ORIGINAL ARTICLE
Poor potential of proliferation and differentiation in bone
marrow mesenchymal stem cells derived from children
with severe aplastic anemia
Yu-Hua Chao&Ching-Tien Peng&Horng-Jyh Harn&
Chin-Kan Chan&Kang-Hsi Wu
Received: 30 June 2009 / Accepted: 21 December 2009 / Published online: 19 January 2010 # Springer-Verlag 2010
Abstract The pathogenesis of severe aplastic anemia (SAA) has not been completely understood, and insufficiency of the hematopoietic microenvironment can be an important factor. Here, we compared the basic properties of mesenchymal stem cells (MSCs), a major component of bone marrow microen-vironment, from five SAA children with those of MSCs from five controls. Although MSCs from SAA children and controls were similar in morphology and immunophenotypic profile, SAA MSCs had slower expansion rate and smaller cumulative population doubling (1.83±1.21 vs 3.36±0.87; p=0.046), indicating lower proliferative capacity. After osteogenic induction, SAA MSCs showed lower alkaline
phosphatase activity (optical density, 1.46±0.04 vs 2.27± 0.32;p=0.013), less intense von Kossa staining, and lower gene expression of core binding factorα1 (0.0015±0.0005 vs 0.0056±0.0017;p=0.013). Following adipogenic induction, SAA MSCs showed less intense Oil red O staining (optical density, 0.86±0.22 vs 1.73±0.42; p=0.013) and lower lipoprotein lipase expression (0.0105±0.0074 vs 0.0527± 0.0254; p=0.013). These findings provided evidence that defects in bone marrow MSCs of SAA children do exist.
Keywords Aplastic anemia . Mesenchymal stem cells . Hematopoiesis . Bone marrow failure . Microenvironment
Y.-H. Chao
Department of Pediatrics, Tungs’ Taichung MetroHarbor Hospital,
Taichung, Taiwan Y.-H. Chao
Graduate Institute of Clinical Medical Science, China Medical University,
Taichung, Taiwan
C.-T. Peng
:
K.-H. WuDepartment of Pediatrics, China Medical University Hospital, Taichung, Taiwan
C.-T. Peng
Department of Biotechnology and Bioinformatics, Asia University,
Taichung, Taiwan H.-J. Harn
Center for Neuropsychiatry, China Medical University Hospital, Taichung, Taiwan
H.-J. Harn
Department of Pathology, China Medical University Hospital, Taichung, Taiwan
C.-K. Chan
Graduate Institute of Clinical Medical Science, Chang Gung University,
Taoyuan, Taiwan
C.-K. Chan
Department of Pediatrics, Taoyuan General Hospital, Taoyuan, Taiwan
K.-H. Wu
Stem Cell Research Laboratory, Department of Medical Research, China Medical University Hospital,
Taichung, Taiwan
K.-H. Wu (*)
No. 2 Yuh-Der Road North District, Taichung 404, Taiwan
Introduction
Childhood acquired aplastic anemia, characterized by failure of hematopoiesis, is rare and potentially life-threatening with an annual incidence of one to six per million [1–3]. Severe aplastic anemia (SAA) is defined as profound bone marrow (BM) hypocellularity and marked peripheral blood pancyto-penia. Despite of many putative etiologies, a specific cause cannot be identified in most children and is termed “idiopathic SAA.” Significant advances have been made in the management of the disease, including allogeneic stem cell transplantation and immunosuppressive therapy [2–4]. However, the mechanism by which idiopathic SAA develops has not been completely elucidated. Although many studies demonstrated the association of immune-mediated pathogen-esis, up to 30% of patients do not have detectable evidence for an underlying immune basis and not respond to immunosuppressive therapy [2]. Therefore, other mechanisms do exist.
