PHYSIOLOGIA PLANTARUM 106: 253 – 261. 1999 Copyright © Physiologia Plantarum1999
ISSN0031-9317
Printed in Ireland — all rights reser6ed
Molecular characterization and expression of starch granule-bound
starch synthase in the sink and source tissues of sweet potato
Shu-Jen Wang, Kai-Wun Yeh and Chia-Yin Tsai*
Department of Botany, National Taiwan Uni6ersity, Taipei, Taiwan *Corresponding author, e-mail:tsaicy@ccms.ntu.edu.tw
Received 14 April 1999
This GBSSI gene was well expressed in tuberous roots, leaves, In an effort to study regulation of starch synthesis in the
source and sink tissues, a cDNA clone for starch granule- and stems, but not in roots. However, mechanisms involved in regulating the expression of this gene were different between bound starch synthase (GBSSI), which encodes a 67-kDa
protein, was isolated from a cDNA library prepared from tuberous roots and leaves. In tuberous roots, the synthesis of tuberous roots of sweet potato (Ipomoea batatas Lam. cv. GBSSI transcript increased coordinately with tuberous root
Tainong 62). This GBSSI protein contains a signal peptide of expansion; nevertheless, accumulation rates of GBSSI protein in starch granules remained constant regardless of tuberous 77 amino acids, and the mature protein has a molecular mass
of about 59 kDa. The mature protein shares 75 – 85% se- root sizes, suggesting an involvement of post-transcriptional regulation for the synthesis of this protein. The levels of quence identity with GBSSIs of dicotyledonous plants and
about 70% identity with those of monocotyledons. The se- GBSSI transcript were investigated in photosynthetic tissues
during diurnal cycles, and the results suggest that the tran-quence of the signal peptide, on the other hand, differs
sub-stantially from those of the dicotyledons and monocotyledons scription of the GBSSI gene in leaves is controlled by the endogenous circadian rhythm.
studied, although their hydropathic profiles are all similar.
Several waxy mutants have been identified that contain amylose-free starch, and these mutants are deficient in the GBSS activity (Nelson and Tsai 1964, Hovenkamp-Her-melink et al. 1987); furthermore, the activity of GBSS is proportional to the number of Wx allele (Tsai 1974), sug-gesting the importance of GBSS in amylose synthesis. Stud-ies using an antisense technique also confirm the importance of GBSS in amylose formation (Visser et al. 1991a, Salehuz-zaman et al. 1993). GBSS can be classified into different types based on the molecular mass and localization: (1) GBSSI with a molecular mass of 58 – 60 kDa is tightly bound to starch granules and offers the largest proportion of total GBSS activity (Shure et al. 1983, Vos-Scheperkeuter et al. 1986, Dry et al. 1992). In some plant species, multiple isoforms of GBSSI have been identified and their expres-sions are tissue-specific (Denyer et al. 1997, Nakamura et al. 1998, Tomlinson et al. 1998). Studies on a waxy mutant of wheat and low-amylose (lam) mutant of pea indicate that
Introduction
Starch synthase (EC 2.4.1.21) is an important enzyme re-sponsible for the biosynthesis of starch in plant tissues (DeFekete et al. 1960, Nelson and Tsai 1964). This enzyme includes two major forms, namely, starch granule-bound starch synthase (GBSS) and soluble starch synthase (SSS). GBSS is also known as the WAXY protein, which is tightly associated with starch granules (Tsai 1974), while SSS can be found in the soluble fraction of amyloplasts or the stroma of chloroplasts (Preiss 1988). Biochemical properties of GBSS and SSS are different. GBSS transfers glucose units from adenosine 5%-diphosphate (ADP)-glucose or uridine 5%-diphosphate (UDP)-glucose to non-reducing ends of a-1,4 glucose polymers, although the rate of transfer from ADP-glucose is higher than from UDP-glucose; in contrast, SSS uses ADP-glucose as the sole substrate (Tsai 1974). A previous study showed that GBSS preferred amy-lose over amylopectin as a primer while SSS would only add the glucose unit into amylopectin (Tsai 1973).
GBSSI plays little function in non-storage organs; instead, another GBSSI-like protein replaces the GBSSI to synthe-size amylose in those tissues (Denyer et al. 1997, Naka-mura et al. 1998, Tomlinson et al. 1998). However, a single GBSSI isoform is responsible for amylose synthesis in tubers, leaves, roots, and pollens of potato (Jacobsen et al. 1989). Although GBSSI may exist in different isoforms, these observations clearly indicate the involvement of GB-SSI in amylose formation. The waxy mutants of potato, barley, and maize characterized so far are all associated with a loss of GBSSI activity (Hovenkamp-Hermelink et al. 1987, Hylton et al. 1996). (2) GBSSII (also referred to as SSII) with a molecular mass of 77 – 79 kDa is present in starch granules as well as in the soluble fraction of plastids (Dry et al. 1992, Denyer et al. 1993, 1995, Ed-wards et al. 1995, 1996, Hylton et al. 1996, Craig et al. 1998). In contrast to GBSSI, the role of GBSSII remains ambiguous. A reduction of this activity in potato tubers did not affect the ratio of amylose to amylopectin (Ed-wards et al. 1995); however, a study on rugosus5 (rug5) mutant of pea suggests that GBSSII might play an impor-tant role for determining amylopectin structure and starch granule morphology (Craig et al. 1998).
In addition, another type of starch synthase with a molecular mass of 140 kDa, SSIII, has been purified from potato tubers (Marshall et al. 1996). SSIII contributes about 80% of the activity found in the soluble fraction, and it may also bind to starch granules. The antisense-SSIII transgenic potato did not show a change in the starch content nor in the amylose-to-amylopectin ratio in tubers; however, the morphology of starch granules and the amylopectin branching patterns were significantly dif-ferent from those of the wild type (Abel et al. 1996, Mar-shall et al. 1996, Edwards et al. 1999).
