Characterization of ADG1, an Arabidopsis locus encoding
for ADPG pyrophosphorylase small subunit, demonstrates
that the presence of the small subunit is required for large
subunit stability
Shue-Mei Wang1, Wei-ling Lue2, Tien-Shin Yu2, Jih-Hau Long1, Chen-Nai Wang2, Klaus Eimert2and Jychian Chen2,*
1Department of Botany, National Taiwan University,
Taipei, Taiwan, Republic of China, and
2Institute of Molecular Biology, Academia Sinica, Taipei,
Taiwan, Republic of China
Summary
Two mutants of Arabidopsis have been isolated that affect ADPG pyrophosphorylase (ADGase) activity. Previously, it has been shown that ADG2 encodes the large subunit of ADGase. This study characterizes the adg1 mutant phenotype and ADG1 gene structure. RNA blot analyses indicate that the adg1-1 mutant accumulates transcripts encoding both the large and small subunits of ADGase, while the adg1-2 mutant accumulates only large subunit transcripts. RFLP analysis and complementation of adg1 mutants with the ADGase small subunit gene demonstrate that ADG1 encodes the small subunit. Sequence analysis indicates that adg1-1 represents a missense mutation within the gene. Western blot analysis confirms that adg1 mutants contain neither the large nor the small subunit proteins, suggesting that the presence of functional small subunits is required for large subunit stability.
Introduction
ADP-glucose pyrophosphorylase (ATP:α -glucose-1-phos-phate adenylyl transferase, EC 2.7.7.27; ADGase) is a key regulatory enzyme in the starch biosynthetic pathway of plants (for review, see Preiss, 1991). Within chloroplasts and amyloplasts, the enzyme catalyzes the synthesis of ADP-glucose and pyrophosphate from glucose-1-phos-phate and ATP. ADP-glucose functions as the glucosyl donor forα-glucan synthesis by various starch synthases (Preiss, 1991). ADGase from almost all higher plant sources is activated by 3-phosphoglyceric acid (3PGA) and inhibited by orthophosphate (Plaxton and Preiss, 1987).
ADGase from all sources is found to be a tetrameric
Received 21 March 1997; revised 5 September 1997; accepted 11 September 1997.
*For correspondence (fax1886 2 7826085; e-mail mbjchen@ccvax.sinica.edu.tw).
63 structure (Smith-White and Preiss, 1992) with a size ranging from about 200–240 kDa (Copeland and Preiss, 1981; Preiss
et al., 1987). However, the bacterial enzyme is
homo-tetrameric in structure (Haugen et al., 1976), whereas the plant enzyme comprises two subunits (Morell et al., 1987; Okita et al., 1990; Preiss, 1991). Immunological studies of
Arabidopsis (Lin et al., 1988a, b), wheat, rice and maize
(Krishnan et al., 1986, Preiss et al., 1990), Chlamydomonas (Iglesias et al., 1994) and pea (Hylton and Smith, 1992) demonstrated that the two subunits differed in size. It was suggested that the native ADGase of plants formed as heterotetramer with two large and two small subunits (Preiss, 1988). Expression of both potato ADGase subunits in the same Escherichia coli cell leads to assembly in vivo into active homo-tetramers, while the large subunit expressed on its own leads to very little enzyme activity (Iglesias et al., 1993). However, the detailed assembly process of ADGase has not been well characterized.
Starch accumulation is severely affected in plants that are deficient in ADGase activity. This has been shown in
Arabidopsis leaves (Lin et al., 1988a, b), maize endosperm
(Weaver et al., 1972), pea cotyledons (Smith et al., 1989),
Chlamydomonas (Van den Koornhuyse et al., 1996), and
in tubers of potato plants expressing antisense constructs of an ADGase gene (Muller-Rober et al., 1992). The
Arabi-dopsis adg2 mutant has only ~5% ADGase activity but
accumulates ~40% of wild-type starch levels (Lin et al., 1988b). Western blot analysis has shown that the adg2 mutant is deficient in the large subunit protein (Lin et al., 1988b), and this suggests that the functional ADGase that is present in the adg2 mutant is a homo-tetramer of small subunits. This suggestion was confirmed by Li and Preiss (1992). By molecular analysis and complementation of the mutant with the wild-type large subunit gene, we have shown that ADG2 encodes the ADGase large subunit (Wang
et al., 1997). DNA sequence analysis indicated that adg2-1
is a missense mutation (glycine 118 changed to glutamic acid, G118E) in a domain (PAVPXG) proposed to be involved in the interaction of ADGase small and large subunits. This change is thought to be important both for allosteric regulation and for enzyme assembly (Wang et al., 1997).