Mesenchymal stem cells (MSCs), first described by Friedenstein et al. [5], play an important role in providing the specialized BM microenvironment for hematopoietic stem cell (HSC) survival and differentiation [6–11]. MSC dysfunction may result in the impairment of hematopoiesis, but data focusing on the role of MSCs in the pathophys-iology of SAA are limited [12–16]. As the so-called stem cells, MSCs are capable of proliferating and differentiating into mesenchyme-lineage cells, and many studies used these properties as indicators to assess MSCs. Until now, no information about the basic characteristics of morphology, immunophenotyping, proliferative capacity, and differenti-ation potential of SAA MSCs has been reported. In order to clarify pathophysiology of SAA and to identify character-istic changes of SAA MSCs, we compared these basic properties of BM MSCs derived from SAA children and controls.
Design and methods
Materials
BM cells were obtained from iliac crest aspirates. Idiopathic SAA was defined as pancytopenia and hypocellular BM after excluding any other underlying diseases. To diagnose SAA, BM cellularity of less than 25% and at least two of the following criteria must be fulfilled: absolute neutrophil count less than 0.5×109/l, platelet count less than 20×109/l, and reticulocyte less than 1% [2, 3,17]. Control subjects were patients who received BM examination for diseases other than hematological diseases with pathological proof of normal BM. All patients were previously untreated and aged less than 18 years old. The institutional review
board of Tungs’ Taichung MetroHarbor Hospital ap-proved this protocol, and written informed consents were obtained from the parents or legal guardians of the patients.
Cell culture
Mononuclear cells were isolated from BM aspirates by Ficoll-Paque density centrifugation (1.077 g/ml; Amersham Biosciences, Uppsala, Sweden) and then seeded in low-glucose Dulbecco’s modified Eagle medium (DMEM; Gibco, Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% antibiotic– antimycotic (Gibco). Cells were incubated at 37°C with 5% CO2in a humidified atmosphere. After 48 h, medium
with suspension of nonadherent cells was discarded, and fresh medium was added. Thereafter, medium was replaced twice a week. When reaching 80–90% conflu-ence, cells were detached with 0.25% trypsin–EDTA (Gibco) and replated at a concentration of 8.5×103/cm2 in 10-cm dishes.
Immunophenotypic analysis
Cultured MSCs (passage 4) were detached, washed, and resuspended in phosphate-buffered saline (Gibco). After fixing and blocking, the cells were immunolabeled with the following mouse antihuman antibodies: fluorescein isothiocyanate-conjugated CD45 (FITC-CD45; BD Biosciences, San Jose, CA, USA), phycoerythrin-conjugated CD14 (PE-CD14; BD Biosciences), FITC-CD34 (BD Biosciences), FITC-CD105 (Serotec, Oxford, UK), PE-CD73 (BD Pharmigen, San Diego, CA, USA), and FITC-CD44 (BD Pharmigen). The nonspecific mouse IgG (BD Biosciences) served as isotype control. Data were analyzed by flow cytometry (FACSCalibur; BD Bio-sciences) with CellQuest software.
Proliferative capacity
Yield of cells at each passage was enumerated using Trypan blue (Gibco) to exclude dead cells. The population doubling (PD) of cultured MSCs was calculated according to the equation: PD=log2 (the number of viable cells at
harvest/the number of seeded cells). The cumulative PD was the sum of PD from passages 4 to 6.
Osteogenic and adipogenic potential
To evaluate differentiation potential, cultured MSCs (third passage, at 80–90% confluence) were subjected to osteogenic and adipogenic differentiation in vitro. Cells were detached from culture dishes and replated in 60-mm dishes for further studies.
To promote osteogenic differentiation, cells were incu-bated in DMEM supplemented with 10% FBS, 10 mM β-glycerophosphate (Sigma, St Louis, MO, USA), 0.1 μM dexamethasone (Sigma), and 0.2 mM ascorbic acid (Sigma) for 3 weeks. On day 21, cultures were stained for alkaline phosphatase (ALP; Sigma) activity and mineralized deposits were detected by von Kossa stain (Cedarlane, Ontario, Canada). To quantify ALP activity, 2 ml of 0.05 N NaOH in ethanol was added to each dish after ALP activity stain, and the extraction was measured by spectro-photometry (Ultrospec 1100 pro; Amersham Biosciences) at 550 nm.
