Prospect and Challenges for the Development of Multivalent Vaccines against Hand, Foot and Mouth Diseases
Chia-Chyi Liu1, Yen-Hung Chow1, Pele Chong1,2 and Michel Klein3
1Vaccine R&D Center, National Health Research Institutes, Zhunan Town, Miaoli County 350, Taiwan
2 Graduate Institute of Immunology, China Medical University, Taichung, Taiwan. 3 VaxiBio Inc., Toronto, Ontario, Canada.
Correspondence:
Pele Chong, PhD., 35 Keyan Road, Zhunan Town, Miaoli County, Vaccine R&D Center, National Health Research Institutes, Taiwan (pelechong@nhri.org.tw).
Michel H. Klein, MD., 54 Strathgowan Avenue, Toronto, Ontario, Canada, M4N 1B9 (michel.klein@umontreal.ca).
Key Words: Human Enterovirus A (HEV-A); Hand, Foot and Mouth Diseases; Enterovirus 71; Coxsackievirus, inactivated whole virion vaccine; serum-free culture technology; cross-neutralizing antibody; immunodominant epitopes; waning immunity.
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19
Abstract
Enterovirus 71 (EV71), an emerging neurotropic virus and coxsackieviruses (CV) are the major
causative agents of hand, foot and mouth diseases (HFMD). These viruses have become a serious
public health threat in the Asia Pacific region. Formalin-inactivated EV71 (FI-EV71) vaccines have
been developed, evaluated in human clinical trials and were found to elicit full protection against
EV71. Their failure to prevent CVA16 infections could compromise the acceptability of monovalent
EV71 vaccines. Bivalent FI-EV71/FI-CVA16 vaccines have been found to elicit strong neutralizing
antibody responses against both viruses in animal models but did not protect against CVA6 and
CVA10 viral infections in cell culture neutralization assay. In this review, we discuss the critical
bottlenecks in the development of multivalent HFMD vaccines, including the selection of vaccine
strains, animal models to assess vaccine potency, the definition of end-points for efficacy trials, and
the need for improved manufacturing processes to produce affordable vaccines.
Introduction
Hand, foot and mouth disease (HFMD) is a self-limiting infection characterized by vesicular exanthema of the hands, feet, mouth and buttocks caused by human enterovirus (HEV-A/B) infections of infants and young children. However, coxsackievirus (CV) and Enterovirus 71 (EV71) infections have been associated with severe clinical complications and have become a major public health problem across the Asia-Pacific region [1-3]. Clinical manifestations of HFMD caused by HEV-A viruses are indistinguishable. However, EV71 is a neuroinvasive virus responsible for 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41
herpangina, aseptic meningitis, cerebella ataxia, poliomyelitis-like paralysis, acute encephalomyelitis, cardiopulmonary failure and death. Furthermore, EV71 infections expose to the risk of long-term impairment of neurological development and cognitive functions [1-4]. CVA16 infections that are usually mild can also lead to severe hyper angina, and major neurological complications and fatal outcomes [5]. Although outbreaks of EV71 and CVA16 may alternate, these two viruses often co-circulate [5, 6].
EV71 and coxsackieviruses are non-enveloped RNA viruses of the Piconaviridae family. The viral particles contain a single positive-sense RNA molecule and four capsid proteins VP1, VP2, VP3 and VP4 [2]. VP1 has often been used for EV71 molecular genotyping and epidemiological monitoring. EV71 were originally classified into three genotypes A, B, and C, and further subdivided into B1-B5 and C1-C5 subgenotypes [1-3]. B5 and C4 isolates were recently identified during epidemics in Malaysia, Singapore, Taiwan, Thailand and China [7-8]. However, Bessaud et al., [9] using genetic distance analysis and phylogenetic investigations compared 3,346 VP1 nucleotide sequences from GenBank with those of EV71 isolated from recent HFMD outbreaks in Africa. They found that some these recently-reported isolates did not fall into genotypes A-C but clustered into three additional groups, including one Indian genotype D and 2 African genotypes E and F. Their results reveal a wide genetic diversity of EV71 and suggest that the number of circulating genotypes might be underestimated particularly in developing countries where EV71 epidemiology has been poorly studied. Based on molecular epidemiology surveillance in Japan, Singapore, Taiwan and China, EV71, CVA6, CVA10 and CVA16 are the most common viruses causing HFMD in children [3, 8]. Because of the major impact of HFMD on the health care and daycare systems and the need to control widespread panic reactions in the population during epidemics, the development of efficacious vaccines to prevent HFMD outbreaks has been a national priority in some Asian countries [3, 10]. Based on the success of the poliovirus vaccine [11], inactivated EV71 vaccines will be the first ones available in the near future.
