Dear Dr. Author(s),
- Please modify your manuscript as per the reviewer recommendations on this copy (ONLY) and resubmit it along with your reply to the reviewer comments, to continue with its processing.
- Please highlight the modified areas
- Authors shall NOT change other settings and/or formats of this version. Original Article
KMJ-043/013
Use of Proton Pump Inhibitors Correlates with Increased Risk of Pancreatic Cancer: A Case-Control Study in Taiwan
Running head: proton pump inhibitors and pancreatic cancer
Shih-Wei Lai1,2, Fung-Chang Sung3,4, Cheng-Li Lin 3,4, Kuan-Fu Liao5,6
(The first two authors contributed equally to this study.)
1School of Medicine, and 3Department of Public Health, China Medical University, Taichung,
Taiwan
2Department of Family Medicine, and 4Management Office for Health Data, China Medical
University Hospital, Taichung, Taiwan
5Graduate Institute of Integrated Medicine, China Medical University, Taichung, Taiwan 6Department of Internal Medicine, Taichung Tzu Chi General Hospital, Taichung, Taiwan
Corresponding author:
Kuan-Fu Liao, Department of Internal Medicine, Taichung Tzu Chi General Hospital, No.66, Sec. 1, Fongsing Road, Tanzi District, Taichung City, 427, Taiwan
Phone: 886-4-2205-2121 Fax: 886-4-2203-3986
E-mail: kuanfuliao@yahoo.com.tw
ABSTRACT (144 Words)
Objective: This study investigated whether use of proton pump inhibitors (PPIs) enhances the
risk of pancreatic cancer.
Design: From a random sample of National Health Insurance claims data of Taiwan.
Subjects: We identified 977 patients aged 20 years or older with newly diagnosed pancreatic
cancer as the case group in 2000-2010. The control group consisted of 3908 subjects without pancreatic cancer selected from the same sample.
Main outcome measure: The history of using PPIs and other comorbidities were compared
between cases and controls.
Results: After adjustment for
confounders
,
multivariable
logistic regression analysis showed that pancreatic cancer had strong association with PPIs use (OR 9.28, 95% CI 7.77-11.08). Among PPI drugs, those using esomeprazole were at the highest risk with an OR of 12.1 (95% CI 9.76-15.0).Conclusions: Taking PPIs correlates with increased risk of pancreatic cancer. The risk may
greater for those taking esomeprazole.
INTRODUCTION
Pancreatic cancer is an important global burden of cancer because of low survival rate. Among cancer related deaths, it is the eighth most common cause of deaths (266000 deaths, 3.5% of the total) worldwide, the fourth in the US and the eighth in Taiwan. The etiology of pancreatic cancer remains unclear. Studies have implicated that smoking, drinking, coffee consumption, obesity, family history, medications and pancreatitis as factors associated with this disease. Lowenfels et al. in an Europian international cohort study found that the incidence ratio of
pancreatic cancer in patients with pancreatitis was 26.3 higher than expected. A recent study
found the risk of pancreatic cancer increased in patients with gastric ulcer.
Proton pump inhibitors (PPIs), a class of drugs that reduce gastric acid secretion, are commonly prescribed to manage peptic ulcer diseases. Their long-term effects on cancer risk have been widely discussed. Long-term omeprazole treatment may lead to hypergastrinemia and profound hypochlorhydria in response to the reduced gastric acid secretion. Hypergastrinemia is found to be associated with digestive tract malignancies. The earlier experimental studies have identified gastrin receptors in human pancreatic cancer cells, and gastrin can stimulate the growth of human pancreatic cancer cells in culture. Thus, we hypothesized that use of PPIs may lead to hypergastrinemia, which might correlate with increased risk of pancreatic cancer. To date, no evidence is available about the role of PPIs on pancreatic cancer risk in Taiwan. Therefore, we conducted a case-control study to explore whether there is an association between PPIs use and pancreatic cancer risk.
MATERIALS AND METHODS Study population
This case-control study used the claims data of the National Health Insurance of Taiwan. The program has been detailed in previous studies. In brief, this universal insurance program has a coverage rate of more than 99% in this country[17]. The database consisted of a random sample
with 1,000,000 insured persons, being established by the Taiwan National Health Research Institute. The database included information on insured demographic status, ambulatory care and inpatient care, and medicine prescribed. International Classification of Diseases (ICD) 9th
Revision-Clinical Modification (ICD-9) was used to identify diagnosis. Data files can be linked with scrambled identification to secure privacy of patients.
