δ
13C and N Contents of Two Aquatic Plants, Spaganium fallax and
Schenoplectus mucronatus, in a Subtropic Mountainous Lake
Wen-Yuan Kao(1,2*)
1. Institute of Ecology and Evolutionary Biology, 1 Roosevelt Rd., Sec. 4, Taipei 106, Taiwan. 2. Department of Life Science, 1 Roosevelt Rd., Sec. 4, Taipei 106, Taiwan.
* Coresponding author. Email: wykao@ntu.edu.tw
(Manuscript received 3 September 2009; accepted 2 November 2009)
ABSTRACT: Spaganium fallax and Schoenoplectus mucronatus subsp. robustus are emergent monocot plants dominating a subtropical mountainous lake, the Yuanyang Lake (YYL), in Taiwan. The photosynthetic pathways and the ecophysiology of these two species were studied in this study. I first analyzed δ13C of fractions of S. fallax (submerged leaves and roots of seedlings, and submerged and emergent parts of leaves, and roots of mature plants) and S. mucronatus (emergent culms and roots of mature plants) to identify their photosynthetic pathway. I then investigated monthly variation in δ13C and nitrogen content of emergent parts of the two species during 2003 to evaluate the integrated photosynthetic response. The leaf carbon isotope ratio of both emergent plants was within the range of most C3 plants indicating that they used C3 photosynthetic pathway. Similar pattern of seasonal variation in leaf nitrogen content and δ13C values was found. Spring leaves (or culms in S. mucronatus) had significantly higher leaf nitrogen content and more positive δ13C values than summer leaves (or culms). Consequently, there are significantly positive correlations between δ13C and leaf nitrogen content in both species. The result suggests that changes in photosynthetic capacity might contribute to the seasonal variation in δ13C in both species. In comparison between two species, S. fallax had significantly more positive δ13C and higher leaf nitrogen content than S. mucronatus during most of the sampling months. The higher leaf nitrogen content found in S. fallax might also contribute to its more positive δ13C values than S. mucronatus.
KEY WORDS: Yuanyang Lake Natural Preserve, Spaganium fallax, Schoenoplectus mucronatus subsp. robustus, nitrogen content, stable carbon isotope ratio.
INTRODUCTION
Because of loss of suitable habitats, the number of aquatic plant species worldwide has greatly reduced in the past decades. Improving our understanding of aquatic plants and their habitats is a critical first step in helping to combat the losses. Unfortunately, compared to ecological studies on terrestrial plants, those of aquatic plants are relatively rare. Research on aquatic plants distributed in mountain lake is even less.
Sparganium, a genus of monocot plant containing
about 20 species, is distributed mainly in temperate regions. There is only one species of Sparganium, i.e. S.
fallax, in Taiwan and it is distributed in mountain lakes at
mid elevation (Yang et al., 2001). The Yuanyang Lake (YYL) Nature Preserve is one of the six Taiwan Long-Term Ecological Research sites. One of the reason for declaration of the area as a preserve is to protect the emergent plant S. fallax Graebn. In the lake, S. fallax is neighbored by Schoenoplectus mucronatus subsp.
robustus (Chou et al., 2000), also a monocot plant. Schenoplectus is a genus of about 80 species with a
cosmopolitan distribution. Seven species of
Schenoplectus are reported in Taiwan and are mainly
distributed in wetlands from low to mid elevation (Yang et al., 2001). To my knowledge, the photosynthetic performance of these two species has not been studied.
The objectives of this study were to understand their photosynthetic pathway and to compare the ecophysiology of these two emergent plants in the subtropical mountainous lake, YYL.