42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66
Monovalent EV71 vaccines
I. Recent Success in EV71 Vaccine Development
We recently reviewed [12] the respective merits of several experimental vaccines evaluated in animal models including inactivated, recombinant subunits, virus-like particles, synthetic neutralization epitopes, plasmid DNA-based and live vectored vaccines. Only formalin-inactivated EV71 virions (FI-EV71) formulated in alum elicited cross-virus genotypes neutralizing antibodies in mice, rabbits and non-human primates but induced only weak neutralizing responses against CVA16. From regulatory, economic and market acceptability reasons, FI-EV71 vaccines were first selected for clinical development. Five inactivated EV71 vaccines have been rapidly developed in the past few years (Table 1).
Going through vaccine strain selection, optimization of serum-free culture conditions, downstream chromatographic purification, virus-inactivation, adjuvant formulation and vaccine stability studies, the Vaccine R&D Center of the National Health Research Institutes (NHRI) of Taiwan launched the first FI-EV71 vaccine (EV71vac) human phase I clinical trial in adults in 2010. A single vaccine dose of either 5µg or 10 µg was safe and highly immunogenic [13, 14]. The vaccine elicited 100% seroconversion in naïve volunteers and strong virus neutralizing antibody (VNA) responses (GMT = 210) against the vaccine strain B4 as well as similar VNA titers against B1, B5 and C4a strains in 85% of the vaccinees. In contrast, neutralizing responses against C4b and CVA16 were weak in 20% of the subjects and 90% of the vaccinees did not develop any neutralizing response against a C2 strain. EV71vac significantly enhanced neutralizing titers in subjects with pre-existing antibodies against EV71 and/or CVA16, but a second immunization did not further boost VNA levels induced by priming in most of the volunteers.
67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91
Three inactivated EV71 vaccines based on C4 isolates which predominate in mainland China were independently developed and evaluated by three different Chinese companies (Table 1). The clinical efficacy of these vaccines formulated in alum was assessed in large Phase III trials involving more than 30,000 healthy infants and young children (6 to 35 months of age) who received twice a 1µg dose of vaccines 28 days apart. All three vaccines were found to be safe and well tolerated and provide ≥ 90% protection against EV71-associated disease. In addition, both EV71 vaccines from Beijing Vigoo and Sinovac have been 100% efficacious against EV71-related hospitalizations and severe EV71-associated HFMD cases [15]. Immune sera from subjects immunized with C4-based vaccines were shown to cross-neutralize other circulating EV71 genotypes and subgenotypes associated with epidemics in recent years [15-19]. Furthermore, pre-existing antibodies due to stealth infections of young children did not interfere with the protective efficacy of FI-EV71 vaccines against different EV71 genotypes [13-19]. However, neutralization titers decreased by half after 6 months [18] but the waning of VNA titers did not affect vaccine efficacy. Importantly, the Phase III results suggest that a virus neutralizing antibody titer of 1/16 can serve as a surrogate marker of protection against EV71-related HFMD [18]. In addition, EV71vac was shown to boost neutralizing responses (10- to 100-folds) in volunteers with pre-existing antibodies against EV71 and/or CVA16, probably as a result of anamnestic responses [14]. In spite of differences in seed strains and manufacturing processes, C4-based vaccines have shown batch consistency and efficacy [20] which should facilitate their licensure and market entry in China if there were no stability issue nor production limitations. If successful, national immunization programs could be implemented to build strong herd immunity. Ultimately, studies must be performed to assess the merits of EV71 vaccines in infants less than 6 months of age and determine whether they should ultimately be combined with EPI vaccines. In this regard, Chen et al. [21] reported that the co-administration of FI-EV71 with the commercial pediatric pentavalent vaccine Pediacel (Sanofi Pasteur) did not affect antibody responses against its individual components.
92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116
2. Future challenges for the development of EV71 vaccines.
i. Cross-neutralizing ability of EV71 vaccines and selection of vaccine strains.
A Chinese C4-based vaccine elicited broad cross-neutralizing activity against EV71 C2, C4, C5, B4 and B5 strains [16] whereas the Taiwanese B4-based vaccine strongly cross-neutralized B1, B5, and C4a isolates [14]. It is likely that EV71 genotypes and subgenotypes share common neutralization epitopes. However, the degree of cross-immunity may depend on the vaccine strain since sera from volunteers immunized with the B4 vaccine weakly neutralized C4b strains and did not neutralize a C2 isolate [14]. The cross-neutralizing activity of the C4- and B4-based vaccines against viruses from genotypes D, E and F remains to be evaluated. The risk of inter-typic and intra-typic genetic recombination may lead to the emergence of new pathogens [6] and influence the selection of vaccine strains in the future. Only results from multinational efficacy trials conducted in countries where different EV71 genotypes and subgenotypes circulate will reveal whether a monovalent vaccine can elicit broad protection against EV71-associated HMFD.