Inclusion criteria
First, during the period of 2000-2010, subjects aged 20 years or older who had newly diagnosed
pancreatic cancer
were defined as the study cases (based on ICD-9 codes 157). Totally, 977
cases were selected as the case group. Second, for each
pancreatic cancer
case, four subjects
without
pancreatic cancer
from the same database were randomly selected as the study control
s
(case/control ratio =1:4)
. Totally,
3908
subjects were selected as the
control
group.
Both groups
were matched by gender, age (per 5 years) and index year of diagnosing
pancreatic cancer
.
Thedate of diagnosing pancreatic cancer was defined as the index date. Subjects with pancreatic cancer or any other cancer (ICD-9 codes 140-208) before index date were excluded.
Patients with early-undiagnosed pancreatic cancer initially presenting with abdominal symptoms might have been treated with PPIs. To reduce misclassification, cases receiving the PPIs therapy only within two years before the index date were excluded from the data analyses. In order to explore the effect of medications on pancreatic cancer risk, histamine-2 receptor antagonists, statins, non-statin lipid-lowering drugs, aspirin, other non-steroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors before index date were included.
Co-morbidities potentially associated with pancreatic cancer risk
Co-morbidities before index date potentially associated with pancreatic cancer risk were as follows: acute pancreatitis, chronic pancreatitis, diabetes mellitus, obesity, gallstones, and hepatitis C infection. All were identified with ICD-9 codes.
Statistical analysis
Data analysis first compared between cases and controls for distribution of demographic status, comorbidities and medications received. The Chi-square test and t-test were used to examine the differences. Only the factors found significantly in the crude analysis were further included in multivariable logistic regression analysis to estimate odds ratio (OR) and 95% confidence
interval (CI) for pancreatic cancer. The risk of the cancer was estimated by individual PPI with the adjustment of acute pancreatitis, chronic pancreatitis, diabetes mellitus, obesity, histamine-2 receptor antagonists, statins, non-statin lipid-lowering drugs, and both of aspirin and cyclooxygenase-2 inhibitors. The probability value < 0.05 was considered statistically significant (SAS software version 9.1, SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
Characteristics of the study population
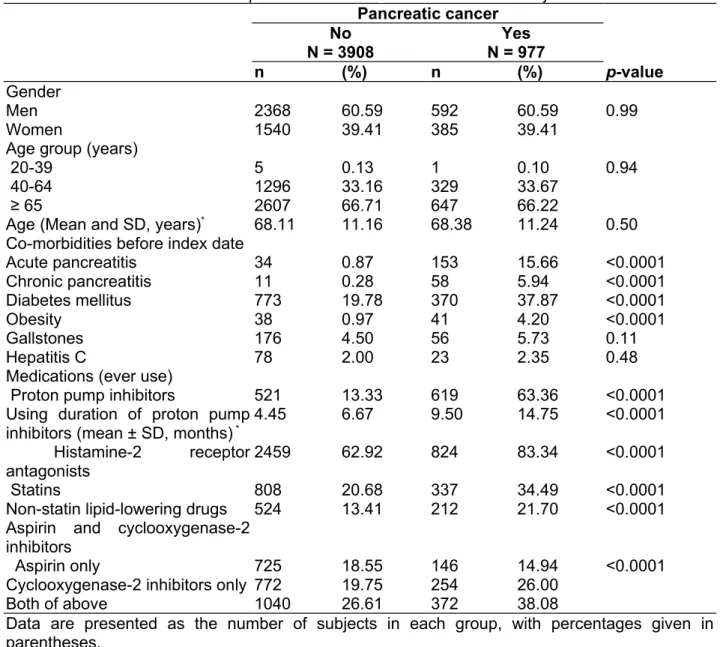
There were 977 patients with pancreatic cancer as cases and 3908 subjects without pancreatic cancer as controls. Table 1 shows that the case group had higher proportions of acute pancreatitis, chronic pancreatitis, diabetes mellitus, obesity, PPIs use, histamine-2 receptor antagonists use, statins use, non-statin lipid-lowering drugs use, and cyclooxygenase-2 inhibitors use. The mean duration of using PPIs was longer in case group than in the control group (9.50 vs. 4.45 months, P <0.0001). Because more than 98% of subjects in both groups had ever used other non-steroidal anti-inflammatory drugs, these drugs were excluded for further analysis (data not shown).