The adg1-1 mutant is a monogenic recessive mutation that has no measurable ADGase activity in leaf extracts (Lin et al., 1988a). The absence of starch in these mutants demonstrates that starch synthesis in the chloroplast is entirely dependent on a pathway involving ADGase.
small and large subunit gene expression (Lin et al., 1988a). Here we show that adg1 is not a regulatory mutation. Rather, it is a mutation in the structural gene encoding the ADGase small subunit. The absence of large subunit protein in adg1 mutants suggests that stability of the large subunit protein is dependent on the presence of the small subunit.
Results
Isolation and characterization of Arabidopsis starch metabolic mutants
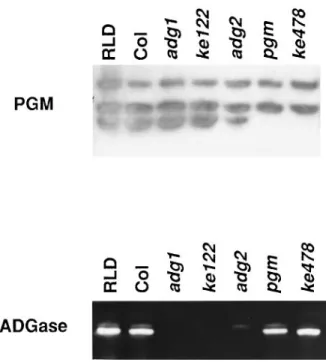
We have isolated Arabidopsis mutants affecting starch metabolism (Eimert et al., 1995). Two of them were isolated on the basis that leaves grown under continuous light did not stain blue with iodine. We measured the starch content in these two mutants (ke122 and ke478 of RLD ecotype) and confirmed that the level of starch accumulation in mutant leaves (0.3 mg starch per g leaf tissue) is signific-antly lower than that of the wild-type (5 mg starch per g leaf tissue).
So far, studies of Arabidopsis starch-deficient mutants have identified two types of defect. Biochemical analysis showed that one class of mutant is deficient in chloroplastic phosphoglucomutase (PGM) activity, e.g. pgm1-1 mutant (Caspar et al., 1985), and the other class perturbs ADGase activity, e.g. adg1 and adg2 mutants. The adg1 mutant has no ADGase activity (Lin et al., 1988a), and the adg2 mutant has decreased ADGase activity (Lin et al., 1988b). We analyzed the PGM and ADGase activity of ke122 and ke478 mutants. As shown in Figure 1, ke478 has the same level of ADGase activity as the wild-type but, similar to
pgm1-1, has lost the chloroplastic PGM activity. In contrast, the
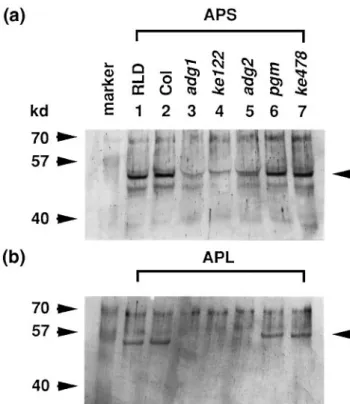
ke122 mutant has normal levels of PGM activity but has lost the ADGase activity (Figure 1). In order to examine the basis for the deficiency of ADGase activity, the presence of ADGase proteins in leaf extracts was detected by West-ern blot analysis. We found that both the small and large subunit proteins were present in the wild-type and the
pgm mutants (Figure 2). In the mutants carrying either adg1-1 or adg1-2 allele, both the small subunit (Figure 2a,
lanes 3 and 4) and large subunit proteins (Figure 2b, lanes 3 and 4) were below the detectable level. The amount of small subunit protein (Figure 2a, lane 5) and large subunit protein (Figure 2b, lane 5) in the adg2 mutant was signific-antly reduced compared with that of the wild-type. Cross-reactive bands were detected by the antibodies. Because these proteins were not affected by the mutations,
Figure 1. Activity gel assay for phosphoglucomutase and ADPG pyrophosphorylase in wild-type and mutant leaf extracts.
Leaf extracts (20µl) from the wild-type (RLD ecotype and Columbia (Col) ecotype), adg1-1 (adg1), adg1-2 (ke122), adg2, pgm1-1 (pgm), and pgm1-2 (ke478) were separated in a 7% native polyacrylamide gel and assayed for phosphoglucomutase (PGM) and ADPG pyrophosphorylase (ADGase) activity.
they may be related proteins that share antigenic sites with the ADGase proteins or E. coli-derived proteins.
Notably, two bands were detected in the ADGase activity assay in both the wild-type and pgm1 mutants (Figure 1). Only the upper band was found in the adg2-1 mutant. Western blot analysis demonstrated that the main activity (lower) band of the wild-type contains both the small and large subunits whereas the minor (upper) band seen in
adg2-1 and wild-type samples contains only the small
subunits. The amount of protein detected by Western blot analysis is roughly correlated to enzyme activity assayed
in vitro, and as such this result is consistent with the fact
that ADGase in adg2-1 is a homo-tetramer of small subunits (Li and Preiss, 1992).