ii. Durability of neutralizing antibody responses and role of cellular immunity
The recent efficacy trials have clearly shown that humoral immunity correlates with protection but wanes after the first six months, raising the issue of a lack of persistence in protective antibody levels. The waning of VNA responses during natural infection may explain the outbreak of epidemics caused by new EV71 genotypes or subgenotypes emerging every 2 to 3 years. Longitudinal studies are necessary to evaluate the role of efficacious EV71 vaccines in controlling antigenic drift, the emergence of new viruses and virus fitness. To prevent a decline in neutralizing titers below 1/16, a third immunization at 18 months is highly recommended for long-lasting protection. Indeed, a booster injection one year after vaccination elicited more than a 10-fold increase in neutralizing antibody titers [14, 22]. The development of mucosal vaccines that are 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141
theoretically attractive may not be necessary since parenteral immunization confers protection. However, prospective studies should be conducted during EV71 and CV epidemics to assess the role of cellular immunity in long-term protection and viral pathogenesis.
Bivalent EV71/CVA16 Vaccines: The first step towards multivalent HFMD vaccines.
EV71 and CVA16 are the most predominant causes of HFMD [5-6]. Their co-circulation increases the risk of co-infection and genetic recombination [5-6, 24]. However, EV71 vaccines did not elicit cross-neutralizing antibody responses against CVA16. The acceptability of the EV71 vaccine may thus be compromised by its inability to significantly reduce the number of clinical cases during HFMD outbreaks. Furthermore, if EV71 immunization programs are successful, CVA16 may become the predominant cause of HFMD in the future. Therefore, the development of a bivalent EV71/CA16 vaccine is highly desirable.
Inactivated CVA16 vaccines and virus-like particles have elicited strong neutralizing antibody responses against homologous and heterologous strains and conferred protection against lethal challenge in neonatal mice [5]. We recently showed that an FI-CVA16 vaccine produced by the same technology as EV71vac could elicit antibodies neutralizing CVA16 but not EV71 [24]. Mice immunized with either FI-EV71 or FI-CVA16 produced antibodies against their immunodominant VP1 neutralization epitope (residues 205-225) [11, 24]. As shown in figure 1, amino acid substitutions in this epitope sequence as well as in other VP1 epitopes involving "hot spot"- residues 97, 167, and 241-260 [25, Liu et al., unpublished data] among EV71 and CVA16 can partially explain why individual FI-EV71 and FI-CVA16 vaccines do not provide cross-immunity.
Experimental bivalent FI-EV71/FI-CVA16 vaccines have elicited balanced protective responses against both viruses [26-27]. Could such a bivalent vaccine prevent most of HFMD outbreaks? Besides EV71 and CVA16, CVA4, CVA6, CVA9 and CVA10 as well as coxsackievirus B are the 142 143 144 145 146 147 148 149 150 151 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166
most common etiologic agents of HFMD [3]. We found that a bivalent vaccine induced strong neutralizing antibody responses against both EV71 and CVA16 in mice and rabbits but did not protect against CVA6 and CVA10 viral infections in a cell culture neutralization assay [Liu et al., unpublished data] likely as a result of structural differences in neutralization epitopes among CVA viruses (Fig. 2). The failure of antisera raised against bivalent the FI-EV71/FI-CVA16 vaccine to cross-neutralize CVA6 and CVA10 indicates that HFMD outbreaks still remain a serious threat to the health of Asian children. In particular, severe outbreaks of atypical cases of HFMD have affected both children and adults worldwide since 2008 [28, 29]. A new CVA6 strain from a distinct genetic lineage was reported to cause high fever, and HMFD mimicking chicken pox with a frequent association of onychomadesis. More detailed disease burden studies on this atypical HFMD are needed to support the rationale for including CVA6 in a multi-valent HFMD vaccine.
Ultimate Need for HFMD Multivalent Vaccines
The critical bottlenecks in the development of multivalent HFMD vaccines are the selection of the vaccine strains; the establishment of reference standards; the availability of assays to quantify antigens and cross-neutralizing antibodies; appropriate animal models; the establishment of end-points for efficacy trials, and solutions for ethical and economic issues.
1. Disease Burden and Potential Vaccine Strains
Information on age-specific incidence rates of HFMD is required to identify disease burden in target populations (6 to 60 months old children), to define clinical endpoints and to calculate sample sizes for efficacy trials. Age-specific incidence rates of EV71-related HFMD severe infections during the 1998 epidemic in Taiwan were reported to be 27.3, 37.1, 30.0, and 23.1 per 100,000 for children aged < 6, 6–11, 12–23, and 24–35 months, respectively [30]. This study suggests that severe 167 168 169 170 171 172 173 174 175 176 177 178 179 180 181 182 183 184 185 186 187 188 189 190 191
HFMD would not be suitable as clinical endpoint since its frequency is too low and trials would require a huge sample size at each clinical site. Alternatively, mild illness such as herpangina and HFMD could be suitable as primary endpoints. However, reported HFMD cases in some countries seem to include herpangina cases whereas in some other countries they do not, Herpangina and HFMD were separately reported in the national disease surveillance registers in Taiwan and Japan. On the contrary, the national surveillance systems in China and several other countries just report HFMD but not herpangina cases. Surveillance criteria and bias for HFMD and herpangina should be carefully considered. Thus, harmonization of the different surveillance systems for data collections and laboratory procedures is critical to validate the number of HFMD cases reported worldwide.