Although the role of GBSSI in starch synthesis has been established, expression and regulation of this gene in the sink and source tissues remain unclear. Assimilate availability from the source and the ability of the sink to accumulate starch have been recognized as two major fac-tors for yield determination of agronomic crops. Leaf is an important source tissue in which starch formed during the day is mobilized at night to provide the carbon source for starch synthesis in the sink. Storage sink is a non-pho-tosynthetic tissue where starch deposition is a major bio-chemical process during development and maturation. Thus, a positive interaction between the two tissues should favor starch accumulation in the sink. A reduction of starch synthesis in sink tissues not only reduces yield but also produces important pleiotropic effects during develop-ment (Tsai 1983, Martin and Smith 1995). For example, the inhibition of starch synthesis in the sink may result in a high osmotic potential and reduce the content of storage protein (Tsai et al. 1978, Lee and Tsai 1985, Mu¨ller-Ro¨ber et al. 1992). Since source-sink interactions play such a vital role in determining yield (Tsai and Tsai 1990) and GBSS is important for starch synthesis, efforts were made to investigate regulatory mechanisms involved in controlling the expression of the GBSS gene in the sink and source tissues of sweet potato.
Materials and methods Plant material
Sweet potato (Ipomoea batatas Lam. cv. Tainong 62) was grown at Tauyan District Agricultural Improvement sta-tion in northern Taiwan. Sweet potato was planted in July 1996, and tuberous roots of different sizes were harvested in September. After harvest, these tuberous roots were brought into laboratory for processing. For photoperiod experiments, plants were grown and maintained at 28°C in a growth chamber under 16 h light/8 h dark. To initiate the study, some plants were moved to separate chambers after the 8 h dark period and then treated continuously with 24 h light or 24 h dark, respectively. Leaf samples were then harvested at the time intervals indicated.
Isolation of DNA and RNA from sweet potato
Genomic DNA was isolated according to the procedure of Dellaporta et al. (1983) with some modifications. A sam-ple of tuberous roots (20 g) was ground in liquid nitro-gen, transferred into a flask containing 50 ml of extraction buffer (100 mM Tris-HCl, pH 8.0, 50 mM ethylenedi-aminetetraacetate [EDTA], 500 mM NaCl, 100 mg ml− 1 proteinase K), and then incubated at room temperature with gentle shaking for 20 min before centrifugation at 12000 g for 10 min. The supernatant received 3 ml of 20% (w/v) sodium dodecyl sulphate (SDS), was incubated at 55°C for 1 h, and then centrifuged at 12000 g for 20 min. After centrifugation, the supernatant was mixed with 0.1 volume of 3 M potassium acetate (pH 5.2) and 0.6 volume of isopropanol; the mixture was kept at − 20°C for 30 min before centrifugation at 12000 g for 10 min at 4°C. The pellet containing DNA was dissolved in 2 ml of TE buffer (10 mM Tris-HCl, pH 8.0, 1 mM EDTA) be-fore re-extracting in succession with equal volume of phe-nol:chloroform:isoamylalcohol (25:24:1, v/v/v) and chloro-form:isoamylalcohol (24:1, v/v). After centrifugation, DNA in the supernatant was precipitated with 5 ml of 100% ethanol and 0.2 ml of 3 M sodium acetate, pH 5.2. Finally, the DNA pellet was dissolved in 0.5 ml of TE buffer or H2O.
RNA was isolated from tuberous roots, leaves, stems, or roots according to the procedure described by Yeh et al. (1991). Poly(A)+RNAs were isolated by oligo(dT) cel-lulose column chromatography (Shennan 1996). For deter-mining expressions of the GBSSI gene, total RNAs (10 mg for each sample) were separated on 1% formaldehyde-agarose gels (Sambrook et al. 1989), and probed with GB-SSI cDNA of sweet potato (spss67 clone). rRNA was used as an internal standard. All experiments were re-peated at least twice.
Preparation of a DNA probe
GBSSI sequences of potato (van der Leij et al. 1991) and cassava (Salehuzzaman et al. 1993) were selected from the GenBank and then aligned by Wisconsin Genetics Com-puter Group (GCG) software to identify conserved
re-gions. Two primers were designed for amplifying a part of sweet potato GBSSI gene that contained the first consen-sus region (BOX 1) of starch synthase genes using reverse transcription-polymerase chain reaction (RT-PCR). The sequence of sense and antisense primers were [5%-TGGAG-CAAAACTGGAGGTCT-3%] and [5%-GTGCCAATCAT-TGGCAATG-3%], respectively (see also Fig. 1). Tuberous root poly(A)+RNAs (0.5 mg) of sweet potato were used as templates, and the first strand cDNAs was synthesized by RAV2 reverse transcriptase (Amersham, Bucking-hamshire, UK). The PCR reaction was completed by Taq DNA polymerase (Promega, Madison, WI, USA). The RT-PCR was conducted according to the procedure de-scribed by Goodbourn (1996). Final amplification gener-ated a 0.4-kb DNA fragment.
Construction and screening of a cDNA library
cDNAs were synthesized from poly(A)+RNAs prepared from tuberous roots of sweet potato by using a GIBCO BRL cDNA synthesis kit. The cDNAs obtained were methylated by EcoRI methylase and ligated to an EcoRI linker. After digestion with EcoRI, the cDNAs were lig-ated into the EcoRI site of lgt10 (Promega). Approxi-mately, 1 × 105 p.f.u. were screened according to the procedure described by Sambrook et al. (1989) using the 0.4-kb RT-PCR fragment as a probe.
