177
Age of Pacific Tarpon, Megalops cyprinoides, at Estuarine Arrival
and Growth during Metamorphosis
Wann-Nian Tzeng*, Chou-En Wu and Yu-Tzu Wang
Department of Zoology, College of Science, National Taiwan University, Taipei, Taiwan 106, R.O.C. Fax: 886-2-23636837. E-mail: wnt@ccms.ntu.edu.tw
(Accepted March 18, 1998)
Wann-Nian Tzeng, Chou-En Wu and Yu-Tzu Wang (1998) Age of Pacific tarpon, Megalops cyprinoides, at estuarine arrival and growth during metamorphosis. Zoological Studies 37(3): 177-183. The age of Pacific tarpon, Megalops cyprinoides (Broussonet), at estuarine arrival was estimated by counting otolith growth increments from specimens collected in the Gongshytyan Brook estuary of northern Taiwan on 15 to 24 September 1995. The daily deposition of otolith growth increments provided a mean to estimate age of individuals. Tarpon at estuarine arrival were in the 1st metamorphic stage. Their body lengths ranged between 17.8 and 32.9 mm TL (mean = 25.6 mm TL) and ages between 20 and 39 d (mean = 28.5 d). A metamorphic mark within the otolith indicated that the fish had begun to metamorphose several days before arriving at the estuary. Negative correlations between age estimates, and both somatic and otolith growth rates indicate that faster-growing tarpon arrived at the estuary earlier than did slower-growing ones. Otolith growth continued while body length was reduced during metamorphosis. The duration of metamorphosis for Pacific tarpon was estimated to be approximately 14 d.
Key words: Megalops cyprinoides, Otolith, Age, Growth, Metamorphosis.
*To whom correspondence and reprint requests should be addressed.
T
arpon, or ox-eye herring, Megalops cyp-rinoides (Broussonet), Megalopidae, Elopiformes, are widely distributed in tropical and subtropical areas of the Indo-Pacific Ocean (Nelson 1984). Like Atlantic tarpon, M. atlanticus, this species probably spawns in coastal waters (Crabtree et al. 1992). Tarpon has a leptocephalus larval stage, and juveniles frequently enter fresh water (Merrick and Schmida 1984, Tzeng and Yu 1986, Coates 1987). Leptocephalus larvae of M. cyprinoides occur in the Gongshytyan Brook estuary of Taiwan from summer to autumn (Tzeng and Yu 1986). Until now, there has been only fragmentary knowl-edge of the life history of this species (Tsukamoto and Okiyama 1993 1997).Since the daily formation of growth increments in fish otoliths was reported by Pannella (1971), aging of larval and juvenile fish by counting the growth increments has become a commonly used
technique for studying the early life history of fish. Examination of otolith microstructure has proved to be a useful tool for estimating spawning and hatch-ing dates (Ralston 1976, Struhsaker and Uchiyama 1976, Miller and Storck 1984), growth rate (Campana 1984, Volk et al. 1984, Penney and Evans 1985, Tzeng 1990), and transitions in life history (Radtke and Dean 1982, Neilson et al. 1985, Victor 1986, Chambers and Leggett 1987, Tzeng and Tsai 1994, Tzeng 1995a, Cheng and Tzeng 1996). The growth increments in otoliths of Pacific tarpon have been shown to be deposited on a daily basis (Tsukamoto and Okiyama 1993). Thus, it is possible to determine the age and growth rate of tarpon by counting otolith growth increments.
This study attempts to clarify the age of tarpon at estuarine arrival in northern Taiwan and growth during metamorphosis.