Two stable carbon isotopes, 12C and 13C, are found in nature. The relative content of 12C and 13C in a matter is generally expressed as δ13C values. During the process of photosynthetic carboxylation, the carboxylation enzyme ribulose bisphosphate carboxylase (rubisco) discriminates against 13C more than phosphoenol pyruvate carboxylase (O’Leary, 1981; Roeske and O’Leary, 1984). Consequently, C3 plants contain less 13C than C4 plants. Among plants measured, δ13C values of C3 plants vary from -34 to -23 ‰, and those of C4 plants approximately -15 to -7 ‰ (O’Leary, 1988). CAM plants use the same two carboxylating enzymes as do C4 plants. Hence, obligate-CAM species tend to have δ13C values similar to C4 plants, while facultative-CAM plants intermediate between C4 and C3 plants (-22 to -10 ‰). Compared to those of terrestrial plants, δ13C values of submerged plants are more complicated by the possible different forms of C utilized and the variation in δ13C of these sources C (Keeley, 1988). Analyses of δ13C of submerged plants might provide information about their carbon source in addition to the photosynthetic pathways. In addition for providing a powerful method for determining the pathway of CO2 fixation of terrestrial
Fig. 1. The location of Yuanyang Lake Natural Preserve in Taiwan.
plants, stable carbon isotopes analysis has also become a widely used tool in plant physiological ecology after the demonstration that δ13C values in C
3 plants are a reliable long-term measure of the ratio of intercellular (Ci) to ambient (Ca) atmospheric concentrations of CO2 (Farquhar et al., 1982). The ratio of Ci/Ca, estimated from gas exchange measurement, is often used as an indicator of instantaneous photosynthetic water use efficiency (Jones, 1992).
To obtain an integrated picture of physiological and year-round photosynthetic responses of S. fallax and S.
mucronatus in the YYL, in addition to analyze δ13C values of fractions of the two emergent plants, I also investigated monthly variation in the ratio and nitrogen contents in emergent part of the two plants. Specifically, I asked following questions. (1) What are the δ13C values of the two aquatic plants? Are there variation in δ13C in plant parts and compartments of these two aquatic plants? (2) Is there seasonal variation in the δ13C in the emergent part of the two aquatic plants? If so, what factors might contribute to the seasonal variation?
MATERIALS AND METHODS
Schoenoplectus mucronatus subsp. robustus and Sparganium fallax were sampled from the Yuanyang
Lake, Taiwan (Fig. 1). The Yuanyang Lake Natural Preserve (24º 35’ N, 121º 24’ E) is located in the northern part of Hsinchu County in Taiwan. The monthly mean air temperature ranged from 5 to 17.5 ºC, and the mean annual rainfall was ca. 3000 mm (Hwang et al., 1996). The climate of the area is classified as temperate heavy moist (Liu and Hsu, 1973). The Yuanyang Lake (YYL), an oligotropic lake at elevation of 1670m, is at the edge of the Nature Reserve. S. fallax and S.
mucronatus dominated the lake habitat with the former
covering ca. 1.6 ha and the later 0.6 ha of the area (Hwang et al., 1996). In YYL, S. fallax grew in water
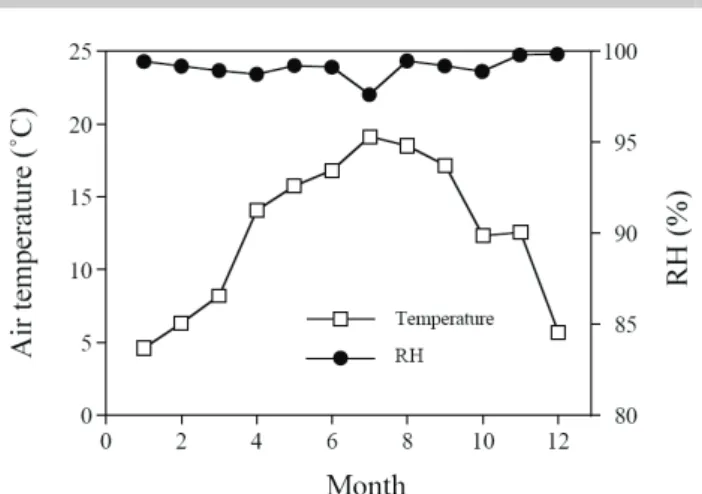
Fig. 2. The monthly average of air temperature and relative humidity at Yuanyang Lake, 2003.
less than 2 m deep, seedlings were submerged in the water, while mature plants had emergent leaves with some lower part remaining submerged. S. mucronatus populations occupied marsh area and at the borders of the lake (Huang et al., 1996). In contrast to S. fallax, most of populations of S. mucronatus with only roots immersed in the water, while shoot were above water surface. The leaves of S. mucronatus are much reduced and culms are the dominant part (Yang et al., 2001).
