J . CHEM. SOC., CHEM. COMMUN., 1991 1023
Conversion of the Cluster Core Structure via CO Elimination and Activation of the
Coordinated Hydrocarbon Ligand. Synthesis and Reactions of
WOs3Cp(
CO)
o(
CMeCMeCCP h
)
Yun Chi,*a Hui-Ying H s u , ~ Shie-Ming Pengt b and Gene-Hsiang Leeb
a Department of Chemistry, National Tsing Hua University, Hsinchu 30043, Taiwan b Department of Chemistry, National Taiwan University, Taipei 10764, Taiwan
Reaction of WCp(C0)3C=CPh with O S ~ ( C O ) , ~ ( C ~ M ~ ~ ) produced the butterfly cluster W O S ~ C ~ ( C O ) , ~ ( C M ~ C M ~ C C P ~ ) 1 in moderate yield; treatment of 1 with Me3N0 followed by thermolysis in refluxing toluene yielded the spiked
triangular cluster W O S ~ C ~ ( C O ) ~ ( C L - H ) [ C M ~ ~ M ~ ~ C ( ~ ~ - ~ ~ ~ - ~ ~ H ~ ) ] 3 as a major product; on further thermolysis, complex 3 reconverted to a butterfly cluster W O S ~ C ~ ( C O ) ~ [ C M ~ C M ~ C H C ( ~ ~ - T ~ X ~ H ~ ) ] 5 and then to a tetrahedral cluster W O S ~ C ~ ( C ~ ) ~ [ C M ~ C M ~ C H C ( ~ * - ~ ~ - ~ ~ H ~ ) ] 6 via hydride migration followed by loss of CO; the structures of complexes 3, 5 and 6 have been determined by X-ray diffraction.
Systematically increasing the nuclearity of cluster compounds and transformation of the cluster core geometries continues to
be a challenging and important task in the chemistry of transition metal cluster complexes. 1 We have been exploring
the use of group 6 mononuclear metal acetylide2 and hydride3 complexes in the stepwise synthesis of heterometallic cluster complexes; the preparation of the butterfly cluster complex W O S ~ C ~ ( C O ) ~ ~ ( C M ~ C M ~ C C P ~ ) 1 containing a multi-site bound C4 hydrocarbon ligand has been achieved. Herein we report the activation of this C4 hydrocarbon ligand via C O elimination and the sequential conversion of the cluster core from the spiked triangle to the tetrahedral arrangement.
Treatment of WCp(C0)3C=CPh4 and O S ~ ( C O ) ~ ~ ( C ~ M ~ ~ ) ~ in 1 : 1 molar ratio and in refluxing toluene (110 "C, 40 min)
yielded two condensation products W O S ~ C ~ ( C O ) ~ ~ ( C M ~ - CMeCCPh) 1 (41%) and W O S ~ C ~ ( C O ) ~ ( C C P ~ C M ~ C M ~ ) 2 (33%) (Scheme 1). Both complexes 1 and 2 were charac-
terized by mass, IR and NMR spectroscopy.$ The exact
molecular structures of these complexes were established by comparing their IR spectra with those of the structur- ally characterized cluster complexes W O S ~ C ~ ( C O ) ~ O [ C - (C02Et)C(C02Et)CCPh] and WOs3Cp(CO),[CCPhC- (Tol)C(Tol)], respectively.6 It is evident that the tetranuclear butterfly cluster complex 1 was produced by coupling of the coordinated alkyne with the a-carbon of the incoming acetylide ligand, while complex 2 was produced by coupling with the (3-carbon. Both complexes display some interesting reactivity patterns and we here focus on the transformation of the cluster cores starting from complex 1.
Treatment of 1 with Me3N0 (1.1 mol equiv.) in a mixture of acetonitrile-dichloromethane at ambient temperature fol- lowed by heating in refluxing toluene produced a burgundy- coloured solution within ten minutes. Following TLC separa- tion (hexane-dichloromethane 4 : 1) and purification by re- crystallization, we obtained a wine-red cluster WOs3Cp( C0)g-
(pH)[CMeCMeCC( p2-qz-C6H4)] 3 in 48% yield, in addition
t For crystallographic enquiries.