For induction of adipogenic differentiation, cells were grown in DMEM supplemented with 10% FBS, 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine (Sigma), 0.1 mM indomethacin (Sigma), and 10μg/ml insulin (Novo Nordisk A/S, Bagsværd, Denmark) for 2 weeks. On day14, adipogenic differentiation was demonstrated by intracellular accumulation of lipid droplets stainable with oil red O (Sigma). The dye content was eluted by ethanol and quantified spectrophotometrically.
Differentiation was verified further by real-time polymerase chain reaction (Q-PCR) for the assessment of lineage-specific genes as core binding factorα1 (Cbfa1) for osteocytes and lipoprotein lipase for adipocytes. MSCs cultured in osteogenic and adipogenic induction medium were harvested on days21 and 14, respectively. Total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instruction. Concentration of the RNA samples was estimated spectrophotometrically at OD 260/280, and cDNA was synthesized using MMLV reverse transcriptase (Epicenter Biotechnologies, Madison, WI, USA) in the presence of oligo-dT primer (Promega, Madison, WI, USA). The sequences of PCR primers were as follows: Cbfa1 [18], sense CATGGCGGGTAACGATGAA-3′ and antisense 5′-CGGCCCACAAATCTCAGATC-3′; lipoprotein lipase [19], sense 5′-ATGGAGAGCAAAGCCCTGCTC-3′ and antisense 5′-TACAGGGCGGCCACAAGTTTT-3′. The expression of β-actin (sense 5′-TGTGGATCAGCAAGCAGGAGTA-3′ and antisense 5′-CAAGAAAGGGTGTAACGCAACTAAG-3′) was used as an internal control to normalize specific gene expression in each sample [18]. Q-PCR was performed using cDNA samples with SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA) and carried out in the ABI 7300 Real-Time PCR system (Applied Biosystems).
Statistical analysis
Data analysis was performed using SPSS 14.0 for Windows. Kolmogorov–Smirnov Z test was used for comparison of the two groups. Statistical value of p<0.05 was considered significant.
Results
Morphology and immunophenotypic profile
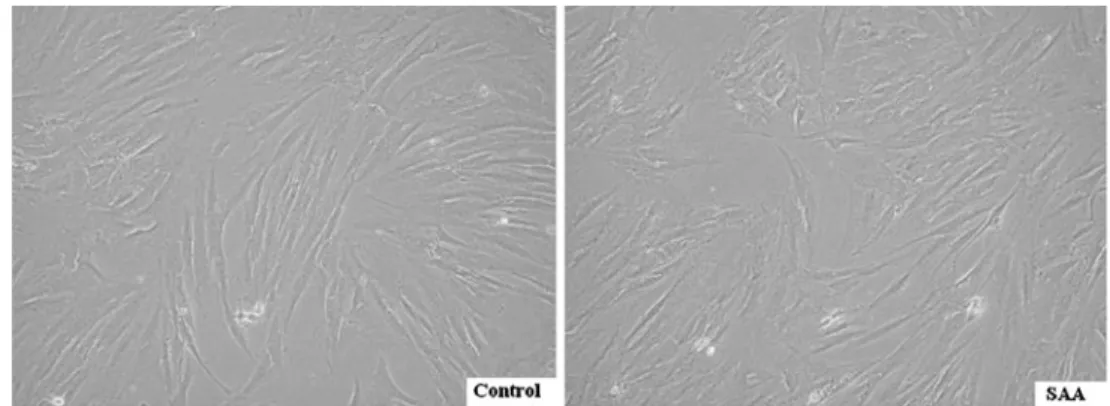
A total of ten children, five SAA patients and five controls, were enrolled in the study. Clinical data of these children are outlined in Tables 1 and 2, and all BM aspirates were obtained at the time of diagnosis without concomitant medication. The average age was 11.9 and 11.6 years old, respectively. In vitro, MSCs of SAA and control group shared a similar spindle-shaped morphology (Fig.1). Both revealed a consistent immunophenotypic profile which was negative for CD45, CD14, and CD34, and positive for CD105, CD73, and CD44 (Table 3). No significant difference was noted in the expression of any single surface marker between the two groups.