Association between co-morbidities, medications and pancreatic cancer risk
After adjustment for multiple confounders that were found significantly in the crude analysis,
multivariable logistic regression analysis showed that the adjusted OR of pancreatic cancer was
9.28 for the group with PPIs use (95% CI 7.77, 11.08), as compared to the group with non-use of PPIs. In addition, acute pancreatitis (OR 16.32, 95% CI 10.24, 26.02), chronic pancreatitis (OR 2.27, 95% CI 1.00, 5.15), diabetes mellitus (OR 1.54, 95% CI 1.27, 1.87), obesity (OR 2.59, 95% CI 1.54, 4.36), and use of histamine-2 receptor antagonists (OR 1.90, 95% CI 1.53,
2.35), were significantly associated with pancreatic cancer risk (Table 2).
Sub-analysis of association between individual proton pump inhibitors and pancreatic cancer risk
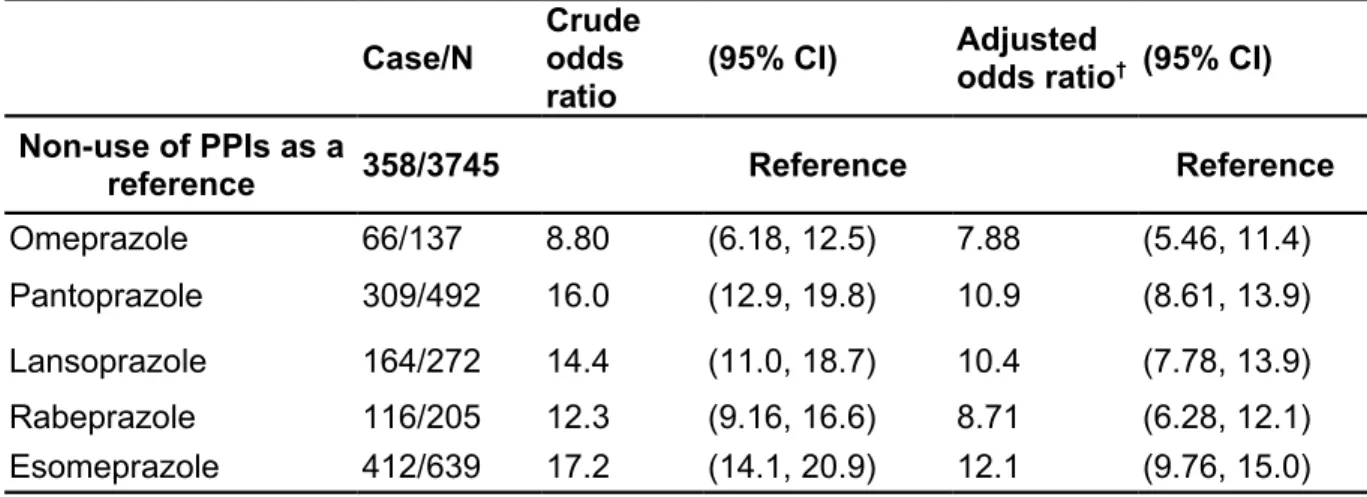
In sub-analysis, use of omeprazole (OR 7.88, 95% CI 5.46, 11.4), pantoprazole (OR 10.9, 95% CI 8.61, 13.9), lansoprazole (OR 10.4, 95% CI 7.78, 13.9), rabeprazole (OR 8.71, 95% CI 6.28, 12.1), or esomeprazole (OR 12.1, 95% CI 9.76, 15.0), could be associated with increased risk of pancreatic cancer (Table 3).
DISCUSSION
So far, only one observational study with large sample size from UK general practice research database (GPRD) has reported that PPIs use was not associated with pancreatic cancer risk (OR 1.02, 95% CI 0.85-1.22), no matter using duration or dosage. Because some subjects might have underlying early-undiagnosed pancreatic cancer who initially presented with abdominal symptoms and received PPIs treatment, to reduce this confounding effect, subjects who have used PPIs only within two years before index date were excluded from this present study. In this present study, we found the overall risk of pancreatic cancer might be increased to 9-fold among PPIs-users. To date, only few studies can be referenced, so we cannot provide a plausible explanation about the strong discrepancies of above results. The postulated pathophysiological basis linking hypergastrinemia and pancreatic cancer is that gastrin receptors in human pancreatic cancer cells, and gastrin can stimulate the growth of human pancreatic cancer cells in culture. Additionally, bacterial overgrowth and generation of nitrosamines secondary to gastric acid suppression may also contribute to human pancreatic carcinogenesis in vitro. Therefore, our finding is compatible with the prior hypothesis that use of PPIs might cause hypergastrinemia and gastric acid suppression, which might correlate with increased risk of pancreatic cancer. Nevertheless, one point needs to be discussed. Because of the lag time between diagnosing date of pancreatic cancer and onset of pancreatic cancer, we could not make sure whether PPIs use was before or after onset of pancreatic cancer, even though subjects who have used PPIs only within two years before index date were excluded from the analysis. Thus, whether PPIs use is really causality for pancreatic cancer risk or only a coincidence for treating abdominal symptoms of early-undiagnosed pancreatic cancer cannot be determined in this present study.