Based on the current surveillance data, there is an EV71 epidemic every 3 years. In Japan, Taiwan and Singapore CVA6, CVA10 and CVA16 were the prevalent viruses causing HFMD in the last few years. Recent outbreaks of CVA6-associated HFMD occurred in Finland (2008), Taiwan (2010) and China (2013) [5]. Unlike EV71 and CVA16 which infect cells via the human scavenger receptor class B member 2 hsSCARB2 [31], CVA6 and CVA10 which can also cause severe HFMD [32] use hsSCARB2-independent pathways [33]. Furthermore, a pair-wise amino acid sequence analysis revealed sequence differences in VP1 among CVA2, CVA3, CVA4, CVA5, CVA6, CVA7, CVA8, CVA10, CVA12, CVA14, CVA16 and EV71 to be 28 to 40% [34]. Multiple HEV-A serotypes can co-circulate during sporadic outbreaks and individuals may be infected with two or more serotypes [34-35]. Genetic recombination between different genotypes and serotypes is well documented [5-6]. It is thus predictable that the genotypes and subgenotypes of prevalent viruses causing HFMD in Asia Pacific will change with time and that novel genotypes will emerge. To speed up the licensure of HFMD vaccines, multinational, randomized, controlled efficacy trials are necessary to prove their efficacy in the targeted age group. Asian countries have not yet harmonized their HEV-A surveillance systems. A global surveillance network including Africa for enterovirus outbreaks similar to the WHO global influenza surveillance and response system is urgently needed 192 193 194 195 196 197 198 199 200 201 202 203 204 205 206 207 208 209 210 211 212 213 214 215 216
to monitor immune responses to HFMD vaccines in the future. Based on current epidemiology data, it is reasonable to propose that EV71, CVA6, CVA10 and CVA16 should be the core viruses to be included in multivalent HFMD vaccines.
2. Animal Models and Assays
The first criterion to select potent HFMD vaccine candidates is their ability to elicit strong cross-genotype and serotype neutralizing antibody responses. Currently, the various assays to quantify vaccine antigens and measure neutralizing antibodies titers need to be harmonized and standardized to compare vaccine potency in animal models, gain broad approval by regulatory authorities and monitor clinical trials. To overcome these deficiencies, we have developed and standardized a micro-neutralization assay and an EV71 particle-based ELISA that could be used to quickly screen pre-immune sera from young children since a negative result at a 1/2000 serum dilution correlates with neutralizing titers levels <8 [14,36-37].
Appropriate animal models to understand HEV-A pathogenesis and evaluate the potency and consistency of vaccine batches are not yet available [37-38]. Rats, rabbit sand macaques develop antibody responses to EV71 vaccines similar to those observed in humans [13-14]. However, non-human primate models are not suitable for assessing the neurovirulence and the risk of cardiopulmonary complications associated with severe EV71 infections [38]. Furthermore, their use is limited by ethical and economic considerations. Neonatal suckling mice and improved mouse models using adapted strains and immunodeficient animals do not mimic human infections [38]. NHRI [39] and Fujii et al. [40] have successfully developed transgenic mice carrying the human receptor hsSCARB2. In this promising model, HFMD-like skin rashes were observed in transgenic animals infected with clinical EV71 isolates of the B4 and B5 subgenotypes, and severe limb paralysis and death occurred in animals inoculated with EV71 C2 and C4 genotypes as well as with CVA16. The presence of EV71 in tissues and CNS was accompanied by the up-regulation of pro-inflammatory mediators (CXCL10, CCL3, TNF-α, and IL-6), and correlated with the recruitment of 217 218 219 220 221 222 223 224 225 226 227 228 229 230 231 232 233 234 235 236 237 238 239 240 241
T lymphocytes and disease severity [39-40]. In addition, passive administration of the monoclonal anti-EV71 VP1 neutralizing antibody N3 [41] reduced symptoms induced by EV71 B5 infection and protected the transgenic mice against EV71 C2-induced severe limb paralysis and death. Protection was associated with a reduction in viral load in the brain, spinal cord, and limb muscles as well as in pro-inflammatory mediators in tissues. The transgenic mouse model once standardized will be useful to assess the efficacy and cross-protective ability of vaccines against HEV-A using hsSCARB-2 as receptor and to evaluate immunotherapeutic strategies.
3. Regulatory, Ethical and Economic Considerations
Since HFMD affects children from developing countries, an ideal vaccine should be inexpensive, safe, compatible with large-scale production, easy to administer and acceptable to parents. Only a few vaccine companies in the Asia-Pacific region have the capability to take a vaccine from research to product launch. More efforts and more cooperation between R&D institutes and companies are urgently needed to improve the current downstream processes.