Subcloning and sequencing
The RT-PCR product (0.4-kb DNA fragment) was ligated into the SmaI site of pGEM3Z (Promega). The selected cDNA clones were subcloned into the EcoRI site of pGEM7Z (Promega) before unidirectional deletion with exonuclease III (ExoIII) and S1 nuclease to obtain differ-ent sizes of nested deletion clones (Henikoff 1984). DNA sequences were determined according to the method of Sanger et al. (1977) and analysis was performed using the Wisconsin GCG Software Package version 9.0 (Devereux et al. 1984).
Southern and northern blot analyses
For the Southern blot analysis, sweet potato genomic DNA (10mg) was digested with EcoRI, HindIII, or XbaI, and the digested DNA was separated on a 0.65% agarose gel and blotted onto a nitrocellulose membrane (Amer-sham). For the northern blot analysis, total RNAs (10 mg) were separated on 1.0% formaldehyde-agarose gels (Sam-brook et al. 1989). The DNA probe was radioactively labeled with a-32P-dCTP using a random primer labeling kit (Amersham). After hybridization, the membranes were washed twice with 2× SSC (1 l of 20× SSC stock solu-tion contained 175.3 g of NaCl and 88.2 g of sodium citrate, pH 7.0) containing 0.1% (w/v) SDS at room
tem-perature for 30 min and twice with 0.1× SSC containing 0.1% (w/v) SDS at 55°C for 30 min (Sambrook et al. 1989).
Expression of GBSSI in Escherichia coli
The coding region of GBSSI cDNA was amplified by PCR using the following primer pairs: sense primer [5%-TCGGATCCATGGCGACTATAACTGC-3%], and anti-sense primer [5%-TCGAATTCGGTGGAGTAGCGAC-GTT-3%]. The amplified fragment was constructed into BamHI-EcoRI cloning sites of pET-21a vector (Novagen, Madison, WI, USA). This constructed plasmid was trans-ferred into E. coli strain BL21 (DE3) and incubated in Luria broth (LB) medium containing ampicillin (50 mg ml− 1) as a selective antibiotic. Expression of the target gene was induced by 1 mM isopropyl b-D
-thiogalactopy-ranoside for 3 h.
Isolation of starch granules and GBSSI enzyme
Tissue samples (2 g) were powdered in liquid nitrogen with a mortar and pestle, and subsequently ground with 10 ml of buffer (50 mM Tris-HCl, pH 7.5, 10 mM EDTA, 2 mM dithiothreitol [DTT]) before filtering through two layers of Miracloth (Calbiochem, La Jolla, CA, USA) and centrifug-ing at 12000 g for 10 min at 4°C. The pellet includcentrifug-ing starch granules was washed 3 times with ice-cold extraction buffer followed by 4 times with cold acetone (Tsai 1974). For SDS-polyacrylamide gel analyses, the starch granule-bound proteins were extracted from 15 mg of starch granules by SDS-sample buffer (Salehuzzaman et al. 1993) before elec-trophoresis on 7.5% SDS-polyacrylamide gels (Laemmli 1970).
Western blot analysis and N-terminal sequencing of the GBSSI protein
Proteins were separated on a 7.5% SDS-polyacrylamide gel and transferred to nitrocellulose membrane (Amersham) for western blot analysis using an antibody raised against the potato GBSSI (kindly provided by Dr R. Visser). For N-terminal sequencing of the GBSSI protein, GBSSI was purified from starch granules by separation on 7.5% SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). GB-SSI was excised from the membrane and an N-terminal amino acid sequence obtained using a Perkin Elmer (Nor-walk, CT, USA) Applied Biosystem 477A protein sequenator.
In vivo labeling of tuberous roots
Small discs (each disc weighed about 2 g with a dimension of 1 × 0.5 × 0.3 cm) were cut from sweet potato tuberous roots, incubated in 5 ml shaking buffer (0.1% [w/v] sucrose, 5 mM phosphate buffer, pH 6.0, 50 mg ml− 1 chlorampheni-col), and gently shaken at 25°C for 30 min. Subsequently, the sample discs were shaken for 4 h after 1.85 MBq of 35S-methionine (37 PBq mol− 1; Amersham) was added.
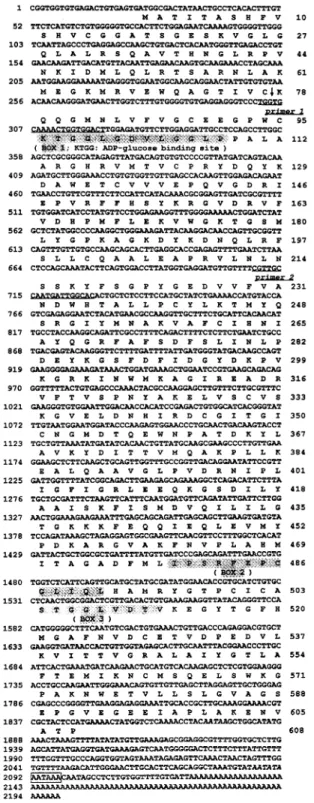
Fig. 1. Nucleotide and deduced amino acid sequence of the cDNA (spss67) encoding sweet potato GBSSI. The conserved regions of starch synthases are shaded and labeled BOX 1, 2, and 3; ¡ indicates the signal peptide cutting site. The typical polyadenylation signal is boxed. Primer pairs for generating 0.4-kb DNA probes (a part of GBSSI gene) are underlined and shown as primer 1 and 2. The nucleotide sequence has been assigned accession number U44126 in the GenBank database.
bound proteins (Salehuzzaman et al. 1993). The extracted proteins were analyzed by one-dimensional SDS-polyacryl-amide gel electrophoresis according to Laemmli (1970). After electrophoresis, the gel was fixed in a solution containing 10% (w/v) trichloroacetic acid, 10% (v/v) glacial acetic acid and 30% (v/v) methanol. Radioactive protein bands were detected by fluorography (Larskey and Mills 1975). The dried gel was exposed to Kodak BioMax film (Eastman-Kodak, Rochester, NY, USA) at − 70°C for 5 days.