Some limitations should be discussed. First, there was no record of body mass index due to inherited limitation of this database. Thus, we defined obesity by using ICD-9 codes. This could lead to underestimate the prevalence of obesity. Second, because there is no other study supporting such an association between PPIs use and pancreatic cancer, interpretation of our findings should be careful. Third, it is not clear whether our findings can be extrapolated to a Caucasian population or not.
We conclude that although residual confounding may have affected the results, PPIs use is associated with a markedly increased risk of pancreatic cancer in Taiwan. Further studies are needed to confirm the role of PPIs on pancreatic cancer risk.
ACKNOWLEDGEMENTS
The authors thank the National Health Research Institute in Taiwan for providing the insurance claims data.
Conflict of Interest Statement: The authors disclose no conflicts of interest
Funding: This study was supported in part by Taiwan Department of Health Clinical Trial and
Research Center of Excellence (DOH102-TD-B-111-004)
and China Medical University
Hospital (Grant number 1MS1)
.The funding agency did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.REFERENCES
1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127:2893-917.
2. Siegel R, Naishadham D and Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10-29.
3. Department of Health. Taiwan: Main Causes of Death in 2011. http://www.doh.gov.tw. [cited in 2013 March].
4. Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, Dimagno EP, Andren-Sandberg A and Domellof L. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993; 328:1433-7.
5. Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML and Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009; 301:2553-62.
6. Hidalgo M. Pancreatic cancer. N Engl J Med. 2010; 362:1605-17.
7. Bao Y, Spiegelman D, Li R, Giovannucci E, Fuchs CS and Michaud DS. History of peptic ulcer disease and pancreatic cancer risk in men. Gastroenterology. 2010; 138:541-9.
8. Klinkenberg-Knol EC, Festen HP, Jansen JB, Lamers CB, Nelis F, Snel P, Luckers A, Dekkers CP, Havu N and Meuwissen SG. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med. 1994; 121:161-7. 9. Ligumsky M, Lysy J, Siguencia G and Friedlander Y. Effect of long-term, continuous
versus alternate-day omeprazole therapy on serum gastrin in patients treated for reflux esophagitis. J Clin Gastroenterol. 2001; 33:32-5.
10. Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N and Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998; 115:275-80.
oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006; 55:1538-44.
12. Chao C and Hellmich MR. Gastrin, inflammation, and carcinogenesis. Curr Opin Endocrinol Diabetes Obes. 2010; 17:33-9.
13. Smith JP, Liu G, Soundararajan V, McLaughlin PJ and Zagon IS. Identification and characterization of CCK-B/gastrin receptors in human pancreatic cancer cell lines. Am J Physiol. 1994; 266:R277-83.
14. Smith JP, Fantaskey AP, Liu G and Zagon IS. Identification of gastrin as a growth peptide in human pancreatic cancer. Am J Physiol. 1995; 268:R135-41.
15. Lai SW, Liao KF, Liao CC, Muo CH, Liu CS and Sung FC. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore). 2010; 89:295-9.
16. Lai SW, Muo CH, Liao KF, Sung FC and Chen PC. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in taiwan. Am J Gastroenterol. 2011; 106:1697-704.
17. Liao KF, Lai SW, Li CI and Chen WC. Diabetes mellitus correlates with increased risk of pancreatic cancer: a population-based cohort study in Taiwan. J Gastroenterol Hepatol. 2012; 27:709-13.
18. Bradley MC, Murray LJ, Cantwell MM and Hughes CM. Proton pump inhibitors and histamine-2-receptor antagonists and pancreatic cancer risk: a nested case-control study. Br J Cancer. 2012; 106:233-9.
19. Parsa I, Marsh WH and Sutton AL. An in vitro model of human pancreas carcinogenesis: effects of nitroso compounds. Cancer. 1981; 47:1543-51.