MATERIALS AND METHODS Experimental design
To understand the age of tarpon at estuarine arrival, fish were collected daily by use of a net set against the tidal current on the flood tide in the Gongshytyan Brook estuary over the period of 15 to 24 September 1995. The net was similar to that used by Tzeng (1995b). The fish collected were immediately preserved in 95% alcohol. Total length was measured to the nearest 0.1 mm after approximately 2-wk fixation. Pacific tarpon devel-ops in 4 phases: leptocephalus phase, 1st and 2nd metamorphic phases, and juvenile phase, as deter-mined based on the position of dorsal and anal fins, gas bladder shape, body shape and size, pig-mentation, dentition, ossification of the endoskel-eton, and growth rate (Sato and Yasuda 1980). Most of the larvae collected in the estuary were in the 1st metamorphic stage. The larvae decrease in length to their smallest size at the end of the 2nd metamorphic phase.
To study somatic and otolith growth during the metamorphosis stages, 340 tarpon at the 1st meta-morphic phase were acclimated for 1 d at a salinity of 0.7‰, similar to that of the estuary where the fish were collected. Fish were then randomly di-vided into 2 groups and reared further in freshwater (< 0.5‰) or 35‰ seawater. The fish were reared in 20-l growth chambers under a photoperiod of 12L: 12D at a temperature of 28 °C, similar to am-bient conditions. They were fed to satiation once a day on the nauplii of the brine shrimp Artemia salina. Five fish from each type of salinity treat-ment were sacrificed daily throughout the 24-d rearing period. Total length of each fish was mea-sured to the nearest 0.1 mm after a 2-wk fixation.
Measurement of maximum radius and growth increments in otoliths
Growth increments in otoliths of both wild and laboratory-reared fish were examined with a trans-mitted light microscope at 200-600 × magnification. Otolith radius along the maximal axis was mea-sured with a computer-aided image processing system (LV-2). The age of wild fish at estuarine arrival was estimated by counting from the otolith growth increments, based on the age validation results of laboratory-reared fish.
Data analysis
Differences in mean age and size at estuarine
arrival among sampling dates were tested with Scheffe's multiple range analysis (Sokal and Rohlf 1981). The relationship between new otolith incre-ments and rearing days, and the relationship be-tween fish length and age at estuarine arrival for wild fish were fitted with linear regressions. So-matic and otolith growth rates were derived by di-viding daily age into total length and otolith radius, respectively. The relationship between ages at es-tuarine arrival and fish and otolith growth rates, and the relationship between otolith radius and days elapsed after rearing were fitted with exponential equations. Changes in fish length during the rear-ing period were fitted with a quadratic equation to delineate the timing and minimum size of fish at the completion of metamorphosis.
RESULTS Daily growth increment
Otoliths of 1st metamorphic stage fish col-lected from the estuary were translucent, with an opaque zone forming the outer peripheral layer (Fig. 1a). Both translucent and opaque zones are composed of an incremental (light band) and a
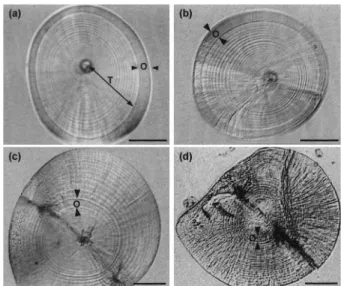
dis-Fig. 1. Daily growth increments in otoliths of Pacific tarpon,
Megalops cyprinoides. (a): Otolith showing translucent (T) and opaque (O) zones from a 1st metamorphic stage wild-caught tarpon; (b)-(d): image-enhanced otoliths showing daily growth increments in T and O zones and new increments deposited during the laboratory-reared period [(a) and (b): the same fish, 28.3 mm TL, (c): 18.91 mm TL after 13-d rearing, and (d): 20.95 mm TL (22 d)]. Scale bar = 30 µm (a, b), 50 µm (c), and 100 µm (d).
continuous zone (dark bank). Increment width in the translucent part is narrower around the primor-dium and becomes wider outward. There are sev-eral fine increments in the opaque zone which can be seen with image enhancement (Fig. 1b). The number of increments in the opaque zone ranged from 1 to 8 with a mean of 3.9 ± 1.2 (n = 117).