Due to intellectual property (IP) rights and proprietary technologies, information on the influence of culture medium and production systems on vaccine yields is totally missing. Currently, roller-bottles, cell factories and microcarrier bioreactors are available for up-stream cell-culture bioprocesses. Both the roller-bottle and cell factory technologies used to produce current clinical lots are easy to implement and operate, although labor intensive. Developing countries could start implementing these technologies for large-scale vaccine production.
Clinical trials have revealed that two administration of 1µg dose of EV71 vaccine achieved efficacy. We have recently reported [12] that a 40-liter pilot-scale production batch could yield 50,000 1µg doses of FI-EV71 at a cost of 0.4 US dollar/dose. Using large GMP facility, several millions of doses could be easily and cheaply produced annually.
However, the large-scale production of EV71 and multivalent HFMD vaccines will require an 242 243 244 245 246 247 248 249 250 251 252 253 254 255 256 257 258 259 260 261 262 263 264 265 266
improvement of the current manufacturing processes. The use of bioreactors, micro-carriers and perfusion technology [42] could increase cell growth by one order of magnitude. Other technologies have also been recently developed to improve virus yields. Transfection of more viral receptor genes into host cells to increase virus infectivity at lower multiplicity of infection and/or removal of genes inhibiting viral replication are promising approaches to enhance viral infection of host cells. Reverse-genetic technology can also be used to improve virus yields by inserting specific protease cleavage sites to enhance virus infectivity or engineer temperature-sensitive mutants. Such improvements in virus production should significantly increase cell-based HFMD vaccine production. The best approach to lower the production cost of EV71 vaccines is to use a simple and efficient downstream chromatographic purification step that co-purifies immunogenic defective and infectious virus particles [12]. In contrast, FI-CVA16 [23] as well as FI-CVA6 and FI-CVA10 had to be prepared from infectious particles obtained in low yield by sucrose gradient ultracentrifugation in order to elicit strong neutralizing antibody responses in experimental animals [Liu et al., unpublished results].
Conclusions and Perspectives
A two-dose regimen vaccination with inactivated C4-based EV71 vaccines protects against EV71-associated HFMD. Multinational efficacy trials are now necessary to fully assess the degree of cross-immunity EV71 vaccines can elicit against circulating genotypes and subgenotypes. Continuous worldwide epidemiological surveillance is critical to identify changes in the genotypes/subgenotypes of circulating isolates and to detect the emergence of new enterovirus variants resulting from genomic recombination in order to guide the selection of future vaccine strains. Harmonization and standardization of immunogens, immunoassays and animal models are urgently required at the international level to compare the potency of vaccine candidates and determine which manufacturing process yields the most potent vaccines. The development of a 267 268 269 270 271 272 273 274 275 276 277 278 279 280 281 282 283 284 285 286 287 288 289 290 291
bivalent EV71/CVA16 vaccine should be the first step towards that of a multivalent HFMD vaccine. This will require an improvement of large-scale manufacturing processes to produce sufficient quantities of vaccine doses at an affordable cost. Lee at al. [43] recently forecasted that routine immunization with a 70% efficacious EV71 vaccine sold at $25 US dollars per dose would be of great economic value. With this profit margin and the new emerging vaccine markets in the Asia-Pacific region, the global vaccine companies might become interested in manufacturing EV71 and multivalent HFMD vaccines.
Acknowledgements
This work was supported by a grant (No. 98A1-VCSP01-014) from the National Science Council (NSC) of Taiwan. 292 293 294 295 296 297 298 299 300 301 302
References
1. McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev, 2002; 26:91-107.
2. Solomon T, Lewthwarte P, Perera D, Cordosa MJ, McMinn P, Ooi MH. Virology,
epidemiology, pathogenesis and control of enterovirus 71. Lancet Infect Dis, 2010; 10:778-790. 3. Lee MS, Tseng FC, Chi CY, Wang JR, Chong P, Su IJ. Challenges to Licensure of Enterovirus
71 Vaccines. PLoS Negl Trop Dis, 2012; 6(8): e1737.
4. McMinn PC. Enterovirus Vaccines for an Emerging Cause of Brain-Stem Encephalitis. N Engl J Med, 2014; 370:792-794.
5. Mao Q, Wang Y, Yao X, Bian L, Wu X, Xu M, et al. Coxsackievirus A16 : Epidemiology, diagnosis and vaccine. Hum Vaccin Immunother, 2014; 10:360-367.
6. Yip CCY, Lau SKP, Woo PCY, Yuen KY. Human enterovirus 71 epidemics: what’s next? Emerg Health Threats J, 2013; Sep 10; 6:19780. Epub 2013 Sep 10.
7. Chan YF, Sam IC, Abubakar S. Phylogenetic designation of EV71 genotypes and subgenotypes using complete genome sequences. Infect Genet Evol, 2010; 10:404-412.
