Analysis of the Steps Involved in Dengue Virus Entry into Host Cells
Shan-Ling Hung,*,1
Pei-Lun Lee,* Hsiao-Wang Chen,* Li-Kuang Chen,† Chuan-Liang Kao,‡ and Chwan-Chuen King§ *Institute of Oral Biology, National Yang-Ming University, Taipei, †Department of Immunology, Tsu-Chi Medical College, Hualien,
‡School of Medical Technology, College of Medicine and Division of Infectious Disease, and §Institute of Epidemiology,
College of Public Health, National Taiwan University, Taipei, Taiwan, Republic of China Received June 24, 1998; returned to author for revision November 7, 1998; accepted February 2, 1999
The initial steps of dengue viral entry have been divided into adsorption and penetration using acid glycine treatment to inactivate extracellular virus after attachment to baby hamster kidney (BHK) cells but prior to penetration. First, we showed that virus infection was accomplished within 2 h after adsorption. Second, the assay was used to examine the properties of dengue envelope E protein-specific monoclonal antibodies (MAbs), lectins, and heparin. We found that three MAbs, 17-2, 46-9, and 51-3, may neutralize dengue 2 virus (DEN-2) through inhibition of not only viral attachment but also of penetration. However, one MAb, 56-3.1, interfered specifically with attachment. Therefore, the functional domains of E protein involved in attachment and penetration may be different. Moreover, studies with lectins indicated that carbohydrates, especially a-mannose residues, present on the virion glycoproteins may contribute to binding and penetration of the virus into BHK and mosquito C6/36 cells. Finally, virus infectivity was inhibited by heparin through its blocking effects at both virus attachment and penetration. This suggests that cell surface heparan sulfate functions in both viral attachment and penetration of DEN-2 virus. In conclusion, our results further elucidated some aspects of the dengue virus entry process. © 1999 Academic Press
INTRODUCTION
Dengue virus, a member of the family Flaviviridae, genus Flavivirus, is an important human pathogen caus-ing dengue fever (DF) and the more severe forms, den-gue hemorrhagic fever (DHF) or denden-gue shock syndrome (DSS) (Monath, 1986). Defining how viruses interact with the cell surface is important for explaining the pathogen-esis of dengue viruses. The initial events required for productive dengue infection have not been described in as much detail as for other flaviviruses, such as West Nile virus (WNV). Studies using electron microscopy have indicated that attachment is a temperature-inde-pendent process that occurs at both 4 and 37°C, whereas viral penetration proceeds only at 37°C. Pene-tration can occur by membrane fusion in mosquito C6/36 cells or by receptor-mediated endocytosis in monocytes (Barth, 1992; Hase et al., 1989). In the presence of sub-neutralizing amounts of antibody, Fc receptors also me-diate attachment and uptake of dengue viruses into cer-tain target cells (e.g., monocytes and macrophages) (Gol-lins and Porterfield, 1985; Mady et al., 1993). This entry mechanism, termed antibody-dependent enhancement (ADE), may play a role in development of DHF and DSS as a consequence of sequential infections with different
dengue serotypes (reviewed in Halstead, 1988). How-ever, ADE does not explain infection of cells without Fc receptors nor does it explain primary infection in patients lacking dengue antibody.
The viral envelope E protein of dengue virus, which is embedded in a lipid bilayer, may mediate virus attach-ment and penetration into cells. E protein is both a target and a modulator of the host immune response. E protein contains two sites for N-linked glycosylation; the mature glycoprotein has a molecular weight (MW) of approxi-mately 60,000 (Smith and Wright, 1985). Use of each glycosylation site in mosquito cells appears to depend on the serotype of the virus (Johnson et al., 1994). Muta-tions of N-linked glysosylation sites of E protein may affect virus-mediated membrane fusion and neuroviru-lence (Guirakhoo et al., 1993; Kawano et al., 1993; Sanchez and Ruiz, 1996). However, the functional signif-icance of the attached carbohydrates themselves has not been determined.
The identification and isolation of a specific cell sur-face receptor for dengue viruses are important for un-derstanding their biology. Chen et al. (1996b) found that recombinant E protein bound specifically to Vero, CHO, endothelial, and glial cells. In addition, this binding has been associated with heparan sulfate or glycosamino-glycans (GAGs) (Chen et al., 1997). These complex charged carbohydrates are found both on the cell sur-face and in the extracellular matrix (Chen et al., 1996b; 1997). Thus, highly sulfated heparan sulfate on the cell
1To whom correspondence and reprint requests should be
ad-dressed at Institute of Oral Biology, National Yang-Ming University, No. 155, Sec. 2, Li-Nong St., Shih-Pai, Taipei, Taiwan, R.O.C. Fax: 886-2-28264053. E-mail: slhung@ym.edu.tw.
Article ID viro.1999.9633, available online at http://www.idealibrary.com on
0042-6822/99 $30.00 Copyright © 1999 by Academic Press All rights of reproduction in any form reserved.
surface may serve as the initial attachment receptor for DEN-2 virus (Chen et al., 1997). However, the precise mechanism of the blocking by heparin in the entry step is not clear. Additional receptors for dengue viruses may be present. For example, DEN-2 virus can infect cultured monocytes through a cellular receptor that is trypsin-sensitive; therefore, it is proteinaceous in nature (Daughaday et al., 1981).
In this report, we defined the initial events of viral entry and the roles played by viral and cellular components in these processes in detail. The initial steps of dengue viral entry have been divided into adsorption and pene-tration by briefly exposing the virus–cell monolayers to acid glycine treatment (Highlander et al., 1987; Long et
al., 1990). This assay was used to examine the effect of
anti-E protein neutralizing antibodies on the entry pro-cess. We found that anti-E protein neutralizing antibodies may inhibit not only viral attachment but also penetration. Studies using lectins suggest that carbohydrates on the virions may play roles in dengue virus binding and pen-etration into BHK and mosquito C6/36 cells. Finally, we found that heparin, a related GAG, blocks virus infection at both the attachment and the penetration steps, sug-gesting a role for cell-surface GAGs in both processes.