The F1progeny of test crosses between ke478 and
pgm1-1 have no chloroplast PGM activity, indicating that ke478
is allelic to 1. ke478 was therefore designated
pgm1-2. Test crosses of ke122 and adg1-1 yielded F1 progeny plant with no starch and no ADGase activity, indicating that ke122 is allelic to adg1-1. ke122 was therefore desig-nated adg1-2.
The adg1-1 mutant is a monogenic recessive mutation that has no ADGase activity in leaf extracts. Both the ADGase small and large subunits are absent in the
adg1-1 mutant as shown by Western blot analysis (Lin et al.,
1988a). Similarly, we have found that both subunits are absent in adg1-2 mutants. Previous reports have suggested
Figure 2. Western blot analysis for the ADGase proteins in the wild-type and mutant leaf extracts.
(a) Leaf extracts containing 20µg proteins from the wild-types (RLD ecotype and Columbia (Col) ecotype), pgm1-1 (pgm) and pgm1-2 (ke478) and 40µg proteins from adg1-1 (adg1), adg1-2 (ke122) and adg2 were separated by 10% SDS–polyacrylamide gel and probed with the antiserum against ADGase small subunit protein (APS).
(b) Leaf extracts containing 20µg proteins for all the samples were probed with the antiserum against the ADGase large subunit protein (APL). The predicted size of ADGase small subunit protein is 49 kDa and the size of the large subunit protein is 52 kDa. Bands of expected size were detected as indicated by the arrows. Cross-reactive bands were detected by the antibodies. They may be related proteins sharing antigenic sites with the ADGase proteins or E. coli-derived proteins.
that this finding demonstrates that ADG1 is a regulatory gene affecting small and large subunit gene expression (Lin et al., 1988a).
Characterization of cDNA and genomic clones of the ADGase small subunit
In order to characterize expression patterns of ADGase small and large subunit transcripts in adg mutants, we isolated and characterized cDNA and genomic clones of
Arabidopsis ADGase small subunit. Previously, four cDNA
clones of ADGase, including one small subunit (APS) and three large subunits (APL1, APL2 and APL3), were isolated by PCR with two primers corresponding to conserved domains (Villand et al., 1993). Using APS and APL1 as probes, two cDNA clones corresponding to the small (APS1A) and one of the large subunits (APL1A) were isolated from a cDNA library prepared with leaf poly(A)1 RNA (Columbia ecotype, Villand et al., 1993). Since mRNA
Figure 3. Restriction maps of Arabidopsis small subunit cDNA and genomic clones.
The transcription orientation of the small subunit of ADGase is indicated with an arrow: X (XbaI), Ss (Sst I), H (HindIII), K (KpnI), R (EcoRI). Sites labeled with an asterisk are derived from the vector; shaded boxes represent transcribed regions.
used for the cDNA library construction was isolated from leaves, both large and small subunit transcripts must be expressed in leaf tissue. Sequence analysis showed that the insert of the small subunit cDNA clone (APS1A) contains 1470 bp of coding region and 202 bp of 39 untranslated region. To clone the complete sequence of the small subunit coding region, we sequenced the small subunit genomic clone (see below). Using the sequence of the 59 genomic region, primers were designed for RT–PCR and full-length cDNA clones (Columbia ecotype) were isolated. cDNA sequence showed that the ADGase small subunit is com-posed of 520 amino acids (GenBank accession number U72351). Genomic clones of the ADGase small subunit were isolated by screening a genomic library (Landsberg
erecta ecotype) with the cDNA probe. Restriction enzyme
mapping and sequence analysis indicated that the genomic clone contains the complete small subunit transcription unit plus 6.4 kb of 59 upstream sequence and 0.4 kb of 39 downstream sequence. The restriction enzyme map and transcription orientation are shown in Figure 3.
Northern blot analysis of ADGase expression
We examined the expression pattern of ADGase transcripts in leaves of wild-type, pgm mutants and the ADGase mutants adg1-1, adg1-2 and adg2-1. Total RNA was isolated from the leaves of plants grown in continuous light and hybridized with the small and large subunit cDNAs of ADGase. As shown in Figure 4, the ADGase large subunit transcript was present in all samples. ADGase small subunit transcripts could be detected in the wild-type, adg1-1 and
adg2-1 samples, but not in the adg1-2 mutant sample. The
absence of small subunit transcripts in adg1-2 leads us to suggest that adg1 mutants may represent a structural mutation in the small subunit gene as opposed to the previously proposed regulatory mutation (Lin et al., 1988a).
RFLP and DNA sequence analyses of the adg1 mutant
We mapped the ADGase small subunit gene using recom-binant inbred lines and the APS cDNA as probe. The
Figure 4. Northern blot analysis of small subunit and large subunit transcripts of ADGase.