Results
Isolation and characterization of starch synthase cDNA clones
A 0.4-kb fragment was obtained by RT-PCR using two primers designed for a consensus region of dicotyledonous GBSS genes, a region which includes the ADP-glucose binding site. This 0.4-kb sweet potato fragment showed a high sequence identity to the corresponding parts of the potato (van der Leij et al. 1991) and cassava (Salehuzzaman et al. 1993) genes (77.6 and 77.7%, respectively). This result indi-cates that the fragment was a partial sequence encompassing the first consensus region of the starch synthase gene in sweet potato. Therefore, it was used as a probe for isolating starch synthase genes in this study. When the tuberous root cDNA library was screened with this probe, six positive clones were isolated from 1 × 105p.f.u. Only one of them contained 2199 bp (spss67) with a full length open reading frame (ORF) encoding 608 amino acids (Fig. 1), while others all contained a partial sequence of this clone. This clone has been assigned accession number U44126 in the GenBank database.
Nucleotide sequencing showed that the spss67 starch syn-thase cDNA clone contained 22 bp in the 5% nontranslated region, 350 bp in the 3% noncoding region, and had an ORF of 1827 bp which encodes a 67-kDa protein with a predicted pI value of 7.46. A typical polyadenylation signal AATAAA is located at 32 nucleotides upstream of the poly(A) tail. Comparison of the deduced amino acid sequence of spss67 with other starch synthases shows a high sequence identity with GBSSI of various plant species (65 – 80%), but a low identity with GBSSII (SSII) and SSIII of potato and with SSS of rice (Table 1). The spss67 cDNA exhibits three consensus regions, BOX 1, 2 and 3, characteristics of starch synthases (Fig. 1). BOX 1, i.e., Lys-Thr-Gly-Gly (KTGG), is known to be in the ADP-glucose binding site. Southern analysis indi-cated that only one copy of GBSSI is present in the sweet potato genome (data not shown).
Characteristics of the GBSSI protein
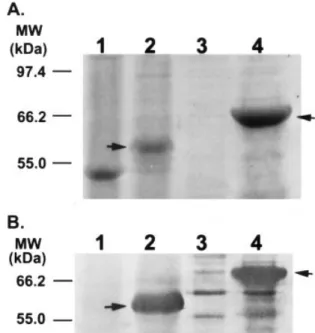
In order to characterize the product of the spss67 clone, spss67 cDNA was constructed into a pET-21a expression vector and transferred into E. coli BL21 (DE3) strain. The transformants over-expressed a 67-kDa protein, which agreed with the molecular mass predicted from the spss67 cDNA, and this protein could be recognized by an anti-serum raised against the GBSSI protein isolated from starch granules of potato (Fig. 2B, lane 4). Although the spss67 After incubation, the discs were washed 5 times with washing
buffer (0.1% sucrose, 5 mM phosphate buffer, pH 6.0, 1 mM methionine) before extraction of starch granules and
granule-Table 1. Sequence comparisons of sweet potato GBSSI cDNA (spss67) and protein with those of other plant species. Data obtained from Wisconsin GCG Software Package version 9.0.
Species (EMBL/GenBank accession num- Nucleotide se- Full length Signal peptide Mature protein Reference bers of the nucleotide sequences) quence identity protein identity identity (%) identity (%)
(%) (%)
GBSSI Dicotyledons
Potato (X58453) 77.0 79.3 46.7 84.0 van der Leij et
al. (1991)
Cassava (X74160) 73.8 73.5 33.8 79.1 Salehuzzaman et
al. (1993)
Pea (X88789) 72.9 70.4 39.4 74.5 Dry et al. (1992)
Monocotyledons
Barley (X07932) 66.4 65.9 30.3 70.7 Rohde et al.
(1988)
Wheat (X57233) 66.1 64.8 30.3 69.4 Clark et al.
(1991)
Rice (X62134) 67.6 65.0 27.5 70.2 Okagaki (1992)
GBSSII (SSII)
Potato (X87988) 56.0 38.7 – – Edwards et al.
(1995) SSIII
Potato (X95759) 66.7 43.2 – – Marshall et al.
(1996) SSS
Rice (D16202) 58.7 39.1 – – Baba et al.
(1993)
cDNA encodes a 67-kDa protein, the mature GBSSI of sweet potato has a molecular mass of only 59 kDa (Fig. 2). Comparison of the N-terminal amino acid sequence of GBSSI deduced from the spss67 cDNA sequence with that of the mature GBSSI isolated from tuberous roots demon-strates that the GBSSI preprotein should contain a transit peptide of 77 amino acids with a molecular mass of about 8.2 kDa. The transit peptide sequence of sweet potato GBSSI shows an identity of 27 – 47% with those of other plant species (Table 1); however, like other GBSSI transit peptides, it has hydrophobic regions in the initiation and end of the peptide (data not shown).