RESULTS Kinetics of viral penetration
To define more accurately the initial events of viral entry, we determined the rate of viral penetration of DEN-2 virus into baby hamster kidney (BHK) cells as described by Long et al. (1990). In this assay, virus, which has entered the cells, is protected from inactivation by acid treatment. Surviving viruses are capable of forming plaques. Thus, penetration is defined as the loss in sensitivity to acid inactivation. BHK cells were used as targets as they are susceptible and commonly used in
vitro for infection by dengue viruses (Malewicz and
Jen-kin, 1979). Cells in 35-mm dishes were infected with DEN-2 virus (PL-046 strain) and incubated at 4°C for 2 h to allow attachment to occur (Fig. 1A). Cells were then washed and shifted to 37°C. At various time points (i.e., 0, 15, 30, 60, 90, and 120 min) after the shift, the infected cells in each dish were treated with acid glycine (pH 3.0) buffer for 1 min to inactivate extracellular virus. The acid was removed and replaced with medium and the cells were incubated at 37°C to allow plaque formation. The percentage of plaque-forming units (PFU) surviving acid treatment was calculated. At zero time, approximately 95% of the attached virus was inactivated by acid treat-ment (Fig. 2). As the time of incubation at 37°C prior to acid treatment increased, more virus became resistant. By 25 min, about 50% of adsorbed virus penetrated into BHK cells, and by 60 min, over 75% of the virus had penetrated the cells. By 2 h, approximately 95% of the
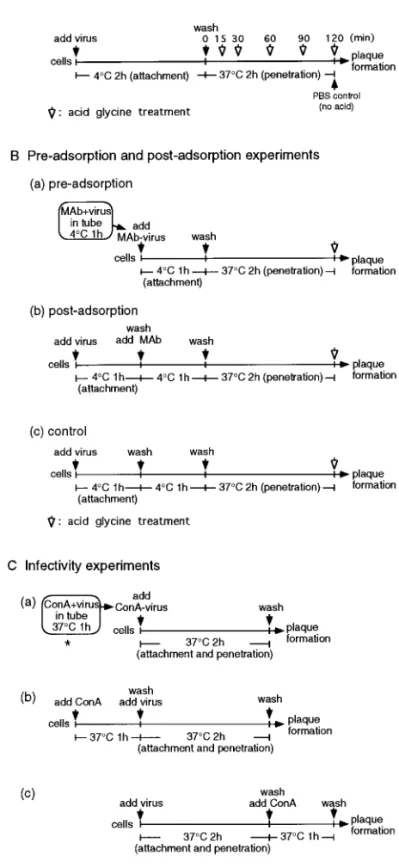
FIG. 1. Schematic representation of the strategies used in this study. (A) Kinetics of viral penetration experiments. Virus samples (100 to 250 PFU) were added to BHK cells and allowed to adsorb to cells for 2 h at 4°C. The cultures were then incubated at 37°C. At the times indicated, cells were washed with acid glycine buffer (pH 3.0) to inactivate extracellular viruses. (B) Preadsorption and postadsorption experi-ments for MAbs. Similar experimental procedures were also used for Con A and heparin in the same manner except that Con A and heparin were used. (C) Infectivity experiments for studying the effect of Con A on viral plaque formation. The effects of WGA, PHA-P, or heparin were also determined under similar conditions. The details are described under Materials and Methods.
virus had penetrated cells. In addition, the results indi-cated that we can easily separate entry steps into at-tachment and penetration using acid glycine treatment. Neutralization of DEN-2 virus by E protein-specific monoclonal antibodies before and after virus adsorption to cell surfaces
To gain insight into the role of E protein in entry, we explored the mechanism of virus neutralization using five MAbs, 17-2, 46-9, 51-3, 55-3, and 56-3.1. These MAbs were specific for E protein of dengue virus as determined by immunoprecipitation and enzyme-linked immunosor-bant assay (ELISA) (data not shown). Four MAbs, 17-2, 46-9, 51-3, and 56-3.1, were able to neutralize dengue viruses when incubated with viruses at 37°C before viral inoculation. However, MAb 55-3 was a nonneutralizing antibody (data not shown; Chen et al., manuscript in preparation).
Here, this panel of MAbs were further examined by measuring their ability to neutralize virus when added before or after virus adsorption to host cells at 4°C. The entry steps were divided into adsorption and penetration using the acid glycine treatment assay as illustrated in Fig. 1B. If the mechanism of neutralization primarily in-volves inhibition of virus attachment, adsorbed virions should be resistant to neutralization. Alternatively, if the mechanism of neutralization involves blocking of a step in infection subsequent to adsorption, these antibodies should be able to neutralize cell surface-bound virions. Pilot studies were conducted to determine whether we could shorten the incubation time at 4°C from 2 h to 1 h. Similar results for virus penetration as shown in Fig. 2
were obtained when the virus was incubated with the cells for 1 h at 4°C (data not shown). Therefore, for the following preadsorption and postadsorption experi-ments, the 4°C incubation time was changed to 1 h.
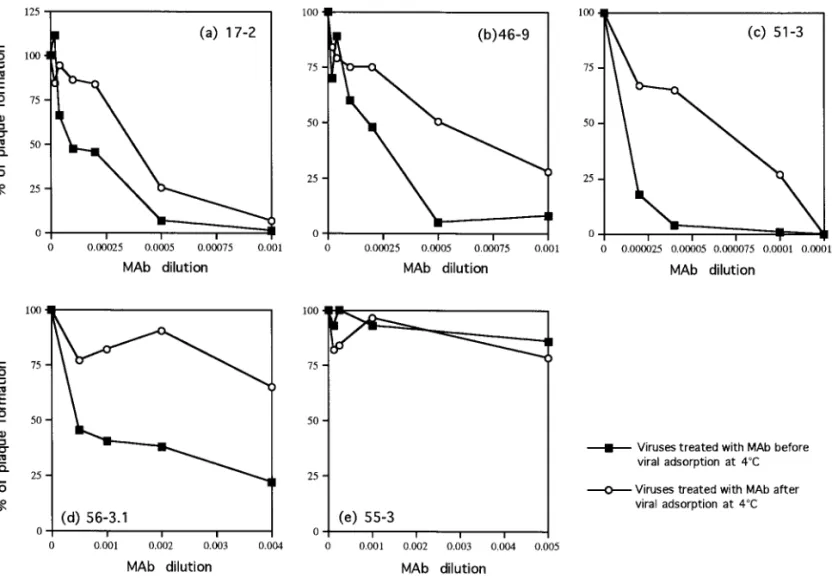
Figure 3 shows the percentage of residual plaque formation for viruses treated with different MAbs added either before [Fig. 1B(a)] or after [Fig. 1B(b)] viral adsorp-tion. First, as expected, none of the antibodies were able to enhance the plaque development of DEN-2 virus sig-nificantly at any concentration tested. The slight increase in PFU of MAb 17-2 is very low (to 110%) (Fig. 3a), and we believe that it is not significant. Second, four of the five MAbs, 17-2, 46-9, 51-3, and 56-3.1, exhibited significant neutralization titers, suggesting that their epitope speci-ficities are localized to a site(s) that contributes to the role of E protein in virus infectivity (Figs. 3a, 3b, 3c, and 3d). Third, three MAbs, 17-2, 46-9, and 51-3, neutralized viruses not only when added before but also when added after viral attachment (Figs. 3a, 3b, and 3c). In these cases, neutralization appears to involve viral pen-etration, a step later than virus attachment. Interestingly, MAb 56-3.1 could neutralize viruses when added before viral adsorption, whereas it had little neutralization activ-ity when added after adsorption (Fig. 3d). Thus, the epitope of MAb 56-3.1 may be involved mainly in viral attachment but appears not to be involved in penetration. MAb 55-3, a nonneutralizing antibody, had no effect on infectivity whether added before or after virus adsorption at 4°C (Fig. 3e). This antibody was also unable to neu-tralize virus infectivity when the entire incubation was carried out at 37°C (data not shown). These results indicated that the mechanism of neutralization of anti-E MAbs involved inhibition of both attachment and virus penetration into cells and strongly supports the concept that E protein plays a direct role in both aspects of the entry process of dengue viruses. It also appears that MAb 56-3.1 directly inhibits the interaction between E and its cell surface receptor and has little effect on penetration.