Proliferative capacity
To prevent hematopoietic cell contamination, which might be present in earlier passages, or the presence of senescent or differentiating MSCs in later passages, we used cells from passages 4 to 6 for the study of growth kinetics. SAA group had slower expansion rate than control group, shown as average PD of each passage (Fig. 2a). Two of five cultures of SAA group stopped proliferating at passages 5 and 6, respectively, whereas all cultures of control group continued to grow well into passage 9. Lower proliferation potential of SAA MSCs was also demonstrated by smaller cumulative PD from passages 4 to 6 (1.83±1.21 vs 3.36± 0.87;p=0.046; Fig.2b).
Differentiation potential
When exposed to osteogenic induction medium, MSCs from SAA children had less robust osteogenic differentia-tion than MSCs from controls as shown by lower ALP activity (Fig.3a). Greater extent of mineralization in control group was also demonstrated by more intense von Kossa stain (Fig. 3b). Under adipogenic condition, SAA MSCs gave rise to less lipid-containing cells. The intracytoplasmic vacuoles of neutral fat can be identified by Oil red O stain (Fig. 3c). The droplets of fat were more and larger within a single adipocyte in control group. Quantitation of dye content of ALP activity stain in the osteogenic cultures (optical density, 1.46±0.04 vs 2.27±0.32; p=0.013) and Oil red O stain in the adipogenic cultures (optical density, 0.86±0.22 vs 1.73±0.42;p=0.013) confirmed the histochemical obser-vations (Fig.4).
Results of Q-PCR analysis presented in Fig.5confirmed the findings of histochemical stains further. After osteogenic induction, MSCs of SAA group expressed lower level of Cbfa1 than MSCs of control group (0.0015±0.0005 vs
0.0056±0.0017;p=0.013). Following adipogenic induction, SAA MSCs showed lower lipoprotein lipase expression (0.0105±0.0074 vs 0.0527±0.0254; p=0.013). The results of Q-PCR analysis were consistent with histochemical findings. However, the proliferative capacity and differenti-ation potential of SAA MSCs from an individual patient did not decrease at the same degree. Probably, it is hard to draw conclusions with only five patients studied, but in our study there was no correlation between the in vitro proliferation or differentiation potential and the response to immunosuppres-sive therapy.
Discussion
MSCs can be characterized by a panel of surface markers, by their in vitro growth pattern and subsequent expansion, and by their multilineage differentiation potential [6,8,9]. Many studies used these basic properties as indicators to identify MSCs from origins other than BM [18–28]. We aimed at these properties of BM MSCs from SAA children and found that SAA MSCs had poor potential of prolifer-ation and differentiprolifer-ation. The alterprolifer-ations may contribute to failure of hematopoiesis and lead to the development of the disease.
The immune-mediated HSC destruction for the patho-genesis of idiopathic SAA has been widely accepted, and many studies have been devoted to the role of T cells in this disease [2,4,29–31]. Dubey et al. found elevated levels of interferon gamma and tumor necrosis factor alpha (TNF-α)
in BM plasma of SAA patients [29], and these cytokines can induce apoptosis of CD34+ BM cells [32]. Hara et al. demonstrated excessive production of TNF-α by BM T cells and higher sensitivity of HSCs to TNF-α in patients with SAA [30]. However, up to 30% of patients have no immune-associated evidence and respond poorly to immu-nosuppressive therapy [2]. Pathogenesis of SAA remains to be determined.