Otoliths of fish reared in the laboratory grew quickly, and consequently increment width in-creased relative to earlier increments (Fig. 1c-d). This provided the means to identify new increments deposited during the rearing period. The number of new increments in otoliths of reared tarpon was positively correlated with the duration of the rearing period (Fig. 2). The regression of the number of new increments (Y) on days elapsed during rearing (X) was calculated as follows:
Y = 1.10 + 1.04 X (1)
(n = 57, r = 0.98).
The slope was not significantly different from 1.0 (t = 1.53, p > 0.05), indicating that new otolith increments were deposited at daily intervals. The intercept was significantly different from zero (t = 3.01, p < 0.01), but not significantly different from 1.0 (t = 0.27, p > 0.05), i.e., the mean number of new increments was one larger than the true age. This is probably due to the 1-d acclimation prior to the rearing experiment. Accordingly, otolith growth increment counts could be used to determine the age of fish in days.
Age and size at estuarine arrival
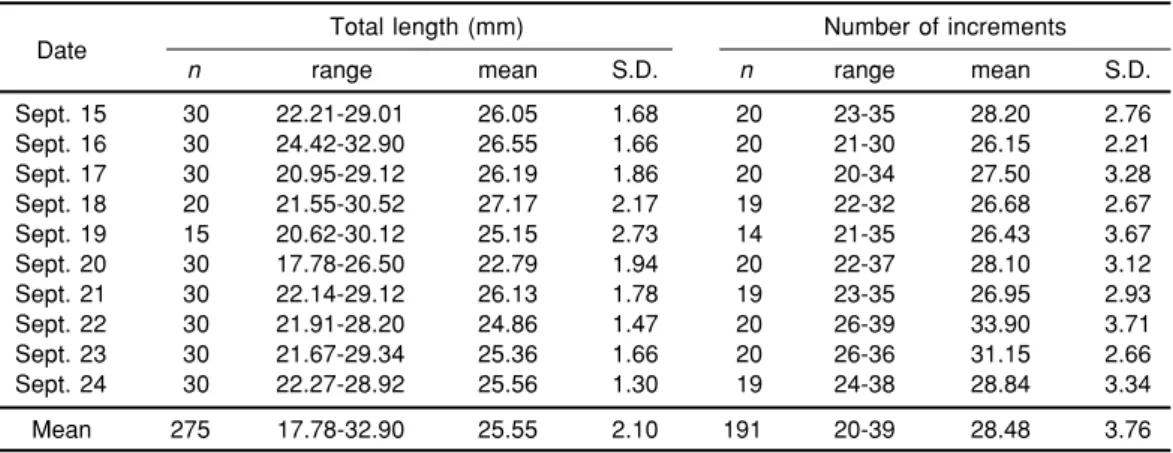
Tarpon at the time of collection were all in the 1st metamorphic phase. Total lengths of the tar-pon ranged from 17.8 to 32.9 mm with an overall mean of 25.6 mm. Mean total lengths of tarpon were significantly different among sampling dates (p < 0.01), being smallest on 20 September (22.79 ± 1.94 mm) and largest on 18 September (27.17 ± 2.17 mm) (Table 1). The ages of tarpon at the time of collection ranged from 20 to 39 d with an overall mean of 28.5 d. Mean ages of tarpon were also significantly different among sampling dates (p < 0.01), being lowest on 16 September (26.15 ± 2.21 d) and highest on 22 September (33.90 ± 3.71 d) (Table 1). Regression of total length on age of tarpon at the time of collection was not significant (r = -0.109, n = 194, p > 0.1) (Fig. 3).
Negative fish growth during metamorphic stage
Tarpon at the beginning of the rearing experi-ments were transparent leptocephali in the 1st metamorphic stage. Mean total lengths of tarpon
Table 1. Mean total length and number of otolith increments of tarpon, Megalops cyprinoides, collected in an estuary of northern Taiwan in September 1995
Total length (mm) Number of increments Date
n range mean S.D. n range mean S.D.