8. Huang SW, Hsu YW, Smith DJ, Kiang D, Tsai HP, Lin KH, et al.Reemergence of enterovirus 71 in 2008 in Taiwan: Dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol, 2009; 47:3653-3662.
9. Bessaud M, Razafindratsimandresy R, Nougairede A, Joffret ML, Deshpande JM, Dubot-Peres A, et al., Molecular comparison and evolutionary analysis of VP1 nucleotide sequences of new Afrian human enterovirus 71 isolates reveal a wide genetic diversity. PLoS ONE 2014; 9(3):e90624.
10. Liang ZL, Mao Q, Gao F, Wang J. Progress on the research and development of human enterovirus 71 (EV71) vaccines. Front Med, 2013; 7(1):111-121.
303 304 305 306 307 308 309 310 311 312 313 314 315 316 317 318 319 320 321 322 323 324 325 326 327
11. Plotkin SA, Vidor E. Poliovirus vaccine-inactivated. 2004 In:Vaccine (4th Edition), Plotkin SA,
Orenstein WA, Offit PA (Eds). WB Saunder Co., PA, USA 625-649
12. Chong P, Hsieh SY, Liu CC, Chou AH, Chang JY, Wu SC, et al. Production of EV71 vaccine candidates. HumVaccin Immunother, 2012; 8:1-9.
13. Cheng A, Fung CP, Liu CC, Lin YT, Tsai HY, Chang SC, et al. A phase 1, randomized, open-label study to evaluate the safety and immunogenicity of enterovirus 71 vaccine. Vaccine 2013; 31:2471-2476.
14. Chou AH, Liu CC, Chang JY, Jiang R, Hsieh YC, Tsao A, et al. Formalin-inactivated EV71 vaccine candidate induced cross-neutralizing antibody responses against subgenotypes B1, B4, B5 and C4A in adult volunteers. PLoS One, 2013; 8(11): e79783.
15. Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med, 2014; 370:818-828.
16. Mao Q, Cheng T, Zhu F, Li J, Wang Y, Li Y, et al. The cross-neutralizing activity of enterovirus 71 subgenotype c4 vaccines in healthy Chineses infants and children. PLoS One, 2013; 8: e79599.
17. Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, et al. An inactivated enterovirus 71 vaccine in health children. N Engl J Med, 2014; 370:829-837.
18. Li JX, Mao QY, Liang ZL, Ji H, Zhu FC. Development of enterovirus 71 vaccines: from the lab bench to Phase III clinical trials. Expert Rev Vaccines, 2014; 13:609-618.
19. Zhu FC, Meng FY, Li JX, Li JX, Li XL, Mao QY, et al. Efficacy, safety and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled phase 3 trial. Lancet, 2013; 381:2024-2032.
20. Chen YJ, Meng FY, Mao QY, Li JX, Wang H, Liang ZL, et al. Clinical evaluation for batch consistency of an inactivated enterovirus 71 vaccine in a large scale phase 3 clinical trial. Hum Vaccin Immunother, 2014; 10(5) (Epub ahead of print).
328 329 330 331 332 333 334 335 336 337 338 339 340 341 342 343 344 345 346 347 348 349 350 351 352
21. Chen CW, Lee YP, Wang YF, Yu CK. Formaldehyde-inactivated human enterovirus 71 vaccine is compatible for co-immunization with a commercial pentavalent vaccine. Vaccine, 2011; 29:2772-2776.
22. Wang S, Li J, Liang Z, Li X, Mao Q, Meng F, et al. A booster dose of an inactivated enterovirus 71 vaccine in Chinese young children: A randomized, double-blind, placebo-controlled clinical trial. J Infect Dis, 2014; Mar 12 (Epub ahead of print).
23. Liu VV, Wu S, Xiong Y, Li T, Wen Z, Yan M, et al. Co-circulation and genomic recombination of coxsackievirus A16 and enterovirus 71 during a large outbreak of hand, foot and mouth disease in central China. PLoS ONE, 2014; 9(4):e96051.
24. Chong P, Guo MS, Lin FH, Hsiao KN, Weng G SY, Chou AH, et al. Immunological and biochemical characterization of coxsackievirus A16 viral particles. PLoS One, 2012; 7(11): e49973.
25. Liu CC, Chou AH, Lien SP, Lin HY, Liu SJ, Chang JY, et al. Identification and characterization of a cross-neutralization epitope of EV71. Vaccine, 2011; 29:4362-73.
26. Cai Y, Ku Z, Liu Q, Leng Q, Huang Z. A combination vaccine comprising of inactivated enterovirus 71 and coxsackievirus A16 elicits a balanced protective immunity against both viruses. Vaccine, 2014; 32:2406-2412.
27. Lin CW, Liu CC, Lu TC, Liu SJ, Chow YH, Chong P, et al. Immunogenicity studies of bivalent inactivated virions of EV71/CVA16 formulated with submicron emulsion systems. Biomed Res Int, 2014. http://dx.doi.org/10.1155/2014/670506.