(a) Northern blot of total leaf RNA (20µg) isolated from the wild-type (RLD ecotype and Columbia (Col) ecotype), adg1-1 (adg1), adg1-2 (ke122), adg2, pgm1-1 (pgm) and pgm1-2 (ke478) plants probed with radioactively labeled small subunit cDNA (APS).
(b) The same blot as shown in (a) hybridized with the large subunit cDNA probe (APL) after recycling the membrane.
(c) The same blot as shown in (a) reprobed with a tubulin cDNA probe.
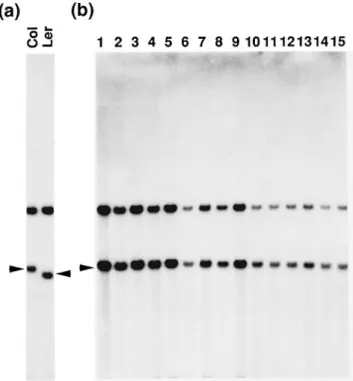
mapping data indicated that the ADGase small subunit gene is located on chromosome 5 at 69.1 cM. If adg1 is an ADGase small subunit gene mutation, we should be able to show linkage of adg1 to the small subunit cDNA probe. To test this hypothesis, we examined RFLPs in selfed F2
progenies of adg1 (Col)3ADG1 (Ler). As shown in Figure 5, we found that in a population of 60 plants that failed to accumulate starch, the APS Col band co-segregated with
Figure 5. RFLP analysis of F2plants of adg1-1 (Col )3ADG1 (Ler). (a) Genomic DNAs (2 µg) isolated from wild-type Columbia (Col) and Landsberg erecta (Ler) ecotypes were digested with Bcl I, Southern blotted and probed with labeled APS cDNA. A polymorphism was detected. (b) Southern blot analysis of genomic DNA isolated from the F2progenies of adg1-1 (Col )3ADG1 (Ler). DNA samples derived from plants that failed to accumulate starch (lanes 1–14) and from wild-type (Col ) plants (lane 15) were digested with BclI, Southern blotted and probed with the APS cDNA.
Figure 6. Protein sequence alignment around the missense mutation site of the adg1-1 allele.
The N-terminal portions of ADGase small subunit sequences of potato, spinach and Arabidopsis were aligned. The sequence of spinach ADGase was determined by protein sequencing and cDNA sequencing. The missense mutation of adg1-1 allele causes the amino acid glycine (92) change to arginine. The PAVPXG domain is shown in bold.
the adg1-1 phenotype. This result suggested that adg1-1 is a mutation in the ADGase small subunit gene.
We confirmed this suggestion by cloning and sequencing both adg1-1 and ADG1 (wild-type) alleles. Sequence com-parison indicated that, in adg1-1, there is a single nucleotide change from G (nucleotide 309) to A causing a missense mutation (glycine 92 mutated to arginine, G92R) in the ADGase small subunit gene. As shown in Figure 6, compar-ison with the N-terminal peptide sequence of mature spinach leaf ADGase small subunit (Morell et al., 1987),
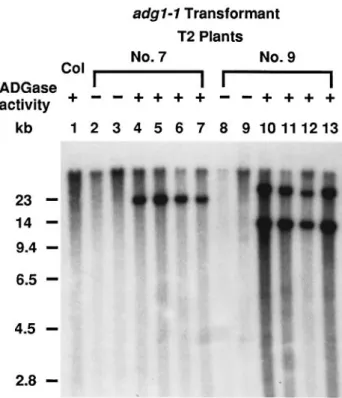
Figure 7. Southern blot analysis of two transgenic lines of adg1-1 carrying the transformed wild-type APS gene.
T2progenies of two representative transgenic lines (No. 7 and No. 9) that co-segregated for kanamycin resistance, wild-type level leaf starch, and wild-type level leaf ADGase activity (labeled as1, lanes 4–7 and 10–13) and kanamycin sensitivity, no leaf starch and no leaf ADGase activity (labeled as –, lanes 2, 3, 8 and 9) were analyzed. Genomic DNA was isolated from leaves of wild-type (Columbia ecotype, lane 1) and transgenic plants, digested with KpnI, Southern blotted, and probed with the APS cDNA. The results showed that transgenic plants with a wild-type phenotype carried the transformed wild-type APS gene.
indicated that this missense mutation is at position 21 of the mature protein.