A survey was conducted in June of 2002 to determine the δ13C values and to investigate if there was variation in δ13C among organs and different growth stage of both plants. Submerged seedlings (n = 3) of S. fallax were sampled and fractionated into submerged leaves and roots while mature plants (n = 3) into emergent part and submersed part of leaves, and roots. In contrast, due to most of populations of S. mucronatus with only roots immersed in the water, while shoot (mainly culm) above water surface, mature plants (n =3) were fractionated into emergent culms and roots. Fraction of samples were rinsed with distilled water, oven dried (60 ºC) for at least 48 hr and then ground to a fine powder with an electric grinder.
To investigate if there was seasonal variation in leaf δ13C, five fully developed, healthy and emergent part from five populations of each species were collected monthly in 2003. Leaves were processed as described above. The air temperature and relative humidity were recorded (Fig. 2) in a meteorological station established in 1992.
Stable carbon isotope and leaf nitrogen content analyses were conducted by a continuous flow system in which an elemental analyzer (NA 1500, Fison, Italy) was connected to an isotope ratio mass spectrometer (Delta S, Finnigan Mat, Germany). δ13C were calculated as: δ13C (‰) = [ (R
sample / Rstandard) – 1] * 1000, where R is the ratio of heavy-to-light isotope. The universally agreed standard for 13C is Pee Dee Belemnite (R = 0.011237) (Hayes, 1983).
Table 1. Mean 13C (‰) values of fractions of sympatric S.
fallax and S. mucronatus plants in Yuanyang Lake, Taiwan.
Plant Mean ± s.e.
S. fallx Seedling Submerged leaf -27.2 ± 0.3a Root -26.5 ± 0.3a Mature plant Emergent leaf -28.6 ± 0.4a Submerged leaf -28.2 ± 0.7ac Root -28.0 ± 0.6ac S. mucronatus Emergent culm -30.8 ± 0.3b Roots -29.3 ± 0.1bc
Means followed by different superscripts are significant at P = 0.05 (Tukey’s test)
RESULTS
δ13C of plant fractions: Table 1 shows δ13C of fractions of S. fallax and S. mucronatus. In S. fallax, no significant difference was found in δ13C values among submerged leaves of seedlings, emergent and submerged leaves of mature plants.
In both species roots had slightly more positive δ13C values than emergent parts, the enrichment was 1-2 ‰ (average 1.5 ‰) in S. mucronatus, while 0.1-1.0 ‰ (average 0.6 ‰) in S. fallax.
In comparison between roots or emergent parts of two species, S. fallax had significantly more positive δ13C values than S.mucronatus.
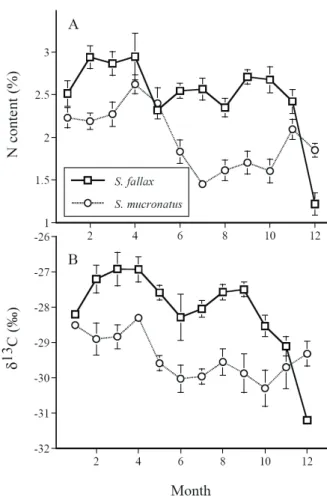
Monthly variation in leaf N content and δ13C: Seasonal variation in nitrogen content and δ13C values of emergent parts was found and the pattern was similar between the two species (Fig. 3). In general, leaves (or culms in S. mucronatus) had higher N content in Spring (Feb., March and April) than in Summer months (June, July and August) (Fig. 3A). The difference in N contents between the two seasons was greater in S. mucronatus than that in S. fallax. In comparison of emergent parts between two species, S. fallax had significantly higher N content than S .mucronatus during most of the sampling months, except in January, April and May both species had similar N contents, while in December S. fallax showed significantly lower N contents than S.
mucronatus.