Primary HSC deficiency, including decrease in number and dysfunction, has also been proposed to account for the development of SAA [33–36]. In vitro long-term BM culture provided evidence for primary HSC dysfunction in the regenerative capacity and in the response to various cytokine stimuli [35,36]. Abnormal telomere shortening of HSCs was found in some patients with SAA [34]. However, allogeneic HSC transplantation cannot cure all patients, suggesting that other mechanisms exist.
Another important concept proposed in the context of SAA is related to deficiency or dysfunction of BM microenvironment. Marrow stromal cells derived from MSCs, including fibroblasts, endothelial cells, and adipocytes, exert the regulatory role in hematopoiesis. They provide an appropriate scaffold and a complex network of cytokines, adhesion molecules, and extracellular matrix proteins [6–11]. Many studies have reported the promotive effect of MSCs for HSC expansion in vitro [10,36–40]. In 2000, Koc et al. found rapid hematopoietic recovery after coinfusion of autologous MSCs at the time of HSC transplantation [41]. Accordingly, Lazarus et al. presented a multicenter trial of 46 patients receiving allogeneic HSCs and MSCs from
HLA-Table 1 Clinical characteristics of five children with severe aplastic anemia
Patient no. Sex Age (years) Treatment Outcome
A1 F 11.6 IST Hematopoiesis recovery after IST
A2 F 11.3 IST; HSCT from MUD No response to IST; hematopoiesis recovery after HSCT with mild chronic GVHD
A3 F 14.7 IST; HSCT from MUD No response to IST; hematopoiesis recovery after HSCT
A4 M 10 IST Hematopoiesis recovery after IST
A5 F 12.2 IST Hematopoiesis recovery after IST
F female, M male, IST immunosuppressive therapy, HSCT hematopoietic stem cell transplantation, MUD matched unrelated donor, GVHD graft versus host disease
Table 2 Clinical characteristics of five controls
Patient no. Sex Age (years) Diagnosis Medication at time of bone marrow examination
C1 M 16.3 Rhabdomyosarcoma Nil
C2 F 5.9 Ewing’s sarcoma Nil
C3 F 15 Rhabdomyosarcoma Nil
C4 M 2.8 Hepatoblastoma Nil
C5 M 17.8 Kikuchi disease Nil
Fig. 1 Morphology. In vitro culture, MSCs of five controls and five SAA children shared a similar spindle-shaped morphology. Magnification of micrographs ×100
Control (n=5) SAA (n=5)
Median (%) Range (%) Median (%) Range (%)
CD45+ 1.47 0.84–2.44 1.92 0.60–3.22 CD14+ 1.09 0.64–1.42 0.94 0.38–2.42 CD34+ 1.17 1.07–1.46 1.63 0.13–2.60 CD105+ 94.46 92.57–95.29 92.86 84.66–96.66 CD73+ 96.55 92.74–97.84 94.59 90.42–95.65 CD44+ 90.86 85.74–97.11 87.21 82.40–94.39 Table 3 Immunophenotypic analysis of mesenchymal stem cells from children with SAA and controls
Fig. 2 Proliferative capacity. a Average PD of the two groups
(n=5 for each group). b
Com-parison of cumulative PD from passages 4 to 6, shown as mean and 95% confidence interval.
Eachcircle represents a subject
identical siblings, and found prompt hematopoietic recovery in most patients, suggesting that the beneficial effect of MSCs on engraftment may relate to their supportive role in the hematopoiesis [42].