Complementation of adg1 mutants with the wild-type ADGase small subunit gene
To further prove that adg1 is an ADGase small subunit mutation, we transformed the adg1 mutants with the wild-type ADGase small subunit gene. The wild-wild-type APS gene (a 7.6 kb Sst I–XbaI fragment) was inserted into pBin19 and transformed into Agrobacterium. adg1-1 and adg1-2 plants were transformed by vacuum infiltration and independent kanamycin-resistant transformants (15 for adg1-1 and four for adg1-2) were selected from the T1seeds. The resulting transformants reversed the starch-deficient phenotype of
adg1 to wild-type. The kanamycin-resistant T1plants were
selfed and T2plants were scored for their ADGase activity.
As shown in Figure 7, T2transformants with wild-type level
of ADGase activity and leaf starch contain the transformed small subunit gene. This result confirms that the adg1 mutant phenotype is caused by a mutation in the small subunit gene.
Discussion
Both the adg1-1 and adg1-2 mutants have no detectable ADGase activity (Figure 1). By RFLP analysis and comple-mentation of the mutant phenotype with the wild-type ADGase small subunit gene, we have shown that ADG1 encodes the ADGase small subunit. The adg1-2 mutant has no detectable ADGase small subunit RNA, thus no detectable small subunit protein, suggesting that this muta-tion perturbs gene expression at the transcripmuta-tional level, either affecting the promoter activity or mRNA stability. The presence of ADGase large subunit RNA in the adg1-2 mutant which has no detectable small subunit RNA indi-cated that the transcription of ADGase large subunit gene is independent of that of the small subunit gene. By Northern blot analysis and ADGase activity gel assays, we showed that pgm1-1 and pgm1-2 mutants accumulate ADGase small and large subunit transcripts (Figure 4) and exhibit ADGase activity (Figure 1) similar to that of the wild-type. In the pgm mutants, both the chloroplastic phosphoglucomutase activity and leaf starch accumulation are defective, suggesting a deficiency of glucose 1-P neces-sary for starch synthesis in the chloroplast. Our results suggest that the expression of ADGase genes is not modu-lated by the glucose-1-P concentration.
We have previously shown that ADG2 encodes the large subunit of ADGase. Complementation analysis suggests that the ADGase large subunit mRNA that accumulates in leaves is primarily transcribed from the ADG2 gene (Wang
et al., 1997). The expression profiles and functions of the
other two copies of the ADGase large subunit genes remain to be determined. Molecular analysis of the adg2-1 allele indicated that it is a missense mutation (G118E) adjacent to a conserved domain (PAV,113–115) involved in the allosteric property of the holoenzyme (Greene et al., 1996). From biochemical/genetic analyses of potato ADGase mutants, Greene et al. (1996) proposed that the PAV allosteric domain of the hetero-tetramer enzyme is formed by interaction of both ADGase small and large subunits. Western blot ana-lysis of ADGase in the adg2-1 mutant indicated that it is composed of a homo-tetramer of small subunits (Li and Preiss, 1992), suggesting that the mutated large subunit protein is not assembled into holoenzyme and is not stably maintained in chloroplasts. Thus, the domain PAVPXG (113–118) is thought to be important both for allosteric regulation and for enzyme assembly (Wang et al., 1997).
Sequence analysis indicated that the adg1-1 mutant contains a missense mutation (G92R) located 21 amino acids upstream of the PAVPXG domain. Although the N-terminal sequence of the mature small subunit protein of
Arabidopsis ADGase has not yet been determined, protein
sequence comparisons with the spinach small subunit mature protein (Figure 6) indicated that the first 20 amino acids differ only by two amino acids (Morell et al., 1987).
stability of unassembled proteins. If the latter proposal is true, the mutation may prevent the small subunit from being folded in a proper conformation for subsequent assembly into an oligomeric structure with the large sub-units. Alternatively, the N-terminal portion of the small subunit may be a part of the domain involved in the interactions of the subunits. The missense mutation G92R may interfere with the interactions between subunits. Since the N-terminal domain is highly conserved among all known ADGase small subunits (Smith-White and Preiss, 1992), this suggestion has credence.
Western blot analysis indicated that both the small and large subunits of ADGase are absent in adg1-1 (Lin et al., 1988a) and adg1-2 mutants (Figure 2). These monogenic recessive adg1 mutants are mutants of the ADGase small subunit and have wild-type large subunit genes. Thus the large subunit genes of adg1 mutants would be transcribed (Figure 4) and translated normally in mutant plants. These results suggest that the presence of functional small sub-units is required for the stability and/or assembly of large subunits and furthermore that large subunit proteins cannot assemble into a functional homo-tetramer in the
adg1-1 and adg1-2 mutants.