Both species also had seasonal variation in δ13C values of emergent parts (Fig. 3B), the pattern was similar to that of nitrogen content. Emergent part had more positive δ13C in spring than in summer. In comparison between two species, S. fallax had significantly more positive δ13C than S. mucronatus during most of the sampling months, except in December during the period S. fallax showed significantly more negative δ13C than S. mucronatus.
Fig. 3. The monthly average of leaf (or culm) nitrogen contents (A) and δ13C value (B) of S. fallax and S.
mucronatus in Yuanyang Lake, 2003.
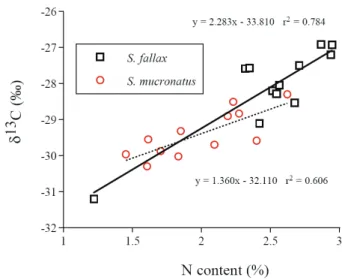
The relationship between N contents and δ13C: When δ13C of emergent parts was plotted against corresponding nitrogen content, significantly positive, linear relationships were found between monthly average N content and δ13C for both species (Fig. 4).
DISCUSSION
Though some aquatic plants have been shown to utilized CAM or C4 photosynthetic pathways when in submerged condition (Robe and Griffiths, 2000, Ueno, 1996), the carbon isotope ratio measured for fractions of both aquatic species (Table 1) or for emergent parts year round (Fig. 3) were all within the range of most C3plants (O’Leary, 1988) indicating that they used C3 photosynthetic pathway. In addition, the result that submerged leaves and emergent leaves of S. fallax had similar δ13C values (Table 1) indicated that the δ13C value of carbon source utilized by submerged leaves was not significantly different from that uptake by emergent leaves.
Fig. 4. Relationships between monthly average of leaf (or culm) nitrogen contents and δ13C value of S. fallax and S.
mucronatus in Yuanyang Lake, 2003.
Both aquatic plants had significantly higher N content in spring than in summer (Fig. 3A). The result is consistent with a previous study by Hwang et al. (1996). The authors found that an increase in nitrogen content of above ground tissue corresponded to the onset of growth of both species in spring. In their study, they also found a similar trend in below ground tissue of the aquatic plants. Accordingly, it is suggested that the increase in nitrogen content of emergent parts in spring was not due to the redistribution of the nutrient between above- and below-ground tissues, however, an increasing in nitrogen uptake might contribute to the result.
Significant differences in leaf δ13C between seasons and between plant species were found in the aquatic plants (Fig. 3B). Farquhar et al. (1982) developed an equation to explain the variation in leaf δ13C value in C
3 plants:
δ13C
leaf = δ13Catm – a – (b-a)* Ci/Ca , where δ13C
atm is the isotopic ratio of CO2 available to leaves, a is the fractionation caused by diffusion (4.4 ‰), b is the fractionation associated with carbon fixation (27 ‰), Ci is the intercellular CO2 concentration and Ca ambient CO2 concentration. According to the equation, δ13C of leaves is enriched when photosynthetic gas exchange is more limited by leaf conductance relative to carboxylation (or photosynthetic demand), i.e. a lower ratio of Ci/Ca. In contrast, if diffusion limitation is reduced, the relative influence of enzymatic discrimination increases favoring 13C depletion during fixation, would result in leaves of more negative δ13C values. Accordingly, leaf δ13C can be used as an indictor reflecting the long-term, integrated effect of factors
affecting inward CO2diffusion (leaf conductance) and CO2 consumption (carboxylation) (Farquhar et al., 1989; Ehleringer et al., 1993). According to the mechanisms of 13C discrimination during photosynthesis, the result that spring leaves (or culms) had more positive δ13C values than summer leaves (or culms) implied that leaves (or culms) in spring time operated at a lower Ci/Ca ratio than those in summer season. A decrease in water availability or a large vapor pressure deficit (VPD) would decrease stomatal conductance and reduce Ci/Ca (Schulze et al., 1987). However, these two conditions do not seem to exist in the study site. Because both plants were rooted in sediment containing saturation water year round, they were unlikely to be limited by soil