Determining the roles of carbohydrates of viral proteins involved in dengue entry
E protein of DEN-2 virus is glycosylated (Smith and Wright, 1985); however, the functions of the carbohy-drates are not clear. To explore the possible roles of carbohydrates on viral proteins in regulating viral entry, the effects of lectins on the infectivity of DEN-2 viruses into BHK cells were first examined under three different conditions as illustrated in Fig. 1C. To examine the ef-fects on the whole entry process, viral infection was carried out at 37°C for 2 h while attachment and pene-tration steps were not dissected. Viruses or BHK cells were treated with lectins including: (1) concanavalin A (Con A, which binds toa-linked terminal mannose
resi-FIG. 2. Rate of penetration of DEN-2 virus. At the times indicated, cells were treated with acid glycine to inactivate extracellular viruses. The results are shown as a percentage of plaque formation when compared with controls in which PBS was substituted for acid glycine buffer.
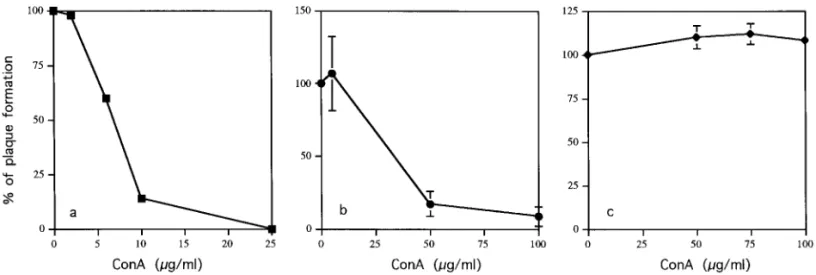
dues on N-linked high-mannose or hybrid glycans); (2) wheat germ agglutinin [WGA, which recognizes acetyl-glucosamine (glcNAcb1-4) on N-linked glycans]; and (3) phytohemagglutinin P (PHA-P, which recognizes oligo-saccharides). The effects of the mannose-specific lectin, Con A, on plaque formation induced by DEN-2 viruses on BHK cells are shown in Fig. 4. Preincubation of virus with Con A for 1 h at 37°C prior to assessment of virus infection [Fig. 1C(a)] blocked plaque formation markedly (approximately 50% inhibition at 7.5mg/ml) (Fig. 4a). Con A also blocked plaque formation when added to BHK cells before viral inoculation [Figs. 1C(b) and 4b]. How-ever, there was no inhibitory effect if Con A was added to cells for 1 h after the initiation of infection [Figs. 1C(c) and 4c]. These results demonstrate that DEN-2 was inactivated when incubated with Con A during the bind-ing of the virus and its fusion with the target cells, but that Con A had no further effect once most of the virus had penetrated. The results suggested that the carbohy-drates of viral protein, probably E protein, may be re-quired for viral infection. Alternatively, aggregates of Con A on virions may affect viral infection through steric
hindrance effect. The other possibility is that Con A binding may affect the conformation of E protein, which results in malfunction of E protein. The results for the effects of WGA and PHA-P on viral infectivity are shown in Figs. 5a and 5b, respectively. Similar to the results of Con A treatment, preincubation of WGA with virus or cells before viral inoculation (but not after) greatly re-duced the viral plaque formation (Fig. 5a). In contrast, there was no inhibitory effect when lectin PHA-P was used (Fig. 5b).
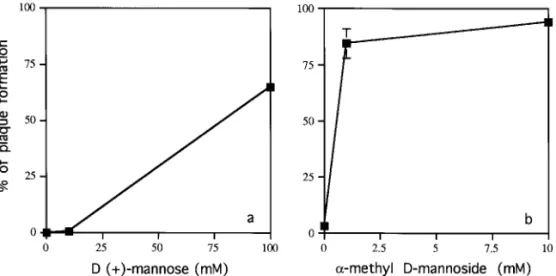
To confirm that the inhibitory effect of Con A was specifically due to binding to a-mannose residues of viral protein, we carried out competition experiments, using D-(1)-mannose or a-methyl-D-mannoside [Fig.
1C(a)]. Both competitors have been used successfully to compete for mannose-specific binding of Con A (Cam-padelli-Fiume et al., 1988; DeGeorge et al., 1985). The inhibitory effect of Con A was blocked by the addition of either of these competitors (Fig. 6). In addition, the a-methyl-D-mannoside competed more efficiently than D-(1)-mannose (compare Figs. 6a and 6b). The results
strongly suggested that the inhibitory effects of Con A on
FIG. 3. Neutralization of viruses by treatment with anti-E-specific monoclonal antibodies (MAbs) before (preadsorption) or after (postadsorption) viral adsorption. DEN-2 virus was incubated with different dilutions of antibodies (a) 17-2, (b) 46-9, (c) 51-3, (d) 56-3.1, or (e) 55-3 before (■) or after (E) viral adsorption at 4°C. The results are reported as a percentage of plaque formation.
viral infection were through binding to mannose residues of viral envelope proteins.
Con A inhibition at the step of attachment and penetration
To further define the mechanisms of the blocking effect of Con A, the inhibitory ability of Con A before or after viral adsorption at 4°C was evaluated. The experimental condi-tions were similar to those for MAb in the preadsorption and postadsorption neutralization experiment, except that Con A was used (Fig. 1B). Approximately 50% of virus was affected when 7.5–8mg/ml of Con A was added after viral
adsorption (Fig. 7). However, if added before viral adsorp-tion, less Con A was required to obtain a similar inhibitory effect. Preincubation of virus with Con A at 4°C appeared to be more effective than preincubation at 37°C (compare Figs. 7 and 4a). In addition, Con A inhibited viral plaque formation when added after viral attachment (Fig. 7). Thus, Con A inhibits both attachment and penetration steps. The effects of Con A on viral entry into mosquito C6/36 cells
The effects of Con A at various concentrations (1.56, 6.25, 25, and 100 mg/ml) on the infectivity of dengue
FIG. 4. Effects of Con A on plaque formation of DEN-2 virus. BHK cells were infected with DEN-2 virus under three different experimental conditions: (a) viruses treated with Con A before virus inoculation; (b) cells treated with Con A before virus inoculation; and (c) cells treated with Con A after virus infection. The results are shown as a percentage of plaque formation compared with the control reactions where infection was carried out in the absence of Con A.