In the adg2 mutant, we observed a reduced amount of both small subunit and large subunit proteins (Figure 2). The presence of small amounts of large subunit proteins that was not observed by Lin et al. (1988b) may reflect the experimental variations of plants affecting the stability of mutated large subunit protein. However, this result did support the suggestion that the stability of small subunit protein in the chloroplast is also affected by the amount of large subunit protein. A similar effect was observed in maize in which the rate of SH2 or BT2 protein turnover is dependent on the presence of BT2 or SH2 protein, respectively (Giroux et al., 1994). It has been suggested that SH2 and BT2 proteins are more susceptible to degradation when the formation of a SH2/BT2 polymer does not occur (Giroux et al., 1994). Similar to Arabidopsis, two groups of maize mutants that affect ADGase activity have been isol-ated (bt2 and sh2). Bt2 encodes the small subunit protein and SH2 encodes the large subunit protein (Preiss et al., 1990). Both genes are expressed specifically in the endosperm. Whereas Arabidopsis adg1 mutants have no detectable small nor large subunits, large subunit protein is detected in maize bt2 mutants in the absence of small subunit proteins (Giroux and Hannah, 1994). It has been suggested that there are other genes expressed in maize endosperm that are homologous to SH2/BT2, i.e. AGP1
suppose that the ADGase proteins detected in the bt2 mutants correspond to the AGP1/AGP2 gene products. Alternatively, large subunit proteins of maize may be able to interact with each other and become stabilized. Differ-ences in the proteolytic activity of endosperm and leaves could also explain why the large subunit protein can accumulate even in the absence of small subunit proteins in bt2 mutants. The underlying mechanism for the presence of ADGase large subunit in maize bt2 mutant requires further analysis.
Existing data from studies of Arabidopsis ADGase showed that wild-type ADGase is mainly composed of two small and two large subunits (Li and Preiss, 1992). In the
absence of the large subunit, small subunit proteins can assemble into a homo-tetrameric quaternary structure (Li and Preiss, 1992) but in the absence of small subunit proteins, large subunit proteins become unstable in the chloroplast (Lin et al., 1988a; Wang et al., 1997). Based on these data, we propose that the ADGase assembly process may be initiated by homodimer formation of small sub-units. The small subunit homodimer then interacts further with large subunit or small subunit proteins. In the absence of the large subunit, however, the homodimers of small subunits would assemble into a homo-tetramer form of ADGase as observed in the adg2-1 mutant. However, this homo-tetramer form of ADGase, has a different allosteric regulatory response to the effectors, in as much as it requires a higher concentration of 3PGA for maximal activation and is more sensitive to inhibition by Pithan the wild-type hetero-tetramer form (Li and Preiss, 1992). Future analysis of the ADGase structure would elucidate the interactions among subunits and its catalytic and regu-latory domains.
Experimental procedures
Plant materials and growth conditions
The M2seeds of ethyl methanesulfonate-treated seeds of Arabi-dopsis thaliana (RLD ecotype) were obtained from Lehle Seeds (Round Rock, TX). adg1-1, adg2-1 and recombinant inbred lines (Lister and Dean, 1993) were obtained from the Arabidopsis Biological Resource Center at Ohio State University (ABRC, Columbus, OH) and Nottingham Arabidopsis Stock Center (NASC, Nottingham, UK). Plants were grown in soil at 23°C under ca. 100µmol quanta m–2sec–1continuous fluorescent light.
Mutant screening
Mutant screening was carried out as described by Caspar et al. (1985). Plants were screened after they developed 4–6 true leaves.
Leaf pieces were cut out, de-pigmented in ethanol or methanol and stained with I2–KI (5.7 mM iodine and 43.4 mM potassium iodide in 0.2 N HCl). Quantitative starch asssays were conducted according to Eimert et al. (1995).
Native gel assay
The ADGase (Lin et al., 1988a) and PGM (Caspar et al., 1985) gel assays were carried out according to previously described methods using 7% polyacrylamide gels. Leaf samples were extracted with extraction buffer (100 mM Tris–HCl pH 7.0, 40 mM
β-mercaptoethanol, 10 mM mgCl2, 100 mM KCl, 15% glycerol, 2 ml per g–1fresh tissue). The crude extract was centrifuged at 14 0003g for 5 min and the activity present in the supernatant fraction was assayed. Quantitative assay of ADPG pyrophosphoryl-ase activity was conducted as described by Sowokinos (1976) in the presence of 2 mM 3-phosphoglyceric acid (3PGA).
General molecular analysis
Standard cloning, Southern blot and Northern blot techniques were used as described by Sambrook et al. (1989). DNA sequencing was performed with double-stranded plasmids, using Sequenase (United States Biochemical).