28. Fujimoto T, Lizuka S, Enomoto M, Abe K, Yamashita K, Hanaoka N, et al., Hand, foot and mouth disease caused by coxsackievirus A6, Japan, 2011. Emerg Infect Dis. 2012; 18:337-339. 29. Feder HM, Bennett N, Modlin JF. Atyptical hand,foot, and mouth disease: a vesiculobullous
eruption caused by coxsackievirus A6. Lancet Infect Dis. 2014; 14:83-86.
30. Chang LY, King CC, Hsu KH, Ning HC, Tsao KC, Li CC, et al. Risk factors of enterovirus 71 353 354 355 356 357 358 359 360 361 362 363 364 365 366 367 368 369 370 371 372 373 374 375 376 377
infection and associated hand, foot and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics, 2002; 109(6): e88.
31. Yamayoshi S, Yamashita Y, Li J, Hanagata N, Minowa T, Takemura T, et al.Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat Med, 2009; 15:798-801.
32. Lu QB, Zhang XA, Wo Y, Xu HM, Li XJ, Wang XJ, et al. Circulation of coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009-2011. PLoS One, 2012; 7(12): e52073. 33. Yamayoshi S, Iizuka S, Yamashita T, Minagawa H, Mizuta K, Okamoto M, et al. Human
SCARB2-dependent infection by Coxsackievirus A7, A14 and A16 and enterovirus 71. J Virol, 2012; 86:5686-5696.
34. Hu YF, Yang F, Du J, Dong J, Zhang T, Wu ZQ, et al. Complete genome analysis of
coxsackievirus A2, A4, A5, and A10 strains isolated from hand, foot, and mouth disease
patients in China revealing frequent recombination of human enterovirus A. J Clinical
Microbiol, 2011; 49 (7): 2426-2434.
35. Zhang T, Du J, Xue Y, Su H, Yang F, Jin Q. Epidemics and Frequent Recombination within
Species in Outbreaks of Human Enterovirus B-Associated Hand, Foot and Mouth Disease in
Shandong China in 2010 and 2011. PLoS One 2013; 8:e67157.
36. Liu CC, Chang HW, Yang G, Chiang JR, Chow YH, Sai IH, et al Development of a
quantitative enzyme linked immunosorbent assay for monitoring the EV71 vaccine
manufacturing process. J Virol Methods, 2011; 176:60-68.
37. Liu CC, Chou AH, Lien SP, Lin HY, Liui SJ, Chang JY, et al. Identification and
characterization of a cross-neutralization epitope of EV71. Vaccine, 2011; 29:4362-73.
38. Wang YF, Yu CK. Animal models of enterovirus 71 infection: applications and limitations. J 378 379 380 381 382 383 384 385 386 387 388 389 390 391 392 393 394 395 396 397 398 399
Biomed Sci, 2014; 21:31-41.
39. Lin YW, Yu SL, Shao HY, Lin HY, Liu CC,Hsiao KN, et al. Human SCARB2 transgenic mice
as an infectious animal model for enterovirus 71. PLoS One, 2013; 8(2): e57591.
40. Fujii K, Nagata N, Sato Y, Ong KC, Wong KT, Yamayoshi S, et al. Transgenic mouse model for
the study of enterovirus 71 neuropathogenesis. Proc Natl Acad Sci USA, 2013;
110:14753-14758.
41. Chang HW, Lin YW, Ho HM, Lin MH, Liu CC, Shao HY, et al. Protective efficacy of VP1-specific neutralizing antibody associated with a reduction of viral load and pro-inflammatory cytokines in human SCARB2-transgenic mice. PLoS One, 2013; 8:269858.
42. Liu CC, Guo MS, Lin FHY, Hsiao KN, Chang KH, Chou AH, et al. Purification and characterization of EV71 viral particles produced from Vero cells grown in a serum-free microcarrier bioreactor system. PLoS One, 2011; 6(5); e200005.
43. Lee BY, Wateska A, Bailey RR, Tai JHY, Bacon KM, Smith KJ. Forecasting the economic value of an enterovirus 71 (EV71) vaccine. Vaccine 2010, 28:7731-7736.
400 401 402 403 404 405 406 407 408 409 410 411 412 413 414
Figure Legends
Figure 1. Comparison of aminoacid sequences of EV71 and CVA16 VP1 proteins. The amino acid sequence of VP1 from the EV71 genotype B4 strain E59 is compared to that of the CVA16 strain 5079. Amino acids involved in neutralization epitopes and “hot-spots” are underlined and highlighted in red, respectively. EV71 E59 strain (GenBank: GQ150746.1) and CVA16_5079 (GenBank: AF177911.1).