FIG. 5. Effects of (a) WGA and (b) PHA-P on infectivity of DEN-2 virus. BHK cells were infected with DEN-2 virus under three different experimental conditions: (F) viruses treated with WGA or PHA-P before virus inoculation; (■) cells treated with WGA or PHA-P before virus inoculation; and ({) cells treated with WGA or PHA-P after virus infection. The results are shown as a percentage of plaque formation compared with the control reactions where infection was carried out in the absence of WGA or PHA-P.
viruses into mosquito C6/36 cells, natural target cells, were also examined (Fig. 8). The experimental conditions were similar, as illustrated in Fig. 1C. Since C6/36 cells did not form visible plaque after dengue infection, we utilized an immunofluorescence assay to detect the ex-pression of viral NS1 protein. BHK cells were examined in the same way for comparison. Under the first experi-mental condition [Fig. 1C(a)], we found that pretreatment of viruses with Con A before viral infection affected the expression of viral antigen, NS1 (Fig. 8). Mock-infected cells showed negative fluorescence results (Figs. 8A and 8D). Preincubation of viruses with 25 (data not shown) or 100 mg/ml of Con A greatly inhibited the expression of
NS1 protein in both C6/36 cells and BHK cells (Figs. 8C and 8F). Under the second experimental condition, when C6/36 or BHK cells were preincubated with 100mg/ml of Con A before viral infection [as in Fig. 1C(b)], the inhib-itory effects of Con A were also observed (data not shown). There were no obvious effects when other con-centrations were used (1.65, 6.25, and 25mg/ml; data not shown). Under the third experimental condition, when Con A was added for 1 h after the initiation of infection [Fig. 1C(c)], there was no difference for each concentra-tion examined (data not shown). The fluorescence data were in agreement with the plaque formation data in showing that virus was inactivated when incubated with Con A during entry, but that once the virus had pene-trated the C6/36 cells, Con A had no further effect. There-fore, the carbohydrates of viral protein may also be required for viral infection into mosquito cells.
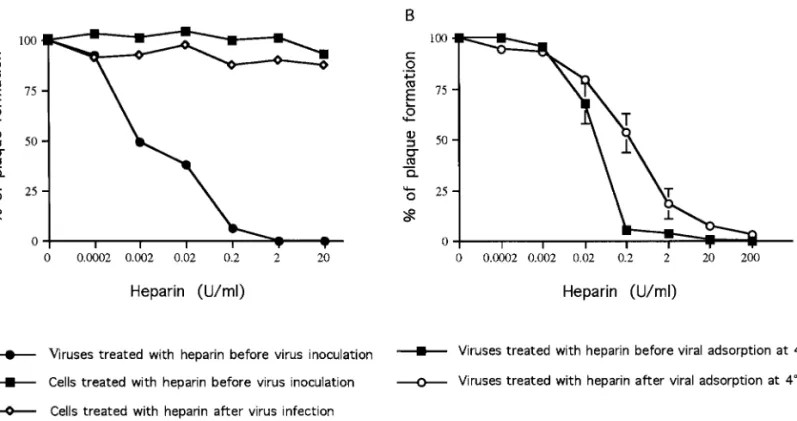
Heparin inhibition at the step of penetration
To define the precise mechanism of the blocking by heparin in the entry step, we assayed the effect of hep-arin on the whole entry process first without dissecting it into attachment and penetration. Similar to the lectin experiments described above, three experimental condi-tions were utilized (Fig. 1C). First, virus was preincubated with heparin at 37°C prior to being added to cells [Figs. 1C(a) and 9A]. Virus was inactivated in a dose-depen-dent manner such that approximately 50% was inacti-vated by incubation with 0.002 U/ml of heparin (equiva-lent to 0.01mg/ml). This suggests that heparin interacts with a viral protein. There was no effect on infectivity when cells were treated with heparin before viral inocu-lation (Figs. 1C(b) and 9A). In addition, no inhibitory effect was observed when heparin was added after the entry events occurred [Figs. 1C(c) and 9A]. Our results suggest that heparin binds to virus and prevents the virus from interaction with a cellular component of BHK cells,
sim-FIG. 6. Blocking the effects of Con A by competitors in the competition assay. (a)D-(1)-mannose and (b)a-methyl-D-mannoside were used as competitors to block the inhibitory effect of Con A (20mg/ml).
FIG. 7. Effects of Con A on preadsorption or postadsorption entry steps of DEN-2 virus. Virus was incubated with different dilutions of Con A before (■) or after (E) viral adsorption at 4°C. The results are reported as a percentage of plaque formation.
ilar to previous findings by Chen et al. (1997) in Vero cells.
To further explore the possible role of heparin at the penetration step, we added it before or after viral attachment at 4°C. We used acid glycine treatment to separate attachment and penetration as for MAb or
Con A (Fig. 1B). We found that more heparin was required to obtain inhibition at 4°C than at 37°C (com-pare Figs. 9A and 9B). However, our results clearly showed that after viral attachment, heparin inhibited viral plaque formation equally efficiently when added before or after attachment (Fig. 9B). Therefore, cell FIG. 8. Effects of Con A on infectivity of DEN-2 virus into C6/36 cells and BHK cells. Infected cells, C6/36 (A to C) and BHK cells (D to F), were tested for dengue NS1 protein expression by using a fluorescence assay. (A and D) Mock-infected cells; (B and E) cells infected with dengue viruses without Con A treatment; and (C and F) virus was incubated with 100mg/ml of Con A before viral inoculation onto cells.
FIG. 9. Effects of heparin on the infectivity and entry steps of DEN-2 virus. (A) Effects of heparin on the infectivity of DEN-2 virus. BHK cells were infected with DEN-2 virus under three different experimental conditions as illustrated in Fig. 1C: (F) viruses were preincubated with different concentrations of heparin before virus inoculation; (■) BHK cells were treated with different concentrations of heparin before virus inoculation; and ({) cells treated with heparin after virus infection. (B) Effects of heparin on preadsorption and postadsorption entry steps of DEN-2 viruses. The experimental conditions are illustrated in Fig. 1B. Virus was incubated with different amounts of heparin before (■) or after (E) viral adsorption at 4°C. The results are shown as a percentage of plaque formation compared with the control reactions where infection was carried out in the absence of heparin.
surface GAGs may play roles in both attachment and penetration of dengue virus.
DISCUSSION
The initial steps in viral infection are attachment and penetration into host cells. Information on the molecular mechanisms concerning these events is accumulating for several virus–host systems, although little is known concerning the molecules involved in the entry of dengue virus into the cell cytoplasm. Dengue viruses are able to attach but not penetrate into cells at 4°C (Hase et al., 1989), whereas penetration requires the temperature to be raised to 37°C. Since acid glycine treatment inacti-vates only virus that has not penetrated into cells, we used this treatment to determine the kinetics of penetra-tion of DEN-2 virus into BHK cells. This assay has been used to separate the attachment and penetration pro-cesses of other viruses such as herpes simplex virus (Highlander et al., 1987), but this is its first application to the study of dengue virus. We found that 50% of the adsorbed virus penetrates into cells within 25 min and more than 75% of the adsorbed virus penetrates within 1 h. However, this treatment cannot distinguish between virus that has fused at the cell membrane and entered the cytoplasm and virus that may have been taken up in vesicles by endocytosis but has yet to cross the mem-brane boundary into the cytoplasm. In fact, both mecha-nisms have been reported for dengue entry (Barth, 1992; Hase et al., 1989). Nevertheless, this method allowed us to easily dissect the entry steps and to further charac-terize the effects of antibodies and other reagents, such as lectins and heparin, on viral attachment and penetra-tion.