Expression of GBSSI in the sink and source tissues
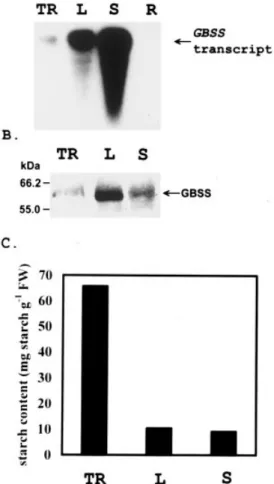
Like tuberous roots and leaves, stems also accumulated starch (Fig. 3C). The level of starch accumulation per unit fresh weight was higher in tuberous roots than in leaves and stems (Fig. 3C). As expected, GBSSI transcripts (Fig. 3A) and proteins (Fig. 3B) were accumulated in tuberous roots, leaves, and stems. In roots, expression of the GBSSI gene was not detectable (Fig. 3A). In tuberous roots, the GBSSI transcript increased along with the size of tubers (Fig. 4A); however, immunoblot analysis showed that protein levels of GBSSI remained relatively constant when isolated from 15 mg of starch granules prepared from tuberous roots of different sizes (Fig. 4B). As shown in Fig. 4C, there is no correlation between the accumulation of GBSSI transcript and protein levels during the development of tuberous roots when expressed on a unit fresh weight basis. In order to determine factors causing the discrepancy between transcript and protein accumulation patterns, the protein accumula-tion rate during development was analyzed by incorporaaccumula-tion
of35S-methionine into GBSSI protein in vivo. The result of in vivo labeling indicated that accumulation rates of GBSSI were similar in tuberous roots of different sizes (Fig. 5). Therefore, these observations suggest that some
post-tran-Fig. 2. Identification of mature GBSSI protein in sweet potato tuberous roots and recombinant GBSSI protein from E. coli. Lane 1, soluble protein fraction from tuberous roots; lane 2, starch granule-bound proteins of tuberous roots; lane 3, inclusion body fraction from the control E. coli strain; lane 4, inclusion body fraction from the GBSSI cDNA (spss67) transformed E. coli strain. Proteins were stained with Coomassie Brilliant Blue on a 7.5% SDS-PAGE (A) and immunoblotted with antiserum against the GBSSI of potato (B). Arrows indicate the GBSSI.
Fig. 3. Expression of GBSSI and starch content in tissues of sweet potato. (A) The levels of GBSSI transcript were determined by northern blot analysis. Total RNAs (10mg) were separated on a 1% formaldehyde-agarose gel, transferred onto nitrocellulose mem-branes and hybridized with32P-labeled GBSSI cDNA (spss67clone)
probe. (B) GBSSI protein of different tissues was detected by antiserum against the GBSSI of potato. (C) Starch content. TR, tuberous root; L, leaf; S, stem; R, root.
Discussion
A cDNA clone (spss67) containing a full length ORF, which encodes a GBSSI protein, was isolated from a tuberous root cDNA library of sweet potato (Fig. 1). This cDNA shared a high sequence identity with that of those coding for starch synthases in other plants (Table 1), and it contained a typical polyadenylation signal located 32 nucleotides up-stream of the poly(A) tail. Sequence analysis indicated that GBSSI of sweet potato contains a transit peptide of 77 amino acids at the N-terminus. The peptide was similar in size to the GBSSI transit peptide of potato (van der Leij et al. 1991), but was larger than that of maize (Klo¨sgen et al. 1986). However, the amino acid sequences of these transit peptides were all rather different (Table 1) although their hydropathic profiles were very similar (data not shown). These observations are consistent with the notion that the secondary structure and hydropathic distributions of transit peptides are more important than the amino acid sequence per se for function (Keegstra et al. 1989).
Unlike the transit peptide, the sequence of the GBSSI mature protein appears to be well conserved. The mature GBSSI of sweet potato shows 70 – 85% amino acid identity with GBSSIs of other plant species. The ADP-glucose bind-ing site (KTGG) of GBSSI, located within BOX 1, agrees
Fig. 4. Expression of the GBSSI gene in tuberous roots. (A) Total RNAs (10mg) of tuberous roots of different sizes were separated on a 1% formaldehyde-agarose gel and probed with32P-labeled GBSSI
cDNA (spss67clone); rRNA was used as an internal control. (B) GBSSI protein was extracted from 15 mg of starch granules and detected by immunoblotting. (C) Relative levels of GBSSI transcript and protein in tuberous roots of different sizes. The content of GBSSI transcript and protein in (C) are expressed on the basis of per unit fresh weight, and relative levels are based on the measure-ment determined for the smallest tuberous root (100%).
scriptional mechanisms were involved in regulating the ex-pression of GBSSI in tuberous roots.
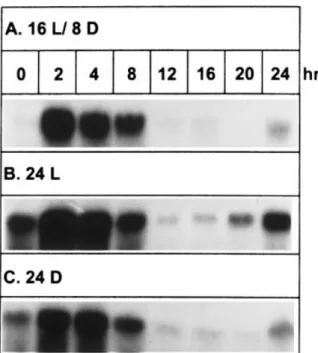
To study the effect of photoperiod on GBSSI gene expres-sion in leaves, the expresexpres-sion pattern of the GBSSI tran-script was determined from plants grown under 16 h light/8 h dark as a control. During the 24-h period, the GBSSI mRNA accumulated abundantly after 2-h treatment of light and then decreased slightly at 8 h, and almost completely disappeared at 12 h (Fig. 6A). However, the transcript reappeared at the end of the 24-h period, i.e., after 8 h dark. In order to determine whether the diurnal expression pattern was controlled by environmental stimuli or circadian rhythm, the 16 h light/8 h dark photoperiod adapted plants were shifted to 24 h continuous light or 24 h continuous dark before RNA isolation. A similar GBSSI expression pattern was observed for these treatments (Fig. 6B,C). Lev-els of rRNA internal standard were similar in all treatments (data not shown).
Fig. 5. Autoradiograph demonstrating the in vivo labeling of GB-SSI protein in tuberous roots of different sizes. Twenty microliters of35S-labeled starch granule-bound proteins (extracted from 2 g of
treated tuberous root discs) were separated on a 10% SDS-gel. Indicates the GBSSI; the signals of labeled GBSSI were confirmed by comparing with the location of mature GBSSI protein identified by immunoblotting and staining with Coomassie Brilliant Blue.
glucose and UDP-glucose as substrates, the substrate bind-ing sequence is identical to that of SSS, which uses ADP-glucose as the sole substrate (Tsai 1973). This result suggests that sequences other than the KTGG might also be involved in determining substrate specificity.