In this study, we demonstrated defects of BM MSCs from SAA children. Although the morphology and surface marker expression of cultured MSCs did not change, SAA MSCs had slower expansion rate and smaller cumulative PD, indicating lower proliferation potential. Besides, only three of five cultures of SAA group retained the ability to grow confluent layers into passage 6, suggesting earlier senescence of SAA MSCs. The significant decrease in osteogenic and adipogenic potential of SAA MSCs was obvious. Even the three cultures which can proliferate beyond passage 6 showed less intense stains after differen-tiation under permissive conditions. The results of gene expression study confirmed the findings of histochemical stains.
Several studies have been conducted on the relationship between MSCs and SAA [12–16]. Hott et al. used long-term culture system to analyze the ability of BM stromal cells to support hematopoiesis and found that stromal layers from three of nine SAA patients failed to maintain normal HSCs [16]. Holmberg et al. evaluated stromal cell function in long-term marrow culture and observed that 6.8% of SAA marrow failed to grow any stromal cells and 42.5% failed to reach complete confluent stromal layers [15]. Bacigulupo et al. found that BM MSCs of SAA patients were deficient in their ability to suppress T cell proliferation and cytokine release, suggesting the lack of MSC immu-noprotection in SAA BM [13]. Wu et al. investigated GATA gene expression of MSCs from chronic aplastic anemia patients and proposed that aberrant expression of these genes in BM MSCs may influence the BM microen-vironment and lead to abnormal hematopoietic regulation [12]. In the current study, we examined the basic character-istics of MSCs and found poor potential of proliferation and differentiation in BM MSCs derived from SAA children. The above studies provide strong evidence for MSC defects in SAA BM.
Rubinstein et al. reported 562 recipients of cord blood HSC transplantation and observed successful engraftment reduced significantly among SAA patients [43]. It is of interest that cord blood MSCs exhibit higher proliferation capacity than bone marrow MSCs in vitro culture [22]. On the other hand, several investigations have found that MSCs cannot be acquired efficiently from umbilical cord blood, suggesting that MSCs are sparse in cord blood [20,
Fig. 3 Osteogenic and adipogenic potential of MSCs from five controls and five SAA children. Osteogenic differentiation was demonstrated by ALP activity a and von Kossa stain b after 3-week induction. Adipogenic differentiation was demonstrated by Oil red O stain c after 2-week induction. Magnification of micrographs ×100
24,26]. As we propose here, MSC defects in SAA patients lead to insufficiency of BM hematopoietic microenvironment. It can be assumed that the lower number of MSCs provided by cord blood during transplantation is the cause of engraftment failure in SAA patients despite of the higher proliferative capacity of cord blood MSCs. Therefore, cotransplant of MSCs and HSCs could be a potential strategy to treat SAA patients.
The present study was of course limited by the small number of patients and the diseases of controls. However, according to our results, BM MSCs derived from children
with SAA had poor potential of proliferation and differen-tiation and these alterations may be important in the pathogenesis of the disease. Subsequently, we try to find genes that participate in proliferation or differentiation of MSCs and express aberrantly in SAA patients. Microarray will be used to find candidate genes, and their expression will be verified further by Q-PCR. Knockdown or knockout experiments will demonstrate their role in the development of the disease. Additional research is required for further understanding of the pathophysiology of SAA and leads to the development of novel treatment modalities.
Fig. 4 Comparison of osteogenic and adipogenic potential by quantification of ALP activity (a) and Oil red O (b) stain spectrophotometrically,
respectively. Mean and 95% confidence interval are illustrated. Eachcircle represents a subject studied
Fig. 5 Comparison of differentiation potential by lineage-specific gene expression, Cbfa1 for osteogenesis (a) and lipoprotein lipase for
Acknowledgments This work was supported partially by grants from the China Medical University Hospital (grant ref. NO. DMR-98-037) and Taoyuan General Hospital Project (PTH9809).