Table 1:Characteristics between pancreatic cancer cases and control subjects Pancreatic cancer No N = 3908 Yes N = 977 n (%) n (%) p-value Gender Men 2368 60.59 592 60.59 0.99 Women 1540 39.41 385 39.41
Age group (years)
20-39 5 0.13 1 0.10 0.94
40-64 1296 33.16 329 33.67
≥ 65 2607 66.71 647 66.22
Age (Mean and SD, years)* 68.11 11.16 68.38 11.24 0.50
Co-morbidities before index date
Acute pancreatitis 34 0.87 153 15.66 <0.0001 Chronic pancreatitis 11 0.28 58 5.94 <0.0001 Diabetes mellitus 773 19.78 370 37.87 <0.0001 Obesity 38 0.97 41 4.20 <0.0001 Gallstones 176 4.50 56 5.73 0.11 Hepatitis C 78 2.00 23 2.35 0.48
Medications (ever use)
Proton pump inhibitors 521 13.33 619 63.36 <0.0001
Using duration of proton pump
inhibitors (mean ± SD, months) * 4.45 6.67 9.50 14.75 <0.0001
Histamine-2 receptor antagonists
2459 62.92 824 83.34 <0.0001
Statins 808 20.68 337 34.49 <0.0001
Non-statin lipid-lowering drugs 524 13.41 212 21.70 <0.0001 Aspirin and cyclooxygenase-2
inhibitors
Aspirin only 725 18.55 146 14.94 <0.0001
Cyclooxygenase-2 inhibitors only 772 19.75 254 26.00
Both of above 1040 26.61 372 38.08
Data are presented as the number of subjects in each group, with percentages given in parentheses.
Table 2: Odds ratio and 95% confidence interval of pancreatic cancer associated with use of
proton pump inhibitors and co-morbidities
Crude Adjusted †
Variables OR (95% CI) OR (95% CI)
Gender (men vs. women) 1.00 (0.87, 1.15) -
-Age (per one year) 1.00 (0.99, 1.01) -
-Co-morbidities before index date (yes vs. no)
Acute pancreatitis 21.16 (14.48, 30.91) 16.32 (10.24, 26.02) Chronic pancreatitis 22.33 (11.68, 42.70) 2.27 (1.00, 5.15) Diabetes mellitus 2.47 (2.13, 2.88) 1.54 (1.27, 1.87) Obesity 4.46 (2.85, 6.98) 2.59 (1.54, 4.36) Gallstones 1.29 (0.95, 1.76) - -Hepatitis C 1.18 (0.74, 1.90) - -Medications
Proton pump inhibitors 11.24 (9.58, 13.18) 9.28 (7.77, 11.08)
Histamine-2 receptor antagonists 3.17 (2.64, 3.82) 1.90 (1.53, 2.35)
Statins 2.02 (1.73, 2.35) 0.97 (0.79, 1.32)
Non-statin lipid-lowering drugs 1.79 (1.50, 2.14) 1.15 (0.91, 1.45) Single treatment on aspirin and/or
cyclooxygenase-2 inhibitors ( vs. non-use of aspirin and non-use of cyclooxygenase-2 inhibitors)
Aspirin only 1.35 (1.07, 1.70) 1.02 (0.79, 1.32)
Cyclooxygenase-2 inhibitors only 2.20 (1.79, 2.70) 1.04 (0.81, 1.34)
Both of above 2.39 (1.98, 2.89) 0.83 (0.65, 1.06)
† Adjusted for acute pancreatitis, chronic pancreatitis, diabetes mellitus, obesity, and histamine-2
receptor antagonists, statins, non-statin lipid-lowering drugs, and both of aspirin and cyclooxygenase-2 inhibitors
Table 3: Risk of pancreatic cancer associated with individual proton pump inhibitors Case/N Crude odds
ratio
(95% CI) Adjusted
odds ratio† (95% CI) Non-use of PPIs as a
reference 358/3745 Reference Reference
Omeprazole 66/137 8.80 (6.18, 12.5) 7.88 (5.46, 11.4)
Pantoprazole 309/492 16.0 (12.9, 19.8) 10.9 (8.61, 13.9)
Lansoprazole 164/272 14.4 (11.0, 18.7) 10.4 (7.78, 13.9)
Rabeprazole 116/205 12.3 (9.16, 16.6) 8.71 (6.28, 12.1)
Esomeprazole 412/639 17.2 (14.1, 20.9) 12.1 (9.76, 15.0)
†Adjusted for acute pancreatitis, chronic pancreatitis, diabetes mellitus, obesity, histamine-2
receptor antagonists, statins, non-statin lipid-lowering drugs, and both of aspirin and cyclooxygenase-2 inhibitors