiobacillus novellus under nutrient-limited mixotrophic conditions."
J. Bacteriol.• 150(3).46-51.
Rands. M. B.• Cooper. D. E.• Woo. C. P.• Fletcher. G. C .• and Rolfe. K. A. (1981). "Compost filters for h2s removal from anaerobic digestion and rendering exhausts." J. Water Pollution Control Fed.• 53(10).
185-189.
Roth. S. H. (1993).Hydrogen sulfide. Handbook of hazardous material.
Academic Press. Inc.• New York. N.Y.
Sublette. K. L.• and Sylvester. N. D. (1987). "Oxidation of hydrogen sulfide by continuous cultures ofThiobacillus denitrijicans." Biotech-nolo Bioengrg.• 29. 753-758.
Tanji. Y.• Kanagawa. T.• and Mikami. E. (1989). "Removal of dimethyl sulfide. methyl mercaptan. and hydrogen sulfide by immobilized Thio-bacillus thioparus TK-m."J. Ferment. Bioengrg.• 67(4). 280-285.
Thompson. M. A.• Kelkar. U. G.• and Vickers. J. C. (1995). "The treat-ment of groundwater containing hydrogen sulfide using microfiltra-tion."Desalination. 102(2).287-291.
Tiwaree. R. S.• Cho. K.S.•Kirai. M.• and Shoda. M. (1992). "Biological deodorization of dimethyl sulfide using different fabrics as the carriers of microorganisms."Appl. Biochem. Biotech.. 32. 135-148.
Vanhoorne. M.• Rouck. A.• and de Bacquer. D. (1995). "Epidemiological study of eye irritation by hydrogen sulphide and/or carbon disulphide exposure in viscose rayon workers." Ann. Occup. Hyg.• 39(3).
307-315.
Yang. Y.• and Allen. E. R. (1994). "Biofiltration control of hydrogen sulfide I. Design and operational parameters." J. Air Waste Mgmt. Assoc.• 44. 863-868.
APPENDIX II. NOTATION
The following symbols are used in this paper:
C.
=
outlet concentration (ppm);Cln
=
logarithmic mean concentration of H2S at inlet and outlet of biofilter (ppm);Co
=
inlet concentration (ppm);F
=
gas flow rate(m3/d);K,
=
half-saturation constant (ppm);Kr
=
reaction rate at TOC(g S/m3per day);K20
=
reaction rate at 20°C (gS/m3per day);L
=
packing height (m);R
=
removal rate (g SId per kg dry bead);Sa
=
column cross section (m2 ); T=
temperature (OC);Vm
=
maximum removal rate (g SId per kg dry bead);<X
=
conversion coefficient (kg dry beadlg S); andI)
=
temperature-dependency coefficient.JOURNAL OF ENVIRONMENTAL ENGINEERING / APRIL 1998/367