Sept. 15 30 22.21-29.01 26.05 1.68 20 23-35 28.20 2.76 Sept. 16 30 24.42-32.90 26.55 1.66 20 21-30 26.15 2.21 Sept. 17 30 20.95-29.12 26.19 1.86 20 20-34 27.50 3.28 Sept. 18 20 21.55-30.52 27.17 2.17 19 22-32 26.68 2.67 Sept. 19 15 20.62-30.12 25.15 2.73 14 21-35 26.43 3.67 Sept. 20 30 17.78-26.50 22.79 1.94 20 22-37 28.10 3.12 Sept. 21 30 22.14-29.12 26.13 1.78 19 23-35 26.95 2.93 Sept. 22 30 21.91-28.20 24.86 1.47 20 26-39 33.90 3.71 Sept. 23 30 21.67-29.34 25.36 1.66 20 26-36 31.15 2.66 Sept. 24 30 22.27-28.92 25.56 1.30 19 24-38 28.84 3.34 Mean 275 17.78-32.90 25.55 2.10 191 20-39 28.48 3.76
Fig. 2. Relationship between number of new increments in
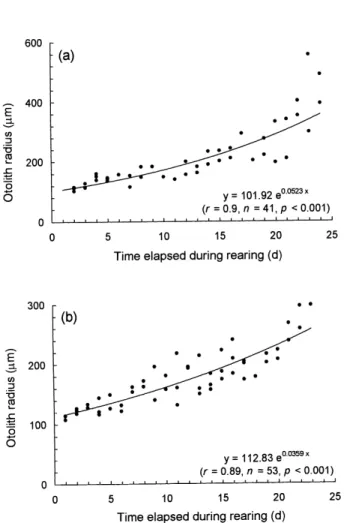
(Fig. 4a) and 18.5 mm at approximately 11 d in freshwater (Fig. 4b), and then increased gradually. The rate of reduction of fish size seems to be inde-pendent of the salinity of the rearing water. The timing of minimum length approximately corre-sponded to the period when the fish completed metamorphosis. On the other hand, otoliths grew continuously during the rearing period (Fig. 5). This indicates that somatic and otolith growth be-came uncoupled during the metamorphic stage.
Growth rate and age at estuarine arrival
Age of tarpon at the time of estuarine arrival was inversely related to both fish and otolith growth rates (Fig. 6). The relationship between age (T, d) and mean somatic growth rate (GL, mm·d-1) was calculated by an exponential growth model:
T = 58.404 e-0.7886 GL (2)
(r = -0.87, n = 194, p < 0.001) reared in both seawater (35‰) as well as fresh
water (< 5‰) decreased gradually in the early pe-riod of the 24-d rearing, diminishing to a minimum size of 18.0 mm at approximately 10 d in seawater
Fig. 4. Somatic growth of tarpon reared in 35‰ seawater (a)
and freshwater (b) at 28 °C.
Fig. 3. Relationship between total length and age in days of
tarpon at estuarine arrival.
Fig. 5. Regression of otolith radius of tarpon on days elapsed
during rearing. The tarpon, the same as those in Fig. 4, were reared in 35‰ seawater (a) and freshwater (b) at 28 °C.