Non-photosynthetic storage organs (sink) and photosyn-thetic leaves (source) are major sites for starch synthesis, but the property and structure of starch produced are different between these two sites (Tomlinson et al. 1997). Expression of some genes involved in the starch synthetic pathway appear to be regulated by different mechanisms in sink and source tissues (Nakata and Okita 1995). Identification and characterization of the GBSSI gene expression enabled us to study regulatory mechanisms of this gene in the two tissues. In addition to tuberous roots and leaves, the GBSSI gene was highly expressed in stems (Fig. 3A). A similar observa-tion was made in potato. Visser et al. (1991b) analyzed the expression of a chimeric GBSSI promoter-GUS gene in transgenic potato, and their results indicated that GUS activity in stems was higher than in leaves. Thus, the obser-vations obtained with sweet potato and potato appear to be different from those obtained with cassava, rice, and maize where the expression of GBSSI is tissue-specific with tran-scripts accumulated primarily in the storage organs, such as tubers and endosperms (Klo¨sgen et al. 1986, Salehuzzaman et al. 1993). It is not clear why stems contained a high level of GBSSI mRNA. We suggest that leaves and stems might share a common mechanism to control GBSSI expression. Changes in the accumulation of GBSSI transcript in stems were similar to those of leaves during diurnal cycles (unpub-lished data), and starch contents were similar in leaves and stems when they were harvested at the same time (Fig. 3C). It should also be noted that relative amounts of GBSSI mRNA in tuberous roots, stems, and leaves could not be determined in an absolute term because the amounts changed independently in each tissue and varied substan-tially depending on stages of sampling.
Tuberous root is a major sink tissue for the storage of starch. As expected, the accumulation of GBSSI transcript increased concomitantly with the size of tuberous roots (Fig. 4A,C). However, the accumulation of GBSSI protein re-mained relatively steady regardless of tuberous root size (Fig. 4B,C). A similar observation was made for the small subunit of ADP-glucose pyrophosphorylase in potato leaves where the protein profile also failed to agree with the accumulation of transcript during photoperiod and sucrose induction (Nakata and Okita 1995). The lack of correlation between GBSSI transcript and protein accumulation might be the result of a post-transcriptional regulation. Since in vivo labeling data (Fig. 5) indicated that accumulation of newly synthesized GBSSI protein in starch granules did not increase with the size of the tuberous roots or the level of transcript, this difference in accumulation patterns might be due to (1) not all GBSSI transcripts were translatable, or (2) some GBSSI proteins synthesized were not partitioned into starch granules. Since this protein could not be detected in the soluble fraction (Fig. 2, lane 1), the second possibility could be ruled out. The first possibility was supported by a preliminary study of in vitro translation using total RNA extracted from tuberous roots of different sizes. The result with starch synthases of other plants, and is similar to the
KXGG found in E. coli and mammalian glycogen synthases (Furukawa et al. 1990). Although GBSS utilizes
ADP-Fig. 6. Expression of the GBSSI gene in leaves under different photoperiods. Plants were cultured under 16 h light/8 h dark as the control (A). Plants were moved to continuous light (B) or dark (C) after being kept under 16 h light/8 h dark condition for 7 days. RNAs were extracted from leaves harvested at the time intervals indicated. Total RNAs (10mg) were separated on a 1% formalde-hyde-agarose gel and probed with32P-labeled GBSSI cDNA (spss67
did not show an increase in the amount of a 67-kDa protein as the accumulation of GBSSI transcript increased (data not shown).
In leaves, starch synthesis and degradation have been shown to be influenced by rates of photosynthesis and photoperiod (Fondy et al. 1989, Li et al. 1992). However, other studies have indicated that starch metabolism is not only controlled by photosynthesis but also by a diurnal circadian component (Britz et al. 1987, Li et al. 1992). In 14 h light/10 h dark photoperiods, temporary starch of sugar beet leaves was accumulated during daytime and began to decrease at the end of the light period. However, the pattern of starch accumulation under continuous irradiance was similar to that of the 14 h light/10 h dark treatment. This circadian pattern could be controlled by enzymes either involved in starch synthesis or degradation. Further studies on carbon alloca-tion in sugar beet leaves indicate that the decrease in starch accumulation was not caused by starch degradation (Li et al. 1992), suggesting that one or more enzymes in the starch synthetic pathway might be involved in regulating the rhythm. However, based on two enzymes studied, activities of ADP-glucose pyrophosphorylase and phosphoADP-glucose isomerase were not implicated in regulating the rhythm of starch metabolism in sugar beet (Li et al. 1992). Our findings that the expression of the GBSSI gene shows a circadian rhythm (Fig. 6) may help to understand how carbon allocation in leaves is subjected to endogenous circadian regulation.