moisture. A large VPD was not expected in the YYL area where the air humidity approached saturation for most of time during the year (Fig. 2). Hence, the more positive leaf (or culm) δ13C in spring was unlikely resulting from drought or VPD effect. An increase in photosynthetic capacity (carboxylation efficiency) would also result in a reduction in Ci/Ca ratio (Korner et al., 1988). The result that there were significantly positive correlation between monthly mean δ13C and leaf (or culm) nitrogen content in both species (Fig. 4) suggests that seasonal variation in δ13C might due to changes in photosynthetic capacity. Accordingly, nutrients may have dictating the effect on leaf (or culm) δ13C of both species. Similarly, Schulze et al. (1998) found that variation in leaf N determined community-averaged leaf δ13C in northern Australia. Compared to low-light grown leaves, high-light grown leaves have high nitrogen content and more positive δ13C values (Ehleringer et al., 1986). Thus, it is possible that seasonal variation in leaf δ13C might be caused by seasonal changes in light intensity. In this study, I did not measure this environmental factor. However, Huang et al. (1996) reported that the area received more daily solar radiation in summer than in spring. Accordingly, the seasonal variation in leaf (or culm) nitrogen content and δ13C was unlikely caused by changes in light intensity. Water temperature is another possible factor would affect the nitrogen metabolism and photosynthetic activity of aquatic plants. In the present study, the water temperature was not monitored. This factor should be included in the future study.
Because majority of leaf nitrogen content is bound in photosynthetic enzyme, leaf photosynthetic capacity is generally linear related to leaf nitrogen content in wild plants (Field and Mooney, 1986). The result that S. falalx had significantly higher leaf N content than S.
mucronatus during most of the sampling period (Fig.
3A) suggests that the former might have a higher photosynthetic capacity than the later. This might also explain why S. fallax had higher annual production than
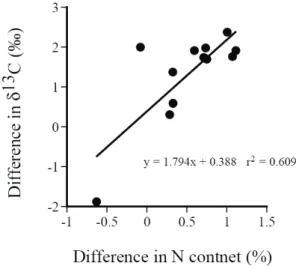
Fig. 5. The relationship between the difference in δ13C and
that in nitrogen content between S. fallax and S.
mucronatus for each sampling month in 2003.
In addition to have a higher N content, S fallax also had significantly more positive δ13C value than S.
mucronatus during most of the sampling period (Fig.
3B). To explore the possible reason for the difference, I analyzed the difference in δ13C (Δδ13C) and that in nitrogen content (ΔN) between the two species for each sampling month. A significantly positive, liner relationship between Δδ13C and ΔN was found (Fig. 4). Thus, higher nitrogen content in S. fallax might contribute to its more positive δ13C values than S.
mucronatus. With higher leaf nitrogen content, S. fallax
might have a higher photosynthetic capacity resulting in a more positive δ13C than S. mucronatus.
In conclusion, stable carbon isotope analysis indicated that the two dominant aquatic plants, S. fallax and S. mucronatus, in YYL used C3 photosynthetic pathway. Though soil moisture availability and atmospheric moisture were constant in the study site, seasonal variations in leaf δ13C and foliar nitrogen content were found in both aquatic plants. There is positive correlation between leaf (or culm) δ13C and leaf (or culm) nitrogen contents implying that changes in photosynthetic capacity contributed to the seasonal variation in δ13C in both species. The higher leaf nitrogen content found in S. fallax might also contribute to its more positive δ13C values than S. mucronatus.
LITERATURE CITED
Berry, J. A. 1989. Studies of mechanisms affecting the fractionation of carbon isotopes in photosynthesis. In: Rundel, P. W. J. R. Ehleringer and K. A. Nagy (eds), Stable Isotopes in Ecological Research. Springer-Verlag, New York, USA. pp. 82-94.
Chou, C.-H., T.-Y. Chen, C.-C. Liao and C.-I Peng. 2000. Long-term ecological research in the Yuanyang Lake forest ecosystem. I. Vegetation composition and analysis. Bot. Bull. Acad. Sin. 41: 61-72.