metamorphic stage (Sato and Yasuda 1980). A peripheral opaque zone related to metamorphosis was also found in otoliths of conger eel, Conger myriaster, and the number of increments in the opaque zone was proposed to represent the ap-proximate duration of metamorphosis of the eel (Lee and Byun 1996). The number of increments in the opaque zone of conger eel otoliths ranged from 53 to 75 increments (days) when the eel was in a temperature range of 10-16 °C, but it de-creased to 22 increments at higher temperatures (18-22 °C) (Asano et al. 1978, Lee and Byun 1996). This indicates that the duration of the meta-morphic stage in conger eel may vary with ambient temperature. Tarpon larvae collected in the estu-ary had not yet completed metamorphosis. The larvae reared in tanks decreased to a minimum size by the end of the 2nd metamorphic phase. Accordingly, the duration of metamorphosis should include both the duration of the opaque zone and the degree of body shrinkage observed during the rearing period. The mean (± SD) duration of the opaque zone in otoliths of tarpon was 3.9 ± 1.2 d. The body length of tarpon reared at 28 °C in either freshwater or seawater diminished to a minimum within approximately 10-11 d. Hence the duration of metamorphosis of the tarpon was approximately 14 d. This duration could not be determined di-rectly from the otolith alone because there were no distinct marks on the otolith corresponding to the timing of the body length minimum. The behavior of Pacific tarpon larvae during the rearing experi-ment was similar to that of the ten-pounder, Elops hawaiensis, as reported by Sato and Yasuda (1980). The larvae in the 1st metamorphic phase initially swam between the surface and middle lay-ers of the aquarium, while the larvae of the 2nd metamorphic phase descended and occasionally moved to the bottom. The rapid behavioral change within this short time implies that Pacific tarpon larvae can adapt to the complicated environment of the estuary during upstream migration.
Early life history
Pacific tarpon, Megalops cyprinoides, at estua-rine arrival were estimated to be 20 to 39 d old with a mean of 28.5 d. Metamorphosis appeared to have commenced several days before arriving at the estuary. These estimated ages are relatively short compared to those of other leptocephalus larvae. For example, Japanese eel elvers arrive at the estuary at approximately 112.8 ± 9.4 to 156.5 ± 13.5 d and had metamorphosed approximately 1 Similarly, the relationship between age (T, d)
and otolith growth rate (GR, µm·d-1) was also cal-culated by an exponential growth model:
T = 54.659 e-0.2566 GR (3)
(r = -0.64, n = 188, p < 0.001).
The inverse relationship between the age of fish at estuarine arrival and their somatic and otolith growth rates indicates that faster-growing fish arrived at the estuary earlier than did slower-growing ones.
DISCUSSION AND CONCLUSIONS Duration of metamorphic stage
An opaque zone containing several fine growth increments was found in the outer layer of otoliths of Pacific tarpon during their upstream migration through the estuary. The opaque zone was prob-ably related to metamorphosis because the tarpon larvae collected in the estuary were at the 1st
Fig. 6. Relationship between age in days and both fish (a) and
mo before estuarine arrival (Tzeng 1990). The dif-ference in the duration of the leptocephalus stage between tarpon and Japanese eel is probably re-lated to the greater distance from the latter's spawning ground. Japanese eel spawn in the open ocean at least 3000 km away from the estuary (Tsukamoto 1992). The lengthy duration of the leptocephalus stage, and differences in the timing of metamorphosis, are the principal factors affect-ing the long-distance dispersal of the eel (Cheng and Tzeng 1996). Atlantic tarpon, Megalops atlanticus, larvae (5.5-24.4 mm; 2-25 d old) were found on the continental shelf and slope of the Florida west coast (Crabtree et al. 1992). Thus, the spawning ground of the Atlantic tarpon was speculated to be close to the coast. However, there is no information concerning the location of the spawning ground or larval distribution of Pacific tarpon. Judging from the age of Pacific tarpon at estuarine arrival, their spawning ground may be close to the estuary studied. Although the duration of oceanic life is different between tarpon and Japanese eel, they show a similar otolith growth pattern during their early life history (Tabeta et al. 1987, Cheng and Tzeng 1996), and body shrink-a g e d u r i n g m e t shrink-a m o r p h o s i s ( T z e n g 1 9 8 5 , Tsukamoto and Umezawa 1992). At present, un-like tarpon, it is still difficult to collect and rear metamorphosing Japanese eel to understand the detailed changes in their somatic and otolith growth during metamorphosis. Accordingly, tarpon is a good model for study of the little known metamor-phic stages in fishes with leptocephalus larvae.