Isolation of genomic and cDNA clones
Arabidopsis ADGase small subunit cDNA clone ALPC10 (APS1A) and large subunit cDNA clone ALPA4 (APL1A), both containing 1.7 kb inserts in pBluescript vector (Stratagene), were isolated from a leaf cDNA library by screening with APS and APL1 probes, respectively (Villand et al., 1993). APS, APL1, APS1A and APL1A plasmids were kindly supplied by Dr Villand. To isolate the corres-ponding genomic clones of ADGase small subunit, a genomic library of Arabidopsis Ler ecotype (constructed as partially digested MobI genomic DNA fragments cloned into the XhoI site ofλFix vector, supplied by ABRC) was screened with the APS1A cDNA insert. A 9 kb Xba I fragment of the small subunit genomic
λclone (APS3B) was further subcloned into pBluescript SK1.
Western blot analysis
Leaf extracts were electrophoresed by 10% SDS–polyacrylamide gels and electroblotted to nytran membranes. Rabbit antiserum was prepared against the E. coli-expressed ADGase small subunit and large subunit proteins and used as probes for the Western blot analyses. The primary antibody was detected with the Vectas-tain ABC kit (Vector Laboratories, Burlingame, CA). The ADGase small subunit protein (Ala340–Ile520) was isolated from an E. coli strain carrying a plasmid with the HindIII–XhoI 0.7 kb fragment of the small subunit cDNA clone (APS1A) inserted into pET30A (Novagen). The ADGase large subunit protein (Glu37–Ile522) was prepared from an E. coli strain carrying a plasmid with an Sst I– XhoI 1.6 kb fragment of the large subunit cDNA clone (APL1A) inserted into pET30A.
RFLP mapping
RFLP mapping was performed according to Chang et al. (1988) and Nam et al. (1989) and the data were analyzed using the JoinMap computer program (Stam, 1993).
Complementation of adg1 mutants with wild-type genes
A 7.6 kb Xba I–Sst I fragment of the ADGase small subunit genomic clone was cloned into XbaI–Sst I sites of pBin19 and denoted pBin19-APS. The resulting T-DNA binary constructs were trans-formed into the Agrobacterium tumefaciens strain pGV2260/C58 rif. adg1-1 and adg1-2 plants were transformed with pBin19-APS by vacuum infiltration (Bechtold et al., 1993). Kanamycin-resistant transformants were selected by germinating seeds on selective medium. The resistant plants were transferred to soil and self-pollinated. Seeds were harvested for subsequent genetic, biochemical and molecular analyses.
Acknowledgments
We thank Jane Langdale for reading the manuscript prior to publication. S.-M.W. and J.C. were supported by the grants from the National Science Council, Taiwan, Republic of China (NSC 82-0418-B-002-376-B03 to S.-M.W. and NSC 84-2311-B-001-033 to J.C.) and Academia Sinica, Taipei, Taiwan (J.C.).
References
Bechtold, N., Ellis, J. and Pelletier, G. (1993) In planta
Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris,316, 1194– 1199.
Caspar, T., Huber, S.C. and Somerville, C. (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana L. deficient in a chloroplast phosphoglucomutase activity. Plant Physiol.79, 11–17. Chang, C., Bowman, J.L., DeJohn, A.W., Lander, E.S. and
Meyerowitz, E.M. (1988) Restriction fragment length polymorphism map for Arabidopsis thaliana. Proc. Natl Acad. Sci. USA,85, 6856–6860.
Copeland, L. and Preiss, J. (1981) Purification of spinach leaf ADPglucose pyrophosphorylase. Plant Physiol.68, 996–1001. Denyer, K., Cunlap, F., Thorbjornsen, T., Keeling, P. and Smith, A.
(1996) The major form of ADP-glucose pyrophosphorylase in maize endosperm is extra-plastidial. Plant Physiol.112, 779–785. Eimert, K., Wang, S.-M., Lue, W-L. and Chen, J. (1995) Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis. Plant Cell,7, 1703–1712. Giroux, M.J. and Hannah, L.C. (1994) ADP-glucose
pyro-phosphorylase in shrunken-2 and brittle-2 mutants of maize. Mol. Gen. Genet.243, 400–408.
Giroux, M.J., Boyer, C., Feix, G. and Hannah, L.C. (1994) Coordinated transcriptional regulation of storage product genes in the maize endosperm. Plant Physiol.106, 713–722. Greene, T.W., Chantler, S.E., Kahn, M.L., Barry, G.F., Preiss, J.
and Okita, T.W. (1996) Mutagenesis of the potato ADPglucose pyrophosphorylase and characterization of an allosteric mutant defective in 3-phosphoglycerate activation. Proc. Natl Acad. Sci. USA,93, 1509–1513.
Haugen, T., Ishaque, A. and Preiss, J. (1976) Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-P adenylyl transferase. J. Biol. Chem.251, 7880–7885.