$ Spectral data for 1: MS (FAB, 184W, 19*Os), mlz 1260 (M+). IR
(C6HI2) v(C0)lcm-1 2077vs, 2055s, 2048vs, 2027m, 2014s, 2010s, 1997m, 1975s, 1968s, 1952vw and 1940m; 1H NMR (400 MHz, CDC13, 294K)67.45(d,2H,JH-H7.4Hz),7.39-7.32(m,3H),5.14(~,5H), 2.98 (s, 3 H) and 1.88 (s, 3 H); 13C NMR (100 MHz, CD2C12, 254 K) 6 217.0 (Jw-c 149 Hz), 186.0 (br), 183.6, 183.3, 183.2 (br), 182.9, 182.4,179.1 (br), 179.0,173.2 (CO), 193.3,156.7,146.6,144.0,134.9
(Me), 23.3 (Me). Spectral data for 2: MS, mlz 1232(M+). IR (C6H12) v(C0)lcm-1 2075s, 2047vs, 2004m, 1993vs, 1972vw, 1958w, 1939w and 1850br,vw; l H NMR (400 MHz, CDC13, 294 K) 6 7.70 (d, 2 H , 5.36 (s, 5 H), 2.47 ( s , 3 H) and 2.28 (s, 3 H); 13C NMR (100 MHz, CD2Clz, 300 K) 6 231.2 (.Iwpc 136 Hz), 187.0, 186.0, 180.8 (br, 3 C), (~PSO-C~HS), 129.2 (o,m-C6Hs), 127.7 (p-c6H5), 90.9 ( 5 c ) , 39.1 JH-H 7.7 Hz), 7.47 (t, 2 H, JH-H 8.4 Hz), 7.39 (t, 1 H , JH-H 7.4 Hz), 177.3 (3 C); 6 237.5 (Jw-c 68 Hz), 156.7 (Jw-c 88 Hz), 137.3, 135.1, (Jw-c 14 Hz), 133.2 (2 C), 129.7, 129.2 (2 C), 120.3, 92.6 (5 C), 30.9
(Me) and 19.2 (Me). Satisfactory elemental analyses were obtained for 1 and 2.
1024 J . CHEM. SOC., CHEM. COMMUN., 1991 1 Me Me Me 3 Me C0l9 5 Scheme 1
to an orange cluster WOs3Cp(CO)g( p3-CPh)( CCMeCMe) 4 (18%).$ Complex 3 was produced by the activation of the phenyl substituent of the C4 hydrocarbon. Its 1H NMR spectrum exhibits four aromatic proton signals within the range 6 8.49-6.59 and a hydride signal at 6 -14.26 with the characteristic tungsten-hydrogen coupling .IWvH 73.8 Hz. In
contrast, complex 4 was formed by C-C bond scission of the carbon chain. Its structure was unambiguously established by comparison of its solution IR v(C0) spectrum with that of the related derivative WOs3Cp(CO)~(p~-CPh)[CC(Tol)C(Tol)] generated from the direct thermal reaction between WCp(CO)&=CPh and O S ~ ( C O ) ~ ~ ( C ~ T O ~ ~ ) . ~
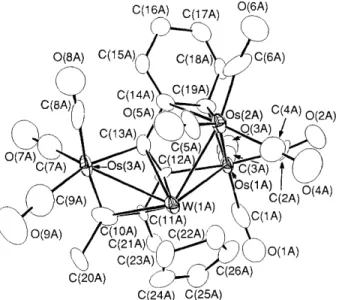
Complex 3 was examined by single-crystal X-ray diffrac- tion.1 The complex consists of two crystallographically distinct, but structurally similar molecules in the lattices; each displays a ‘spiked-triangular’ core arrangement with the tungsten atom located at the centre positions. An ORTEP diagram of one molecule is presented in Fig. 1. The osmium atom Os(3A) and the C4 hydrocarbon backbone are arranged like a metallacyclopentadienyl fragment ,8 which coordinates
to the tungsten atom via two alkenic n-interactions and a W-0s interaction and also links to a metal atom via the
9 Spectral data for 3: MS, mlz 1232 (M+). IR (C6H12) v(CO)/cm-1
2069vw, 2053vs, 2036vs, 1997m, 1987m, 1975s and 1956w; 1H NMR (400 MHz, CDC13,294 K) 6 8.49 (d, 1 H, JH-H 8.2 Hz), 7.59 (d, 1 H, JH-H 8.7 Hz), 7.03 (t, 1 H, JH-H 7.7 Hz), 6.59 (t, 1 H, J H - H 7.3 Hz), Hz); ‘3C NMR (100 MHz, CDC13,253 K) 6 184.9 (3 C), 180.5, 179.8 5.23 (s, 5 H), 2.53 (s, 3 H), 2.46 (s, 3 H) and - 14.26 (s, 1 H, JW-H 73.8 (2 C), 177.2, 175.5, 175.3, 174.0, 156.7, 154.1, 147.8, 141.1, 127.0, 122.8, 117.8, 114.3, 107.0 (Jc-w 11 Hz), 89.0 (5 C), 32.4 (Me), 19.2 (Me). Spectral data for 4: M S , mlz 1232 (M+). IR (C6HI2) v(CO)/cm-l, 2076s, 2044vs, 2035m, 2018s, 1997vw, 1975m, 1959s and 1914br,w; lH NMR (400 MHz, CD2C12, 294 K): 6 7.16 (t, 2 H , JH-H 5 H), 3.39 (s, 3 H) and 2.09 (s, 3 H); 13C NMR (100 MHz, CD2C12, 6 236.1, 192.0, 162.2, 136.7, 130.6, 128.8 (2C), 127.2 (2C), 96.5 (5C), 39.2 (Me) and 32.1 (Me). Satisfactory elemental analyses were obtained for 3 and 4.