GBSSI may appear as several isoforms, and their expres-sion is tissue-specific in some plant species (Denyer et al. 1997, Nakamura et al. 1998, Tomlinson et al. 1998). For example, pea contains GBSSI and GBSSIb with the former isoform expressed in embryos and the latter in leaves and pods (Denyer et al. 1997). GBSSIb is a GBSSI-like protein with different molecular mass. Although antigenic properties are similar between GBSSI and GBSSI-like isoforms, the homol-ogy of nucleotide sequences are not similar enough to produce cross-hybridization (Nakamura et al. 1998). On the other hand, the study on the potato amylose-free (amf ) mutant indicates that amylose is synthesized by the same GBSSI in different tissues (Jacobsen et al. 1989). Our studies demon-strate that the GBSSI prepared from tuberous roots, leaves, and stems are identical in size and could all be recognized by the antiserum against the GBSSI of potato (Fig. 3). Further-more, probing the genomic DNA with GBSSI cDNA pro-duced only one genomic fragment in Southern hybridization (data not shown), and tuberous root GBSSI cDNA could cross-hybridize to the GBSSI mRNA of leaves and stems (Fig. 3). These results suggest that sweet potato contains only one GBSSI. However, regulation of GBSSI expression in the storage organ is different from that of the non-storage tissues. In the storage organ, GBSSI expression is controlled at a transcriptional level during development of tuberous roots, but circadian regulation is the dominant mechanism con-trolling expression of the same gene in non-storage tissues, e.g., leaves and stems.
Acknowledgements – We are grateful to Dr Richard G.F. Visser for
his kind gift of potato GBSSI antibodies, and to Mei-Jung Wu for technical assistance. This research was supported by a grant NSC 85-2311-B-002-043 from the National Science Council of the Re-public of China.
References
Abel GJW, Springer F, Willmitzer L, Kossmann J (1996) Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.). Plant J 10: 981 – 991
Baba T, Nishihara M, Mizuno K, Kawasaki T, Shimada H, Kobayashi E, Ohnishi S, Tanaka K, Arai Y (1993) Identifica-tion, cDNA cloning, and gene expression of soluble starch synthase in rice (Oryza sati6a L.) immature seeds. Plant Physiol 103: 565 – 573
Britz SJ, Hungerford WE, Lee DR (1987) Rhythms during extended dark periods determine rates of net photosynthesis and accumu-lation of starch and soluble sugar in subsequent light periods in leaves of Sorghum. Planta 171: 339 – 345
Clark JR, Robertson M, Ainsworth CC (1991) Nucleotide sequence of a wheat (Triticum aesti6um L.) cDNA clone encoding the waxy protein. Plant Mol Biol 16: 1099 – 1101
Craig J, Lloyd JR, Tomlinson K, Barber L, Edwards A, Wang TL, Martin C, Hedley CL, Smith AM (1998) Mutations in the gene encoding starch synthase II profoundly alter amylopectin struc-ture in pea embryos. Plant Cell 10: 413 – 426
DeFekete MAR, Leloir LF, Cardini CD (1960) Mechanism of starch biosynthesis. Nature 187: 918 – 919
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA mini preparation: Version II. Plant Mol Biol Rep 1: 19
Denyer K, Sidebottom C, Hylton CM, Smith AM (1993) Soluble isoforms of starch synthase and starch-branching enzyme also occur within starch granules in developing pea embryos. Plant J 4: 191 – 198
Denyer K, Hylton CM, Jenner CF, Smith AM (1995) Identification of multiple isoforms of soluble and granule-bound starch syn-thase in developing wheat endosperm. Planta 196: 256 – 265 Denyer K, Barber LM, Edwards EA, Smith AM, Wang TL (1997)
Two isoforms of the GBSSI class of granule-bound starch synthase are differentially expressed in the pea plant (Pisum
sati6um L.). Plant Cell Environ 20: 1566–1572
Devereux J, Haeberli P, Smithies O (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res 12: 387 – 395
Dry I, Smith A, Edwards A, Bhattacharyya M, Dunn P, Martin C (1992) Characterization of cDNAs encoding two isoforms of granule-bound starch synthase which show differential expres-sion in developing storage organs of pea and potato. Plant J 2: 193 – 202
Edwards A, Marshall J, Sidebottom C, Visser RGF, Smith AM, Martin C (1995) Biochemical and molecular characterization of a novel starch synthase from potato tubers. Plant J 8: 283 – 294 Edwards A, Marshall J, Denyer K, Sidebottom C, Visser RGF, Martin C, Smith AM (1996) Evidence that a 77-kDa protein from the starch of pea embryos is an isoform of starch synthase which is both soluble and granule bound. Plant Physiol 112: 89 – 97
Edwards A, Fulton DC, Hylton CM, Jobling SA, Gidley M, Ro¨ssner U, Martin C, Smith AM (1999) A combined reduction in activity of starch synthases II and III of potato has novel effects on the starch of tubers. Plant J 17: 251 – 261
Fondy BR, Geiger DR, Servaites JC (1989) Photosynthesis, carbo-hydrate metabolism, and export in Beta6ulgaris L. and
Phaseo-lus6ulgaris L. during square and sinusoidal light regimes. Plant
Physiol 89: 396 – 402
Furukawa K, Tagaya M, Inouye M, Preiss J, Fukui T (1990) Identification of lysine 15 at the active site in Escherichia coli glycogen synthase. J Biol Chem 266: 2086 – 2090
Goodbourn S (1996) Reverse transcriptase-polymerase chain reac-tion (RT-PCR). In: Docherty K (ed.) Gene Transcripreac-tion – RNA Analysis. John Wiley and Sons Ltd Press, Chichester, pp 68 – 71. ISBN 0-471-96147-7
Henikoff S (1984) Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene 28: 351 – 359