Hylton, C. and Smith, A.M. (1992) The rb mutation of peas causes structural and regulatory changes in ADP glucose pyrophosphorylase from developing embryos. Plant Physiol. 99, 1626–1634.
properties of ADP-glucose pyrophosphorylase from Chlamy-domonas reinhardtii. Plant Physiol.104, 1287–1294.
Krishnan, H.B., Reeves, C.D. and Okita, T.W. (1986) ADPglucose pyrophosphorylase is encoded by different mRNA transcripts in leaf and endosperm of cereals. Plant Physiol.81, 642–645. Li, L. and Preiss, J. (1992) Characterization of ADPglucose
pyrophosphorylase from a starch-deficient mutant of Arabidopsis thaliana (L.). Carbohydrate Res.227, 227–239. Lin, T.-P., Caspar, T., Somerville, C. and Preiss, J. (1988a) Isolation
and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiol.86, 1131–1135.
Lin, T.-P., Caspar, T., Somerville, C. and Preiss, J. (1988b) A starch deficient mutant of Arabidopsis thaliana with low ADPglucose pyrophosphate activity lacks one of the two subunits of the enzyme. Plant Physiol.88, 1175–1181.
Lister, C. and Dean, C. (1993) Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana. Plant J.4, 745–750.
Morell, M.K., Bloom, M., Knowles, V. and Preiss, J. (1987) Subunit structure of spinach leaf ADPglucose pyrophosphorylase. Plant Physiol.85, 182–187.
Muller-Rober, B., Sonnewald, U. and Willmitzer, L. (1992) Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 11, 1229–1238.
Nam, H.-G., Giraudat, J., den Boer, B., Moonan, F., Loos, W., Hauge, B. and Goodman, H.M. (1989) Restriction fragment length polymorphism linkage map of Arabidopsis thaliana. Plant Cell,1, 699–705.
Okita, T.W., Nakata, P.A., Anderson, J.M., Sowokinos, J., Morell, M. and Preiss, J. (1990) The subunit structure of potato tuber ADP-glucose pyrophosphorylase. Plant Physiol.93, 785–790. Plaxton, W.C. and Preiss, J. (1987) Purification and properties of
nonproteolytic degraded ADPglucose pyrophosphorylase from maize endosperm. Plant Physiol.83, 105–112.
Preiss, J. (1988) Biosynthesis of starch and its regulation. In The
GenBank accession number U72351 (ADPG pyrophosphorylase small subunit cDNA).
studies. In Tailoring Genes for Crop Improvement (Bruening, G., Harada, J., Kosuge, T. and Hollaender, A., eds). New York: Plenum Publishing Corp., pp. 133–152.
Preiss, J., Danner, S., Summers, P.S., Morell, M., Barton, C.R., Yang, L. and Nieder, M. (1990) Molecular characterization of the
brittle-2 gene effect on maize endosperm ADPglucose pyrophosphorylase subunits. Plant Physiol.92, 881–885. Sambrook, J., Fritsh, E.F. and Maniatis, T. (1989) Molecular
Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
Smith, A.M., Bettey, M. and Bedford, I.D. (1989) Evidence that the
rb locus alters the starch content of developing pea embryos through an effect on ADP glucose pyrophosphorylase. Plant Physiol.89, 1279–1284.
Smith-White, B.J. and Preiss, J. (1992) Comparison of proteins of ADP-glucose pyrophosphorylase from diverse sources. J. Mol. Evol.34, 449–464.
Sowokinos, J.R. (1976) Pyrophosphorylases in Solanum tuberosum. Plant Physiol.57, 63–68.
Stam, P. (1993) Construction of integrated genetic linkage maps by means of a new computer package: JoinMap. Plant J.3, 739–744.
Van den Koornhuyse, N., Libessart, N., Delrue, B., Zabawinski, C., Decq, A., Iglesias, A., Carton, A., Preiss, J. and Ball, S. (1996) Control of starch composition and structure through substrate supply in the monocellular alga Chlamydomonas reinhardtii. J. Biol. Chem.271, 16 281–16 287.
Villand, P., Olsen, O-A. and Kleczkowski, L.A. (1993) Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana. Plant Mol. Biol. 23, 1279–1284.
Wang, S.-M., Chu, B., Lue, W.-L., Yu, T.-S., Eimert, K. and Chen, J. (1997) adg2-1 represents a missense mutation in the ADPG pyrophosphorylase large subunit gene of Arabidopsis thaliana. Plant J.11, 1121–1126.
Weaver, S.H., Glover, D.V. and Tsai, C.Y. (1972) Nucleoside diphosphate glucose pyrophosphorylase isozymes of developing normal, brittle-2, and shrunken-2 endosperms of Zea mays L. Crop Sci.12, 510–514.