Effect of growth rate on age at estuarine arrival
The age in days of tarpon at estuarine arrival was significantly different between individuals cap-tured on successive days. Ages ranged from 20 to 39 d; the maximum age was approximately twice the minimum. The size of tarpon at estuarine ar-rival being independent of their age, and age in days at estuarine arrival being inversely related to growth rates imply that the age of tarpon at estua-rine arrival was obviously influenced by their growth rates during offshore life. Slower-growing fish apparently metamorphosed later, and faster-growing fish arrived in the estuary earlier, than did slower-growing ones. This phenomenon was also found in winter flounder (Chambers and Leggett 1987) and in Japanese eel (Tzeng 1990).
Acknowledgments: This study was financially
supported by the National Science Council,
Repub-lic of China (Project No. NSC-85-231-11-B-002-032). The authors are grateful to Mr. C. W. Chang for collecting specimens, Miss C. H. Wang for pre-paring the manuscript, and an anonymous reviewer for helpful comments.
REFERENCES
Asano H, Y Kubo, S Yoshimatsu. 1978. On the morphological change and the behavior of the leptocephali of Conger myriaster during the period of rearing experiment. Mem. Faculty Agric., Univ. Kinki 11: 25-31.
Campana SE. 1984. Microstructural growth patterns in the otoliths of larval and juvenile starry flounder, Platichthys stellatus. Can. J. Zool. 62: 1507-1512.
Chambers RC, WC Leggett. 1987. Size and age at metamor-phosis in marine fishes: an analysis of laboratory-reared winter flounder (Pseudopleuronectes americanus) with a review of variation in other species. Can. J. Fish. Aqua. Sci. 44: 1936-1947.
Cheng PW, WN Tzeng. 1996. Timing of metamorphosis and estuarine arrival across the dispersal range of the Japa-nese eel Anguilla japonica. Mar. Ecol. Prog. Ser. 131: 87-96.
Coates D. 1987. Observation on the biology of tarpon, Meg-alops cyprinoides (Broussonet) (Pisces: Megalopidae), in the Sepik River, northern Papua New Guinea. Aust. J. Mar. Fresh. Res. 38: 529-535.
Crabtree RE, EC Cyr, RE Bishop, LM Falkenstein, JM Dean. 1992. Age and growth of tarpon, Megalops atlanticus, larvae in the eastern Gulf of Mexico, with notes on relative abundance and probable spawning areas. Envir. Biol. Fish. 35: 361-370.
Lee TW, JS Byun. 1996. Microstructural growth in otoliths of conger eel (Conger myriaster) leptocephali during the metamorphic stage. Mar. Biol. 125: 259-268.
Merrick JR, GE Schmida. 1984. Australian freshwater fishes -biology and management. Netley, South Australia: Griffin Press.
Miller SJ, T Storck. 1984. Temporal spawning distribution of large mouth bass and young-of-year growth determined from daily otolith rings. Tran. Amer. Fish. Soc. 113: 571-578.
Neilson JD, GH Green, D Bottom. 1985. Estuarine growth of juvenile chinook salmon Oncorhynchus tshawytscha as inferred from otolith microstructure. Can. J. Fish. Aqua. Sci. 42: 899-908.
Nelson JS. 1984. Fish of the world. 2nd ed. New York: John Wiley, 523 pp.
Pannella G. 1971. Fish otoliths: daily growth layers and periodi-cal patterns. Science 173: 1124-1127.
Penney RW, GT Evans. 1985. Growth histories of larval redfish (Sebastes spp.) on an offshore Atlantic fishing bank deter-mined by otolith increment analysis. Can. J. Fish. Aqua. Sci. 42: 1452-1464.