7
Crystal data for 3: C26H15090s3W1, M = 1225.85, monoclinic, space group P21/n,a = 19.596(6), b = 15.288(4), c = 20.352(6)A,
f~ = 118.63(2)”, U = 5352(3) A3, Z = 8, D, = 3.043 gcm-3, F(OO0) = 4358, Nonius CAD-4 diffractometer with Mo-Ka radiation, h = 0.7930A,
~ ( M o - K a ) = 18.65 mm-l. R = 0.050, R, = 0.045 for total 6979 reflections and 5217 reflections with I > 2 . 0 4 4 . Atomic coordinates, bond lengths and angles, and thermal parameters have been deposited at the Cambridge Crystallographic Data Centre for 3, 5 and 6. See
Notice to Authors, Issue No. 1.
7.8Hz),7.05(t, ~ H , J H - H ~ . ~ H Z ) , ~ . ~ O ( ~ , ~ H , J H - H ~ . ~ H Z ) , ~ . ~ ~ ( S ,
294 K) OS-CO 6 185.6, 183.2, 180.7, 179.8, 178.9,176.5, (3C), 172.1;
C ( Z A ) C\Ti5A)
Fig. 1 The molecular drawing of complex 3. Bond lengths
(A):
2.862(2), Os(3a)-W(lA) 2.865(2), Os(lA)-C(12A) 2.10(3), Os(1A)- Os(lA)-0~(2A) 2.836(2), Os(lA)-W(lA) 2.987(2), Os(2A)-W(lA) C(19A) 2.06(3), 0~(2A)-C(14A) 2.36(3), 0~(2A)-C(19A) 2.21(3), Os(3A)-C(lOA) 1.96(3), 0~(3A)-C(13A) 2.15(3), W(1A)-C(1OA) 2.38(3), W( lA)-C( 11A) 2.23(3) , W( 1 A)-C( 12A) 2.19( 3), W (1A)- C(13A) 2.35(3), C(llA)-C(12A) 1.45(3), C(l2A)-C(13A) 1.38(4), C(13A)-C(14A) 1.51(4), C( 14A)-C(19A) 1.41(4), C( 14A)-C( 15A) 1.42(4), C( 15A)-C(16A) 1.40(4), C( 16A)-C(17A) 1.45(4), C( 17A)- C(18A) 1.37(4) and C(18A)-C(19A) 1.47(4).
Fig. 2 The molecular drawing of 5 . Bond lengths
(A):
Os(l)-Os(2) 2.743(1), 0 ~ ( 1 ) - 0 ~ ( 3 ) 2.827(1), OS(1)-W 3.107(1), 0 ~ ( 2 ) - 0 ~ ( 3 ) 2.779(1), 0 ~ ( 3 ) - W 2.680(1), Os(l)-C(15) 2.22(1), Os(2) * * C(14) 2.98(1), 0~(2)-C(15) 2.33(1), 0~(3)-C(10) 2.08(1), 0 ~ ( 3 ) - C ( 13) 2.10( l), W-C(10) 2.30( 1), W-C( 11) 2.33( l ) , W-C( 12) 2.36( l ) , W-C(l3) 2.28(1), C(lO)-C(11) 1.50(2), C(ll)-C(12) 1.42(2), C(12)- C(13) 1.39(2), C(13)-C(14) 1.46(2), C(14)-C(15) 1.41(2), C(14)- C(19) 1.39(2), C(15)-C(16) 1.42(2), C(16)-C(17) 1.38(2), C(17)- C(18) 1.37(2) and C(18)-C(19) 1.38(2).C( 12A)-Os( 1A) bond. The phenyl substituent is now bound to the atom Os(1A) of the WOs2 metal triangle and to the atom Os(2A) via q2-coordination. This same bonding is seen in the pyrolysis products of triosmium and triruthenium phenylphosphine complexes.9 Finally, the bridging hydride is proposed to be associated with the W(lA)-Os(lA) bond, because its bond length is the longest of the three W-0s bonds in the molecule.