References
1. Montane E, Ibanez L, Vidal X, Ballarin E, Puig R, Garcia N, Laporte J-R, Catalan Group for Study of Agranulocytosis and Aplastic Anemia (2008) Epidemiology of aplastic anemia: a
prospective multicenter study. Haematologica 93:518–523
2. Davies JK, Guinan EC (2007) An update on the management of severe idiopathic aplastic anaemia in children. Br J Haematol
136:549–564
3. Kurre P, Johnson FL, Deeg HJ (2005) Diagnosis and treatment of
children with aplastic anemia. Pediatr Blood Cancer 45:770–780
4. Young NS, Calado RT, Scheinberg P (2006) Current concepts in the pathophysiology and treatment of aplastic anemia. Blood
108:2509–2519
5. Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16:381–390
6. Lazennec G, Jorgensen C (2008) Concise review: adult multi-potent stromal cells and cancer: risk or benefit? Stem Cells 26:1387– 1394
7. Sorrentino A, Ferracin M, Castelli G, Biffoni M, Tomaselli G, Baiocchi M, Fatica A, Negrini M, Peschle C, Valtieri M (2008) Isolation and characterization of CD146+ multipotent mesenchymal
stromal cells. Exp Hematol 36:1035–1046
8. Tocci A, Forte L (2003) Mesenchymal stem cell: use and
perspectives. Hematol J 4:92–96
9. Deans RJ, Moseley AB (2000) Mesenchymal stem cells: biology
and potential clinical uses. Exp Hematol 28:875–884
10. Verfaillie CM (1993) Soluble factor(s) produced by human bone marrow stroma increase cytokine-induced proliferation and matura-tion of primitive hematopoietic progenitors while preventing their
terminal differentiation. Blood 82:2045–2053
11. Gordon MY (1988) Extracellular matrix of the marrow microen-vironment. Br J Haematol 70:1–4
12. Wu X, Li Y, Zhu K, Wang Z, Chen S, Yang L (2007) Gata-1, -2 and -3 genes expression in bone marrow microenvironment with chronic aplastic anemia. Hematology 12:331–335
13. Bacigalupo A, Valle M, Podesta M, Pitto A, Zocchi E, De Flora A, Pozzi S, Luchetti S, Frassoni F, Van Lint MT, Piaggio G (2005) T-cell suppression mediated by mesenchymal stem cells is deficient in patients with severe aplastic anemia. Exp Hematol
33:819–827
14. Scopes J, Ismail M, Marks KJ, Rutherford TR, Draycott GS, Pocock C, Gordon-Smith EC, Gibson FM (2001) Correction of stromal cell defect after bone marrow transplantation in aplastic
anaemia. Br J Haematol 115:642–652
15. Holmberg LA, Seidel K, Leisenring W, Torok-Storb B (1994) Aplastic anemia: analysis of stromal cell function in long-term
marrow cultures. Blood 84:3685–3690
16. Hotta T, Kato T, Maeda H, Yamao H, Yamada H, Saito H (1985) Functional changes in marrow stromal cells in aplastic anaemia. Acta Haematol 74:65–69
17. Camitta BM, Thomas ED, Nathan DG, Santos G, Gordon-Smith EC, Gale RP, Rappeport JM, Storb R (1976) Severe aplastic anemia: a prospective study of the effect of early marrow
transplantation on acute mortality. Blood 48:63–70
18. Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM (2006) Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood.