Hovenkamp-Hermelink JHM, Jacobsen E, Ponstein AS, Visser RGF, Vos-Scheperkeuter GH, Bijmolt EW, de Vries JN, With-olt B, Feenstra WJ (1987) Isolation of an amylose-free starch mutant of the potato (Solanum tuberosum L.). Theor Appl Genet 75: 217 – 221
Hylton CM, Denyer K, Keeling PL, Chang MT, Smith AM (1996) The effect of waxy mutations on the granule-bound starch synthases of barley and maize endosperms. Planta 198: 230 – 237
Jacobsen E, Hovenkamp-Hermelink JHM, Krijgsheld HT, Nijdam H, Pijnacker LP, Witholt B, Feenstra WJ (1989) Phenotypic and genotypic characterization of an amylose-free starch mutant of the potato. Euphytica 44: 43 – 48
Keegstra K, Olsen LJ, Theg SM (1989) Chloroplastic precursors and their transport across the envelope membranes. Annu Rev Plant Physiol Plant Mol Biol 40: 471 – 501
Klo¨sgen RB, Gierl A, Schwarz-Sommer Z, Saedler H (1986) Molec-ular analysis of the waxy locus of Zea mays. Mol Gen Genet 203: 237 – 244
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680 – 685 Larskey RA, Mills AD (1975) Quantitative film detection of3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem 56:
335 – 341
Lee L, Tsai CY (1985) Effect of sucrose accumulation on zein synthesis in maize starch-deficient mutants. Phytochemistry 24: 225 – 229
Li B, Geiger DR, Shieh WJ (1992) Evidence for circadian regula-tion of starch and sucrose synthesis in sugar beet leaves. Plant Physiol 99: 1393 – 1399
Marshall J, Sidebottom C, Debet M, Martin C, Smith AM, Ed-wards A (1996) Identification of the major starch synthase in the soluble fraction of potato tubers. Plant Cell 8: 1121 – 1135 Martin C, Smith AM (1995) Starch biosynthesis. Plant Cell 7:
971 – 985
Mu¨ller-Ro¨ber B, Sonnewald U, Willmitzer L (1992) Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11: 1229 – 1238
Nakamura T, Vrinten P, Hayakawa K, Ikeda J (1998) Characteriza-tion of a granule-bound starch synthase isoform found in the pericarp of wheat. Plant Physiol 118: 451 – 459
Nakata PA, Okita TW (1995) Differential regulation of ADP-glu-cose pyrophosphorylase in the sink and source tissues of potato. Plant Physiol 108: 361 – 368
Nelson OE, Tsai CY (1964) Glucose transfer from adenosine diphosphate-glucose to starch in preparations of Waxy seeds. Science 145: 1194 – 1195
Okagaki RJ (1992) Nucleotide sequence of a long cDNA from the rice waxy gene. Plant Mol Biol 19: 513 – 516
Preiss J (1988) Biosynthesis of starch and its regulation. In: Preiss J (ed.) The Biochemistry of Plants, Vol. 14. Academic Press, New York, NY, pp 181 – 254. ISBN 0-12-675414-4
Rohde W, Becker D, Salamini F (1988) Structural analysis of the waxy locus from Hordeum6ulgare. Nucleic Acids Res 16: 7185– 7186
Salehuzzaman SNIM, Jacobsen E, Visser RGF (1993) Isolation and characterization of a cDNA encoding granule-bound starch synthase in cassava (Manihot esculenta Crantz) and its antisense expression in potato. Plant Mol Biol 23: 947 – 962
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. ISBN 0-87969-309-6
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463 – 5467
Shennan KIJ (1996) Isolation of poly(A)+RNA. In: Docherty K
(ed.) Gene transcription – RNA Analysis. John Wiley and Sons Ltd Press, Chichester, pp 18 – 19. ISBN 0-471-96147-7 Shure M, Wessler S, Fedoroff N (1983) Molecular identification
and isolation of the Waxy locus in maize. Cell 35: 225 – 233 Tomlinson K, Craig L, Smith AM (1998) Major differences in
isoform composition of starch synthase between leaves and embryos of pea (Pisum sati6um L.). Planta 204: 86–92 Tomlinson KL, Lloyd JR, Smith AM (1997) Importance of
iso-forms of starch-branching enzyme in determining the structure of starch in pea leaves. Plant J 11: 31 – 43
Tsai CL, Tsai CY (1990) Endosperm modified by cross-pollinating maize to induce changes in dry matter and nitrogen accumula-tion. Crop Sci 30: 804 – 808
Tsai CY (1973) The activities of maizea-1,4 glucan glucosyltrans-ferases in vitro. Bot Bull Acad Sin 14: 125 – 135
Tsai CY (1974) The function of the Waxy locus in starch synthesis in maize endosperm. Biochem Genet 11: 83 – 96
Tsai CY (1983) Genetics of storage protein in maize. In: Janick J (ed.) Plant Breeding Review, Vol. 1. AVI Publishing Co., Inc., Westport, CT, pp 103 – 138. ISBN 0-87055-397-6
Tsai CY, Larkins BA, Glover DV (1978) Interaction of the Opaque-2gene with starch-forming mutant genes on the synthesis of zein in maize endosperm. Biochem Genet 16: 883 – 896
van der Leij FR, Visser RGF, Ponstein AS, Jacobsen E, Feenstra WJ (1991) Sequence of the structural gene for granule-bound starch synthase of potato (Solanum tuberosum L.) and evidence for a single point deletion in the amf allele. Mol Gen Genet 228: 240 – 248
Visser RGF, Somhorst I, Kuipers GJ, Ruys NJ, Feenstra WJ, Jacobsen E (1991a) Inhibition of the expression of the gene for granule-bound starch synthase in potato by antisense constructs. Mol Gen Genet 225: 289 – 296
Visser RGF, Stolte A, Jacobsen E (1991b) Expression of a chi-maeric granule-bound starch synthase-GUS gene in transgenic potato plants. Plant Mol Biol 17: 691 – 699
Vos-Scheperkeuter GH, de Boer W, Visser RGF, Feenstra WJ, Witholt B (1986) Identification of granule-bound starch synthase in potato tubers. Plant Physiol 82: 411 – 416
Yeh KW, Juang RH, Su JC (1991) A rapid and efficient method for RNA isolation from plants with high carbohydrate content. Focus 13: 102 – 103