Dengue E protein is an essential component for initi-ating infection. It mediates virus binding to cell receptors and the subsequent fusion step (Randolph and Stollar, 1990). In our study, efficient virus neutralization by three E-specific MAbs, 17-2, 46-9, and 51-3, was achieved after virus had been adsorbed to the host cell membrane. This indicated that the mechanism of neutralization also in-volved E protein to prevent the penetration of DEN-2 virus. This finding was consistent with the mechanism of virus neutralization that involved blocking of some step(s) after virus attachment, as previously suggested for herpes simplex virus (Highlander et al., 1987). Others have reported that neutralizing antibodies against E pro-teins block binding of DEN-2 virus to monkey kidney (Vero) cells (He et al., 1995). Interestingly, MAb 56-3.1 can neutralize viruses before viral adsorption, whereas it had little neutralization activity when added after adsorption. The results for MAb 56-3.1 suggest that the functional domains involved for attachment and penetration may be different. Further analysis of the epitopes of these anti-bodies will be helpful for mapping the regions on E
proteins that are important for viral entry and for future vaccine development.
The E protein of dengue viruses contains two potential N-linked oligosaccharide sites. Johnson et al. (1994) re-ported that the E proteins of different serotypes (DEN-1 and DEN-2) grown in mosquito cells were heteroge-neous in their utilization of potential glycosylation sites. For example, E protein of the DEN-2 virus is glycosylated only at the first site (Asn-67), whereas the E protein of DEN-1 virus is glycosylated at sites specified by Asn-67 and Asn-153 (Johnson et al., 1994). In addition, glycosyl-ation may also play roles in neurovirulence of dengue infection in mice (Pletnev et al., 1993). Single amino acid substitution of Ile for Thr-155 that ablated the first con-served glycosylation sites in parental E protein of DEN-4 virus yielded a virus strain that was almost as neuroviru-lent as the mouse-adapted mutant (Kawano et al., 1993). Thus the glycosylation site of E protein appears to play a role in neurovirulence of DEN-4 virus (Kawano et al., 1993). The importance of glycosylation of envelope pro-tein on viral entry has also been demonstrated for sev-eral other viruses. For example, variations in the number and position of N-linked glycosylation of viral hemagglu-tinin of influenza virus affect its receptor-binding charac-teristics (Deom et al., 1986; Gambaryan et al., 1998; Gunther et al., 1993; Matrosovich et al., 1997). Moreover, the N-glucan of the envelope protein of human immuno-deficiency virus type 1 (HIV-1) is required for viral infec-tivity and cellular host range selection (Nakayama et al., 1998).
The E proteins from virus grown in mosquito C6/36 cells are likely to contain oligosaccharides of the high-mannose type, which are able to bind to lectins including Con A and WGA (Johnson et al., 1994; Smith and Wright, 1985). Our data suggested that when Con A binds to virions, their infectivity into both BHK and mosquito C6/36 cells was adversely affected at the entry step. Thus, the mannose residues of carbohydrates on viral glycoproteins may also participate in virus entry. For example, the mannose-specific lectin Con A may inter-fere with the direct interaction between carbohydrates of E protein and the cellular receptor(s). Alternatively, Con A may alter the conformation of the E glycoprotein and lead to inactivation. In addition, the effects of Con A may be through steric hindrance. In fact, Con A is not monova-lent and may induce aggregation of virions (Gattegno et
al., 1992; Robinson et al., 1987). Another lectin, WGA, was
also found to inhibit plaque formation when added be-fore viral inoculation. WGA is able to bind E protein; however, it does not bind as effectively as does Con A (Johnson et al., 1994). This may explain why, in our experiments, more WGA than Con A on a weight basis was required for inhibition of plaque formation. There was no inhibitory effect for PHA-P. However, it is not clear whether PHA-P binds E protein or not.
Previously, it has been demonstrated that the lectin pokeweed mitogen (PWN) can enhance dengue virus infection in a mouse macrophage cell line (Hotta and Homma, 1994). Their results raised the possibility that the PWN-mediated increase in viral binding/penetration was due to the exposure of a masked dengue virus receptor (Hotta and Homma, 1994). We did not observe any enhancement with Con A, WGA, or PHA-P on viral plaque formation in BHK cells. Surprisingly, preincuba-tion of DEN-2 virus with a low concentrapreincuba-tion of Con A (1.25mg/ml) before but not after viral infection appeared to enhance the subsequent expression of NS1 protein in both BHK cells and mosquito C6/36 cells (data not shown). The mechanism of this enhancement is not clear, particularly since there was no significant effect on plaque formation by this concentration of Con A. Chen et
al. (1999) found that bacterial lipopolysaccharide inhibits
dengue virus infection of primary human monocytes/ macrophages by blocking for virus entry via a CD14-dependent mechanism. Therefore, future studies should examine the entry events of dengue virus and the effects of various lectins on human monocytes/macrophages. Our preliminary experiments suggest that the effect of Con A on human erythroleukemia cell line K562 may indeed be different from that of BHK or mosquito cells (data not shown). Further analysis of lectins on viral entry into monocytic cells is in progress. Con A can also inhibit the infectivity of other viruses such as HIV (Lifson et al., 1986; Matsui et al., 1994; Pal et al., 1993). The binding of mannose-specific lectins or mannose-binding protein to gp120 inhibits HIV infectivity (Pal et al., 1993). Determin-ing the mechanisms of this inhibition may be helpful for development of antiviral reagents.
Different components of host cell membranes can act as viral receptors (Haywood, 1994). The selective inter-action of animal viruses with a specific cell surface receptor is an essential step in the initiation of a viral infection. This interaction often determines the host range and cellular or tissue tropism of a virus and there-fore plays a key role in determining virus pathogenicity. Little is known about the full nature of the host cell receptor for dengue viruses. In part, the initial receptor for DEN-2 virus may be highly sulfated heparan sulfate (Chen et al., 1997). Cell surface heparan sulfate plays a role in the attachment of several other microorganisms to mammalian cells (Rostand and Esko, 1997); the viral pathogens include herpes simplex virus (HSV) (Shieh et
al., 1992; Spear et al., 1992; WuDunn and Spear, 1989),
HIV-1 (Patel et al., 1993), type O foot-and-mouth disease virus (Jackson et al., 1996), respiratory syncytial virus (Krusat and Streckert, 1997), vaccinia virus (Chung et al., 1998), adeno-associated virus (Summerford and Samul-ski, 1998), and Sindbis virus (Byrnes and Griffin, 1998; Klimstra et al., 1998). However, additional receptors may be required for binding and entry of these viruses
(Mont-gomery et al., 1996). Our results from heparin blocking experiments further suggest that interaction between dengue virus and cell surface GAGs is also important for penetration. Specifically, the binding of heparin to the E protein of DEN-2 virus may alter the ability of E protein to function in both attachment and penetration. Recently, Laquerre et al. (1998) showed that HSV binding to the heparan sulfate receptor is also required in the process of virus penetration. Heparan sulfate may be required to increase binding to a second receptor potentially recog-nized by viral envelope protein or to initiate changes in the envelope of adsorbed virion that trigger the process of envelope fusion with the cellular membrane.