Stem Cells 24:679–685
19. Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC (2006) Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 91:1017–1026
20. Secco M, Zucconi E, Vieira NM, Fogaca LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M (2008) Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells 26:146–150
21. Baksh D, Yao R, Tuan RS (2007) Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells
25:1384–1392
22. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow,
umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301
23. Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD (2005) Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord
blood. Exp Hematol 33:1402–1416
24. Yu M, Xiao Z, Shen L, Li L (2004) Mid-trimester fetal blood-derived adherent cells share characteristics similar to mesenchymal stem cells but full-term umbilical cord blood does not. Br J Haematol 124:666– 675
25. Wang H-S, Hung S-C, Peng S-T, Huang C-C, Wei H-M, Guo Y-J, Fu Y-S, Lai M-C, Chen C-C (2004) Mesenchymal stem cells in
the Wharton’s jelly of the human umbilical cord. Stem Cells
22:1330–1337
26. Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM (2003) Adult bone marrow is a rich source of human
mesenchymal‘stem’ cells but umbilical cord and mobilized adult
blood are not. Br J Haematol 121:368–374
27. in’t Anker PS, Noort WA, Scherjon SA, Kleijburg-van der Keur
C, Kruisselbrink AB, van Bezooijen RL, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE (2003) Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica 88:845–852 28. Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402
29. Dubey S, Shukla P, Nityanand S (2005) Expression of interferon-gamma and tumor necrosis factor-alpha in bone marrow T cells and their levels in bone marrow plasma in patients with aplastic
anemia. Ann Hematol 84:572–577
30. Hara T, Ando K, Tsurumi H, Moriwaki H (2004) Excessive production of tumor necrosis factor-alpha by bone marrow T lymphocytes is essential in causing bone marrow failure in
patients with aplastic anemia. Eur J Haematol 73:10–16
31. Hirano N, Butler MO, Von Bergwelt-Baildon MS, Maecker B, Schultze JL, O’Connor KC, Schur PH, Kojima S, Guinan EC, Nadler LM (2003) Autoantibodies frequently detected in patients with aplastic anemia. Blood 102:4567–4575
32. Maciejewski J, Selleri C, Anderson S, Young NS (1995) Fas antigen expression on CD34+ human marrow cells is induced by interferon gamma and tumor necrosis factor alpha and potentiates
cytokine-mediated hematopoietic suppression in vitro. Blood 85:3183–3190
33. Rizzo S, Scopes J, Elebute MO, Papadaki HA, Gordon-Smith EC, Gibson FM (2002) Stem cell defect in aplastic anemia: reduced long term culture-initiating cells (LTC-IC) in CD34+ cells isolated from aplastic anemia patient bone marrow. Hematol J 3:230–236 34. Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC (1998) Progressive telomere shortening in aplastic
35. Scopes J, Daly S, Atkinson R, Ball SE, Gordon-Smith EC, Gibson FM (1996) Aplastic anemia: evidence for dysfunctional bone marrow progenitor cells and the corrective effect of granulocyte
colony-stimulating factor in vitro. Blood 87:3179–3185
36. Marsh JC, Chang J, Testa NG, Hows JM, Dexter TM (1990) The hematopoietic defect in aplastic anemia assessed by long-term marrow culture. Blood 76:1748–1757
37. Li N, Feugier P, Serrurrier B, Latger-Cannard V, Lesesve J-F, Stoltz J-F, Eljaafari A (2007) Human mesenchymal stem cells improve ex vivo expansion of adult human CD34+ peripheral blood progenitor cells and decrease their allostimulatory capacity.
Exp Hematol 35:507–515
38. Van Overstraeten-Schlogel N, Beguin Y, Gothot A (2006) Role of stromal-derived factor-1 in the hematopoietic-supporting activity
of human mesenchymal stem cells. Eur J Haematol 76:488–493
39. Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, Tang P, Mao N (2004) Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells
from cord blood CD34+ cells. Exp Hematol 32:657–664
40. Wang J-F, Wang L-J, Wu Y-F, Xiang Y, Xie C-G, Jia B-B, Harrington J, McNiece IK (2004) Mesenchymal stem/progenitor
cells in human umbilical cord blood as support for ex vivo expansion of CD34(+) hematopoietic stem cells and for chondrogenic
differen-tiation. Haematologica 89:837–844
41. Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM (2000) Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 18:307–316
42. Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR Jr, Moseley AB, Bacigalupo A (2005) Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in
hemato-logic malignancy patients. Biol Blood Marrow Transplant 11:389–
398
43. Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, Berkowitz RL, Cabbad M, Dobrila NL, Taylor PE, Rosenfield RE, Stevens CE (1998) Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N