Figure 2. Comparison of aminoacid sequences of CVA VP1 proteins. The amino acid sequence of the CVA16 strain 5079 is compared to those of the CVA6 strain M0746 and the CVA10 strain M2014. Amino acids that are involved in neutralization epitopes and that diverge among strains are underlined and highlighted in red, respectively.
415 416 417 418 419 420 421 422 423 424 425 426
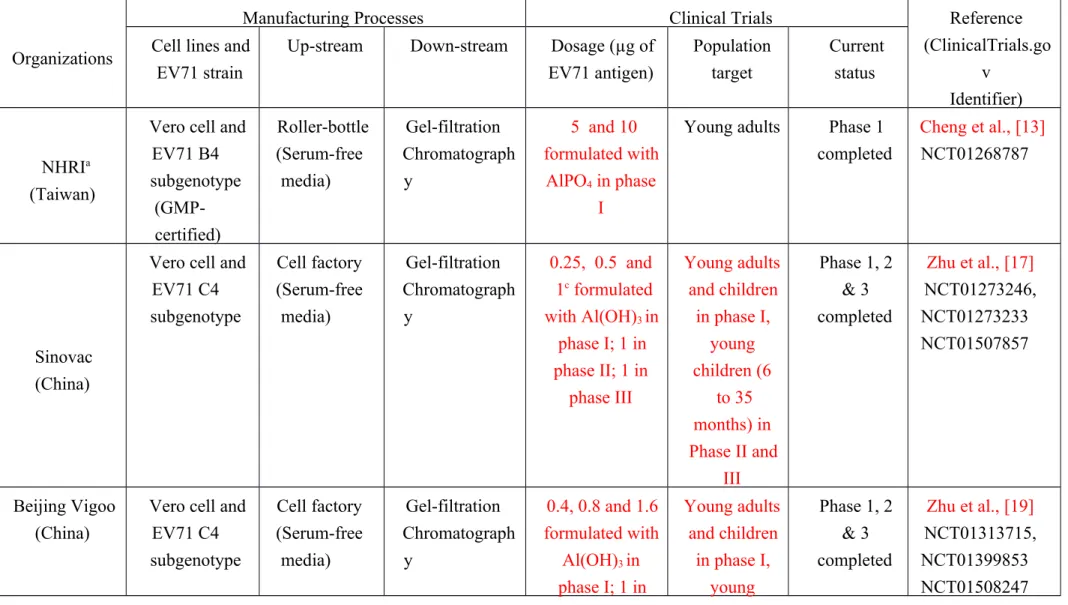
Table 1. FI-EV71 vaccine candidates currently being tested in clinical trials.
Organizations
Manufacturing Processes Clinical Trials Reference
(ClinicalTrials.go v
Identifier) Cell lines and
EV71 strain
Up-stream Down-stream Dosage (µg of EV71 antigen) Population target Current status NHRIa (Taiwan)
Vero cell and EV71 B4 subgenotype (GMP-certified) Roller-bottle (Serum-free media) Gel-filtration Chromatograph y 5 and 10 formulated with AlPO4 in phase I
Young adults Phase 1 completed
Cheng et al., [13] NCT01268787
Sinovac (China)
Vero cell and EV71 C4 subgenotype Cell factory (Serum-free media) Gel-filtration Chromatograph y 0.25, 0.5 and 1c formulated with Al(OH)3 in phase I; 1 in phase II; 1 in phase III Young adults and children in phase I, young children (6 to 35 months) in Phase II and III Phase 1, 2 & 3 completed Zhu et al., [17] NCT01273246, NCT01273233 NCT01507857 Beijing Vigoo (China)
Vero cell and EV71 C4 subgenotype Cell factory (Serum-free media) Gel-filtration Chromatograph y 0.4, 0.8 and 1.6 formulated with Al(OH)3 in phase I; 1 in Young adults and children in phase I, young Phase 1, 2 & 3 completed Zhu et al., [19] NCT01313715, NCT01399853 NCT01508247 427 428
phase II; 1 in phase III children (6 to 35 months) in Phase II and III CAMSb (China) Human diploid cell KMB-17 and EV71 C4 subgenotype Cell factory (Serum-containing media) Gel-filtration Chromatograph y
Unknownd Young adults and children in phase I, young children (6 to 60 months) in Phase II and III Phase 1, 2 & 3 completed Li et al., [16] NCT01391494, NCT01512706, NCT01569581 Inviragen (Singapore)
Vero cell and EV71 B2 subgenotype Cell factory (Serum-free media) Gel-filtration Chromatograph y 0.3 and 3 in phase I Young adults, Phase 1 completed NCT01376479
aNHRI means National Health Research Institutes, Taiwan
bCAMS means Chinese Academy of Medical Sciences, Kunming, China.
cThe antigen dosage is calculated based on the report by Liang et al., [10] that the specific activity of EV71 antigen reference standard is established in China to be 421.1 U/µg.
dThe specific dosage used in these clinical trials by CAMS was not reported. 429 430 431 432 433 434 435