This report addresses interactions between viruses and cells with respect to viral entry. Using the acid glycine method, we demonstrated that both attachment and penetration steps can be affected by three neutral-izing MAb, Con A, or heparin. This assay provided a useful approach in the further study of dengue virus. Whether the same receptor or entry mechanism is uti-lized in all serotypes of dengue viruses or in all tissues infected by the viruses needs further investigation. In-deed, various cell lines vary in their susceptibility to DEN-4 infection and may be determined largely by the presence of a cell receptor capable of binding dengue viral E protein (Anderson et al., 1992). Our results will be valuable for understanding the interactions of dengue viruses with the cell surface. Future research on defining how viral proteins interact with the cell surface will help clarify the pathogenesis of dengue viruses and facilitate the design of antiviral agents that can interfere early in viral infection.
MATERIALS AND METHODS Cell cultures and virus preparation
Aedes albopictus C6/36 cells were grown at 28°C to
confluency in half Dulbecco’s modified minimum essen-tial medium (DMEM) and half Mitsuhashi and Moramo-rosch insect medium (M & M), supplemented with 10% fetal calf serum (FCS, Biological Industries) and antibiot-ics. BHK cells were propagated in RPMI 1640 medium (Gibco) supplemented with 5% FCS and antibiotics. DEN-2 virus (PL-046 strain), a Taiwanese strain obtained from one dengue fever patient in 1987, was kindly pro-vided by the National Institute of Preventive Medicine, Taipei, Taiwan, R.O.C. Viruses were propagated in C6/36 cells and titered on BHK cells at 37°C.
Viral penetration assay
The rate of viral penetration was determined by inac-tivation of extracellular viruses with a low-pH glycine buffer as used by Long et al. (1990) (Fig. 1A). Briefly, BHK cells in 35-mm-diameter tissue culture dishes were
in-oculated with 100 to 250 PFU of virus and incubated at 4°C with gentle rocking for 2 h. Unbound virus was removed by two washes with ice-cold phosphate-buff-ered saline (PBS; pH 7.5). The dishes were overlaid with medium and shifted to 37°C. At various times after the temperature shift, the infected cells were treated with acid glycine (pH 3.0) solution (8 g of NaCl, 0.38 g of KCl, 0.1 g of MgCl2z 6H2O, 0.1 g of CaCl2z 2H2O, and 7.5 g of glycine/L, pH adjusted to 3 with HCl) (Cai et al., 1988) for 1 min at room temperature (RT) to inactivate extracellular viruses and then washed twice with PBS and overlaid with 1% agarose–1.25% FCS–RPMI medium before fur-ther incubation at 37°C for 7 days. Infected monolayers were then fixed with formalin and stained with crystal violet. Plaques, representing intracellular acid-resistant viruses, were counted, and the percentage of PFU sur-viving acid treatment was calculated using the following formula: 1003 number of PFU (acid treated)/number of PFU (PBS control). The number of plaques formed on cells without acid glycine treatment (PBS control) was considered as 100%. Experiments were performed in duplicate for at least two independent experiments and the plaque numbers were averaged.
Monoclonal antibodies to E and NS1 proteins
The procedure for immunization and production of hybridoma cell lines secreting dengue E or NS1 protein-specific monoclonal antibodies (MAbs) has been de-scribed in detail elsewhere (Chen et al., 1996a; Lin et al., 1998). The hybridoma cell lines secreting specific anti-bodies were identified by ELISA and immunoprecipita-tion assay with DEN-2-infected C6/36 cell lysates as previously described (Chen et al., 1996a; Lin et al., 1998). The MAbs against DEN-2 E protein were 17-2 (Lin et al., 1998), 46-9, 51-3, 55-3, and 56-3.1 (Lin et al., 1998). The MAb 8-1 was specific for NS1 protein (Chen et al., 1996a).
Preadsorption and postadsorption virus neutralization experiments
BHK cells were used as target cells instead of cells bearing Fc receptors such as monocytic cells, since antibody-dependent enhancement will make the results with antibodies more difficult to interpret. Pilot studies were conducted to determine whether we could shorten the incubation time at 4°C from 2 h to 1 h. Similar results for virus penetration were obtained when the virus was incubated with the cells for 1 h at 4°C. Therefore, for the following preadsorption and postadsorption experi-ments, the 4°C incubation time was changed to 1 h. For preadsorption virus neutralization, virus (100 to 250 PFU) was preincubated with various dilutions of individual anti-E MAbs for 1 h at 4°C and then added to BHK cell monolayers in six-well trays and incubated at 4°C for 1 h
[Fig. 1B(a)]. For postadsorption virus neutralization ex-periments, virus samples were added directly to the monolayers for 1 h at 4°C [Fig. 1B(b)]. Unadsorbed virus was removed by washing the cells, and bound viruses were incubated with various dilutions of individual MAbs for an additional 1 h at 4°C [Fig. 1B(b)]. The control set of virus samples was plated for 1 h at 4°C, washed, and incubated for an additional 1 h at 4°C [Fig. 1B(c)]. The cells were then washed twice and incubated for an additional 2 h at 37°C under 5% FCS–RPMI to allow penetration to occur. After 2 h at 37°C, virus that had not penetrated was inactivated by the acid glycine (pH 3.0) solution. Cells were then overlaid with 1% agarose–1.25% FCS–RPMI. Plaque formation was determined on day 7. The results are expressed as the percentage of plaques formed in the presence of antibody, that is, the number of PFU obtained with antibody treatment [Figs. 1B(a) and (b)] compared with the value for a control sample of virus incubated without antibody [Fig. 1B(c)].
Treatment of cells with lectins or heparin
Lectins used were Con A (Sigma Chemical Co.), wheat germ agglutinin (WGA) from Triticum vulgaris (Sigma Chemical Co.), and phytohemagglutinin PHA-P from
Phaseolus vulgaris (Sigma Chemical Co.). Heparin, a
heparan sulfate-like molecule, was purchased from Sigma Chemical Co. One milligram of heparin contains approximate 170 units of activity. BHK cell monolayers in six-well tissue culture plates were infected with 0.4 ml (100 to 250 PFU) of virus inoculum under three different experimental conditions. The infectivity experiments ex-amined the effects of various chemicals on the whole entry process. First, virus was preincubated with differ-ent concdiffer-entrations of individual chemicals for 1 h at 37°C [Fig. 1C(a)]. The mixture was then added to BHK cells and incubated for 2 h at 37°C for viral entry (both attach-ment and penetration) to occur. For the second set of treatments, BHK cells were incubated with various amounts of chemicals at 37°C for 1 h and washed twice before virus inoculation [Fig. 1C(b)]. The treated cells were then infected. For the third set, BHK cells were incubated with virus at 37°C for 2 h, the unbound virus was removed, and the cells were then treated with var-ious amounts of chemicals at 37°C for 1 h [Fig. 1C(c)]. In each case, infected cells were then washed and overlaid with 1.25% FCS–RPMI containing 1% agarose. Plaque formation was determined on day 7. The results are shown as a percentage of plaque formation (plus chem-icals) [Figs. 1C(a), (b), and (c)] compared with the indi-vidual control reactions where infection was carried out in the absence of chemicals.