Radtke RL, JM Dean. 1982. Increment formation in the otoliths of embryos, larvae and juvenile of the mummichog, Fundulus heteroclitus. Fish. Bull. US 80: 201-215. Ralston S. 1976. Age determination of a tropical reef
butterflyfish utilizing daily growth rings of otoliths. Fish. Bull. US 74: 990-994.
the ten-pounder, Elops hawaiensis, from Ishigaki Island, Japan. Jpn. J. Ichth. 26: 315-324.
Sokal RR, FJ Rohlf. 1981. Biometry. 2nd ed. San Francisco: WH Freeman.
Struhsaker P, JH Uchiyama. 1976. Age and growth of the nehu, Stolephorus purpureus (Pisces: Engraulidae) from the Hawaiian Islands as indicated by daily growth increments of sagittae. Fish. Bull. US 74: 9-17.
Tabeta O, K Tanaka, J Yamada, WN Tzeng. 1987. Aspects of the early life history of the Japanese eel Anguilla japonica determined from otolith microstructure. Bull. Jpn. Soc. Sci. Fish. 53: 1727-1734.
Tsukamoto K. 1992. Discovery of the spawning area for Japa-nese eel. Nature 356: 789-791.
Tsukamoto K, A Umezawa. 1992. Early life history and oceanic migration of the eel, Anguilla japonica. La Mer 28: 188-198.
Tsukamoto Y, M Okiyama. 1993. Growth during the early life history of the Pacific tarpon, Megalops cyprinoides. Jpn. J. Ichth. 39: 379-386.
Tsukamoto Y, M Okiyama. 1997. Metamorphosis of the Pacific tarpon, Megalops Cyprinoides (Elopiformes, Mega-lopidae) with remarks on development patterns in the Elopomorpha. Bull. Mar. Sci. 60: 23-36.
Tzeng WN. 1985. Immigration timing and activity rhythms of the eel, Anguilla japonica, elvers in the estuary of northern Taiwan with emphasis of environmental influences. Bull. Jpn. Soc. Fish. Oceanogr. 47/48: 11-28.
Tzeng WN. 1990. Relationship between growth rate and age at recruitment of Anguilla japonica elvers in a Taiwan estuary
as inferred from otolith growth increments. Mar. Biol. 107: 75-81.
Tzeng WN. 1995a. Migratory history recorded in otoliths of the Japanese eel, Anguilla japonica, elvers as revealed from SEM and WDS analyses. Zool. Stud. 34 (Supplement 1): 234-236.
Tzeng WN. 1995b. Recruitment of larval and juvenile fishes of the Gong-Shy-Tyan River estuary of Taiwan: relative abundance, species composition and seasonality. In NB Armantrout, ed. Condition of the world's aquatic habitats. Proceedings of the World Fisheries Congress Theme 1. New Delhi: Oxford & IBM Publishing Co. Pvt. Ltd., pp. 360-385.
Tzeng WN, YC Tsai. 1994. Changes in otolith microchemistry of the Japanese eel, Anguilla japonica, during its migration from the ocean to the rivers of Taiwan. J. Fish Biol. 45: 671-683.
Tzeng WN, SY Yu. 1986. Occurrence of the leptocephalus larvae of Elops hawaiensis and Megalops cyprinoides in the Gong-shy-tyan River estuary of north Taiwan with ref-erence to some ecological and taxonomic aspects. Pro-ceedings of the Symposium on Marine Biological Science, Biology Research Center, Nat. Sci. Council Monogr. Se-ries 14, pp. 165-176.
Victor EC. 1986. Delayed metamorphosis with reduced larval growth in a coral reef fish (Thalassoma bifasciatum). Can. J. Fish. Aqua. Sci. 43: 1208-1213.
Volk EC, RC Wissmar, CA Simenstad, DM Eggers. 1984. Rela-tionship between otolith microstructure and the growth of juvenile chum salmon (Oncorhynchus keta) under different prey rations. Can. J. Fish. Aqua. Sci. 41: 126-133.