Ehleringer, J. R., C. B. Field, Z. F. Lin and C.-Y. Kuo. 1986. Leaf carbon isotope and mineral composition in subtropical plants along an irradiance cline. Oecologia 70: 520-526.
Farquhar, G. D., M. H. O’Leary and J. A. Berry. 1982. On the relaltionship between carbon isotope discrimination and the inter-cellular carbon-dioxide concentration in leaves. Aust. J. Plant Physiol. 9: 121-137.
Field, C. and H. Mooney. 1986. The photosynthesis-nitrogen relationship in wild plants. In: Givnish, T. J. (ed.), On the Economy of Plant Form and Function. Cambridge University Press, Cambridge. pp. 25-55.
Hays, J. M. 1983. Practice and principles of isotopic measurements in organic geochemistry. In: Meinschein, W.G. (ed.), Organic Geochemistry of Contemporaneous and Ancient Sediments. Society of Economic Paleontologists and Mineralogists, Bloomington, Indiana. pp. 5-31.
Huang, Y.-H., C.-W. Fan and M.-H. Yin. 1996. Primary production and chemical composition of emergent aquatic macrophytes, Schoenoplectus mucronatus spp. robustus and Sparganium fallax, in Lake Yuan-Yang, Taiwan. Bot. Bull. Acad. Sin. 37: 265-273.
Kao, W.-Y, C.-S. Lu and Y.-C. Chang. 2004. Foliar nutrient dynamics of five dominant plant species in Yuanyang Lake Nature Preserve, Taiwan. Taiwania 49: 49-56.
Keeley, J. E. 1989. Stable carbon isotopes in vernal pool aquatics of differing photosynthetic pathways. In: Rundel, P.W., J.R. Ehleringer and K.A. Nagy (eds.), Stable Isotopes in Ecological Research. Springer-Verlag, New York. pp. 82-94.
Korner, C, G. D. Farquhar and Z. Roksandic. 1988. A global survey of carbon isotope discrimination in plants from high altitude. Oecologia 74: 623-632.
Liu, T. and K.-S. Hsu. 1973. Ecological study on Yuen-yang Lake Natural Area Reserve. Bull. Taiwan For. Res. Inst. 237: 1-32.
Jones, H. G. 1992. Plants and Microclimate. A quantitative approach to environmental plant physiology, Cambridge University Press, Cambridge.
O’Leary, M. H. 1981. Carbon isotope fractionation in plants. Phytochemistry 20: 553-567.
O’Leary, M. H. 1988. Carbon isotopes in photosynthesis. BioScience 38: 328-336.
Robe, W. E. and H. Griffiths. 2000. Physiological and photosynthetic plasticity in the amphibious, freshwater plant, Littorella uniflora, during the transition form aquatic to dry terrestrial environments. Plnat, Cell and Environemnt 23: 1041-1054.
Roeske, C. A. and M. H. O’Leary. 1984. Carbon isotope effects on the enzyme-catalyzed carboxylation of ribulose biphosphate. Biochemstry 23: 6275-6285.
Schulze, E. D., N. C. Turner, T. Gollen and K. A. Shackel. 1987. Stomatal responses to air humidity and to soil drought. In: Zeiger, E., G.D. Farquhar and I.R. Cowan (eds), Stomatal Funciton. Stanford University Press, Standard, California. pp. 311-321.
Schulze, E. D., R. J. Williams, G. D. Farquhar, W. Schulze, J. Langridge, J. M. Miller and B. Wakler. 1998. Carbon and nitrogen isotope discrimination and nitrogen nutrition of trees along a rainfall gradient in northern Australia. Aust. J. Plant Physiol. 25: 413-425.
Ueno, O. 1996. Immunocytochemcial localization of enzymes
involved in the C3 and C4pathways in the photosynthetic cells of an amphibious sedge, Elocharis vivipara. Planta 199: 394-403.
Yang, Y-P., S-H. Yen and C.-K. Lin. 2001. Illustrated Guide to Aquatic Plants of Taiwan. Council of Agriculture, The Executive Yuan of Taiwan.