The effects of Con A and heparin on viral penetration were also analyzed as preadsorption and postadsorption experiments as described above for viral neutralization
experiments (Fig. 1B), except that MAb was replaced with Con A or heparin. The results were expressed as the percentage of plaque formation, that is, the number of PFU obtained with treatment compared with the value for a control sample of virus incubated without Con A or heparin.
Competition assays
D-(1)-Mannose and a-methyl-D-mannoside (Sigma
Chemical Co.) were used as competitors to block the inhibitory effects of Con A. In the presence of 20mg/ml of Con A, 100 to 250 PFU were mixed with different amounts of each competitor and incubated for 37°C for 1 h [Fig. 1C(a)]. The viral mixtures were added to BHK cells and incubated for 2 h at 37°C. Infected cells were then washed and overlaid with 1% agarose–1.25% FCS– RPMI. The percentage of plaque formation was calcu-lated as follows: 100 3 [(the number of PFU in the presence of Con A and competitor)/(the number of PFU in the absence of Con A and competitor)]. The number of PFU formed in the absence of both Con A and competitor was considered 100%, whereas the number of PFU formed in the presence of Con A only was 0%.
Immunofluorescence assay
C6/36 and BHK cell monolayers in 24-well tissue cul-ture plates were infected with virus (m.o.i. of 1) under three different experimental conditions as described in Fig. 1C. The immunofluorescence assay, instead of plaque assay, was conducted to detect the infected C6/36 cells, since C6/36 cells did not form obvious plaque. In addition, C6/36 cells and BHK cells were incubated at 28 and 37°C, respectively. At 4 days postin-fection, the infected cells were examined by indirect immunofluorescence. Cells were fixed with 80% acetone in PBS for 10 min and then saturated with 5% dry milk in PBS for 1 h at RT or overnight at 4°C. The fixed cells were incubated with anti-NS1 MAb 8-1 (1:1000) at 37°C for 1 h and then incubated with goat anti-mouse IgG conjugated to fluorescein isothiocyanate isomer (GAM–FITC) (1:500) at 37°C for 1 h. Between each step, the infected cells were washed twice with PBS. Microscopy was per-formed with a Nikon fluorescence inverted microscope. Experiments were performed in duplicate for three inde-pendent experiments.
ACKNOWLEDGMENTS
This investigation was supported by Grants 85-CNT-CR-501-P and 86-CNT-CR-501-P from the National Health Research Institutes, Taiwan, R.O.C. We thank Drs. Yi-Ling Lin, Ching-Len Liao, Jyh-Hsiung Huang, Yinh-Chang Wu, and Shiau-Ting Hu for helpful discussion. We also thank Drs. Roselyn J. Eisenberg and Gary H. Cohen for helpful com-ments on the preparation of the manuscript.
REFERENCES
Anderson, R., King, A. D., and Innis, B. L. (1992). Correlation of E protein binding with cell susceptibility to dengue 4 virus infection. J. Gen.
Virol. 73, 2155–2159.
Barth, O. M. (1992). Replication of dengue viruses in mosquito cell cultures—A model from ultrastructural observations. Mem. Inst.
Os-waldo Cruz 87(4), 565–574.
Byrnes, A. P., and Griffin, D. E. (1998). Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72(9), 7349–7356.
Cai, W., Person, S., DebRoy, C., and Gu, B. (1988). Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1: An analysis of linker insertion mutants. J. Mol. Biol. 201, 575–588.
Campadelli-Fiume, G., Avitabile, E., Fini, S., Stirpe, D., Arsenakis, M., and Roizman, B. (1988). Herpes simplex virus glycoprotein D is sufficient to induce spontaneous pH-independent fusion in a cell line that constitutively expresses the glycoprotein. Virology 166, 598–602. Chen, L. K., Liao, C. L., Lin, C. G., Lai, S. C., Liu, C. I., Ma, S. H., Huang, Y. Y., and Lin, Y. L. (1996a). Persistence of Japanese encephalitis virus is associated with abnormal expression of the nonstructural protein NS1 in host cells. Virology 217(1), 220–229.
Chen, Y., Maguire, T., Hileman, R. E., Fromm, J. R., Esko, J. D., Linhardt, R. J., and Marks, R. M. (1997). Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3, 866–871.
Chen, Y., Maguire, T., and Marks, R. M. (1996b). Demonstration of binding of dengue virus envelope protein to target cells. J. Virol. 70, 8765–8772.
Chen, Y.-C., Wang, S.-Y., and King, C.-C. (1999). Bacterial lipopolysac-charide inhibits dengue virus infection of primary human monocytes/ macrophages by blockage for virus entry via a CD14-dependent mechanism. J. Virol., 73(4), 2650–2657.
Chung, C. S., Hsiao, J. C., Chang, Y. S., and Chang, W. (1998). A27L protein mediates vaccinia virus interaction with cell surface heparan sulfate. J. Virol. 72(2), 1577–1585.
Daughaday, C. C., Brandt, W. E., McCown, J. M., and Russell, P. K. (1981). Evidence for two mechanisms of dengue virus infection of adherent human monocytes: Trypsin-sensitive virus receptors and trypsin-resistant immune complex receptors. Infect. Immun. 32, 469–473. DeGeorge, J. J., Slepecky, N., and Carbonetto, S. (1985). Concanavalin A
stimulates neuron-substratum adhesion and nerve fiber outgrowth in culture. Dev. Biol. 111, 335–351.
Deom, C. M., Andrew, J. C., and Schulze, I. T. (1986). Host cell-mediated selection of a mutant influenza A virus that has lost a complex oligosaccharide from the tip of the hemagglutinin. Proc. Natl. Acad.
Sci. USA 83, 3771–3775.
Gambaryan, A. S., Marinina, V. P., Tuzikov, A. B., Bovin, N. V., Rudneva, I. A., Sinitsyn, B. V., Shilov, A. A., and Matrosovich, M. N. (1998). Effects of host-dependent glycosylation of hemagglutinin on recep-tor-binding properties of H1N1 human influenza A virus grown in MDCK cells and in embryonated eggs. Virology 247, 170–177. Gattegno, L., Ramdani, A., Jouault, T., Saffar, L., and Gluckman, J. C.
(1992). Lectin-carbohydrate interactions and infectivity of human im-munodeficiency virus type 1 (HIV-1). AIDS Res. Hum. Retroviruses 8(1), 27–37.
Gollins, S. W., and Porterfield, J. S. (1985). Flavivirus infection enhance-ment in macrophages: An electron microscopic study of viral cellular entry. J. Gen. Virol. 66, 1969–1982.
Guirakhoo, F., Hunt, A. R., Lewis, J. G., and Roehrig, J. T. (1993). Selec-tion and partial characterizaSelec-tion of dengue 2 virus mutants that induce fusion at elevated pH. Virology 194, 219–223.
Gunther, I., Glatthaar, B., Doller, G., and Garten, W. (1993). A H1 hem-agglutinin of a human influenza A virus with a carbohydrate-modu-lated receptor binding site and an unusual cleavage site. Virus Res. 27, 147–160.
Halstead, S. B. (1988). Pathogenesis of dengue: Challenges to molec-ular biology. Science 239, 476–481.
Hase, T., Summers, P. L., and Eckels, K. H. (1989). Flavivirus entry into cultured mosquito cells and human peripheral blood monocytes.
Arch. Virol. 104, 129–143.
Haywood, A. M. (1994). Virus receptor: Binding, adhesion strengthen-ing, and changes in viral structure. J. Virol. 68, 1–5.
He, R.-T., Innis, B. L., Nisalak, A., Usawattanakul, W., Wang, S., Kalay-anarooj, S., and Anderson, R. (1995). Antibodies that block virus attachment to Vero cells are a major component of the human neutralizing antibody response against dengue virus type 2. J. Med.
Virol. 45, 451–461.
Highlander, S. L., Sutherland, S. L., Gage, P. J., Johnson, D. C., Levine, M., and Glorioso, J. C. (1987). Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus pene-tration. J. Virol. 61, 3356–3364.
Hotta, H., and Homma, M. (1994). Lectin-mediated enhancement of dengue virus infection in a mouse macrophage cell line Mk1. Arch.
Virol. 134, 51–59.
Jackson, T., Ellard, F. M., Ghazaleh, R. A., Brookes, S. M., Blakemore, W. E., Corteyn, A. H., Stuart, D. I., Newman, W. I., and King, A. Q. (1996). Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70, 5282–5287.
Johnson, A. J., Guirakhoo, F., and Roehrig, J. T. (1994). The envelope glycoproteins of dengue 1 and dengue 2 viruses grown in mosquito cells differ in their utilization of potential glycosylation sites. Virology 203, 241–249.
Kawano, H., Rostapshov, V., Rosen, L., and Lai, C. J. (1993). Genetic determinants of dengue type 4 virus neurovirulence for mice. J. Virol. 67, 6567–6575.
Klimstra, W. B., Ryman, K. D., and Johnston, R. E. (1998). Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72(9), 7357–7366.
Krusat, T., and Streckert, H.-J. (1997). Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 142, 1247– 1254.
Laquerre, S., Argnani, R., Anderson, D. B., Zucchini, S., Manservigi, R., and Glorioso, J. C. (1998). Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72(7), 6119–6130.
Lifson, J., Coutre, S., Huang, E., and Engleman, E. (1986). Role of envelope glycoprotein carbohydrate in human immunodeficiency vi-rus (HIV) infectivity and vivi-rus-induced cell fusion. J. Exp. Med. 1986, 2101–2106.
Lin, Y.-L., Liao, C.-L., Chen, L.-K., Yeh, C.-T., Liu, C.-I., M, S.-H., Huang, Y.-Y., Huang, Y.-L., Kao, C.-L., and King, C.-C. (1998). Study of dengue virus infection in SCID mice engrafted with human K562 cells. J. Virol. 72(12), 9729–9737.
Long, D., Cohen, G. H., Muggeridge, M. I., and Eisenberg, R. J. (1990). Cysteine mutants of herpes simplex virus type 1 glycoprotein D exhibit temperature-sensitive properties in structure and function.
J. Virol. 64, 5542–5552.
Mady, B. J., Kurane, I., Erbe, D. V., Fanger, M. W., and Ennis, F. A. (1993). Neuraminidase augments Fcg receptor II-mediated antibody-depen-dent enhancement of dengue virus infection. J. Gen. Virol. 74, 839– 844.
Malewicz, B., and Jenkin, H. M. (1979). Development of dengue virus plaques under serum-free overlay medium. J. Clin. Microbiol. 9(5), 609–614.
Matrosovich, M. N., Gambaryan, A. S., Teneberg, S., Piskarev, V. E., Yamnikova, S. S., Lvov, D. K., Robertson, J. S., and Karlsson, K.-A. (1997). Avian influenza A virus differ from human viruses by recog-nition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site. Virology 233, 224–234. Matsui, S., Okuno, T., and Shiraki, K. (1994). Functional roles of terminal glycomoieties in varicella-zoster virus infection. Virology 198, 50–58. Monath, T. P. (1986). Pathology of the flaviviruses. In “The Togaviridae and Flaviviridae” (S. Schlesinger and M. J. Schlesinger, Eds.), pp. 375–440. Plenum, New York.
Montgomery, R. I., Warner, M. S., Lum, B. J., and Spear, P. G. (1996). Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87, 427–436.
Nakayama, E. E., Shioda, T., Tatsumi, M., Xin, X., Yu, D., Ohgimoto, S., Kato, A., Sakai, Y., Ohnishi, Y., and Nagai, Y. (1998). Importance of the
N-glycan in the V3 loop of HIV-1 envelope protein for CXCR-4- but not
CCR-5-dependent fusion. FEBS Lett. 426, 367–372.
Pal, R., DeVico, A., Rittenhouse, S., and Sarngadharan, M. G. (1993). Conformational perturbation of the envelope glycoprotein gp120 of human immunodeficiency virus type 1 by soluble CD4 and the lectin succinyl Con A. Virology 194, 833–837.
Patel, M., Yanagishita, M., Roderiquez, G., Bou-Habib, D. C., Oravecz, T., Hascall, B. C., and Norcross, M. A. (1993). Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS
Res. Hum. Retroviruses 9, 167–174.
Pletnev, A. G., Bray, M., and Lai, C. J. (1993). Chimeric tick-borne encephalitis and dengue type 4 viruses: Effects of mutations on neurovirulence in mice. J. Virol. 67, 4956–4963.
Randolph, V. B., and Stollar, V. (1990). Low pH-induced cell fusion in flavivirus-infected Aedes albopictus cell cultures. J. Gen. Virol. 71, 1845–1850.
Robinson, W. E. J., Montefiori, D. C., and Mitchell, W. M. (1987). Evidence that mannosyl residues are involved in human immunodeficiency virus type 1 (HIV-1) pathogenesis. AIDS Res. Hum. Retroviruses 3, 265–282.
Rostand, K. S., and Esko, J. D. (1997). Microbial adherence and invasion through proteoglycans. Infect. Immun. 65, 1–8.
Sanchez, I. J., and Ruiz, B. H. (1996). A single nucleotide change in the E protein gene of dengue virus 2 Mexican strain affects neuroviru-lence in mice. J. Gen. Virol. 77, 2541–2545.
Shieh, M. T., WuDunn, D., Montgomery, R. I., Esko, J. D., and Spear, P. G. (1992). Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116, 1273–1281.
Smith, G. W., and Wright, P. J. (1985). Synthesis of proteins and glyco-proteins in dengue type 2 virus-infected Vero and Aedes albopictus cells. J. Gen. Virol. 66, 559–571.
Spear, P. G., Shieh, M. T., Herold, B. C., WuDunn, D., and Koshy, T. I. (1992). Heparan sulfate glycosaminoglycans as primary cell surface receptors for herpes simplex virus. Adv. Exp. Med. Biol. 313, 341–353. Summerford, C., and Samulski, R. J. (1998). Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72(2), 1438–1445.
WuDunn, D., and Spear, P. G. (1989). Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63, 52–58.