Further pyrolysis of 3 in refluxing toluene as solvent for 2.5 h, inducing the hydride migration to the coordinated C4 hydrocarbon, produced two cluster derivatives W O S ~ C ~ ( C O ) ~ [ C M ~ C M ~ C H C ( p2-q2-C6H4)] 5 and WOs3Cp-
J. CHEM. SOC., CHEM. COMMUN.,
1991
1025respectively (Scheme 1). These complexes underwent revers- ible equilibration in refluxing toluene, because heating a solution of either complex 5 or 6 produced a mixture of both complexes within 30 min.11 In addition, these complexes are stable at room temperature, which allows us to carry out the routine TLC separation and identification by elemental analysis, spectral methods** and X-ray diffraction.??
The structure of 5 adopts an Os3W butterfly core geometry with the tungsten atom sited at one of the 'wingtip' positions (dihedral angle 177.32( 1)'). As indicated by its molecular drawing (Fig. 2), the bridging hydride of 3 is moved to the third carbon atom C(13) of the C4 hydrocarbon linkage, in which all four carbon atoms and the Os(3) atom take up a cyclic, planar disposition similar to complex 3. The phenyl substituent is linked to the C4 linkage via the C(13)-C(14)
bond and from the adjacent carbon atom C(15) which forms an asymmetric bridge over the Os(l)-Os(2) edge. The bonding between the phenyl group and the metal atoms is best described as the formation of Os(1)-C(15) o-bond and of a n-interaction with Os(2) from a delocalized molecular orbital of appropriate symmetry which is centred at C(15). Lewis and Johnson have utilized a similar description to account for the bonding of benzyne moieties in several triosmium com- plexes. 10
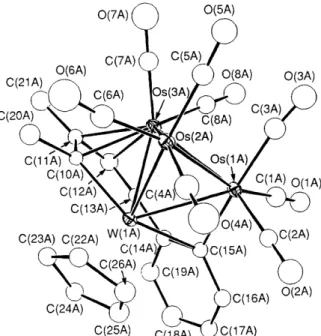
Complex 6 crystallized in the
Pi
space group with two independent molecules in the unit cell. The ORTEP diagram of one molecule is shown in Fig. 3. As expected, the Os3W cluster core consists of a tetrahedral geometry and the C4 fragment adopts a similar type of bonding arrangement ,except that the phenyl group has migrated from an 0s-0s edge to an 0s-W edge. It is possible that the transformation from butterfly to tetrahedral is initiated by the removal of CO
from the tungsten atom and the formation of a new 0s-W bond. The migration of phenyl substituent and fine adjust- ment of the C4 chain plays the secondary role of balancing the number of formal electrons on each individual metal atom.
In summary, our work has provided an interesting example of changing the cluster framework from a butterfly to a spiked triangle, then back to a butterfly and, finally to a tetrahedral
arrangement (Scheme 1). The coordinated hydrocarbon ligand serves as a reservoir to supply two pairs of electrons via
ovtho-metallation and complexation of the phenyl group via n-bonding to compensate the coordinative unsaturation generated during the formation of spiked triangular complex 3. The cluster core undergoes conversion to butterfly and then to tetrahedral via consecutive removal of two pairs of electrons as a result of hydride migration followed by loss of a second CO ligand. This sequence demonstrates some conceiv-
11
Extending the reaction time to six hours induced further loss of CO, giving another Os3W cluster with eight CO ligands.**
Spectral data for 5 : MS, mlz 1232 (M+). IR (CH2C12) v(CO)lcm-l, 2060s, 2022s, 1982 vs, br and 1972m, br; lH NMR (400 MHz, CD2C12, 294 K) 6 8.22 (d, 1 H , JH-H 7.4 Hz), 7.77 (t, 1 H , JH-H 7.3 Hz), 7.26 (d, 1 H, JH-H 7.9 Hz), 6.88 (s, 1 H), 6.55 (t, 1 H , JH-H 7.1 Hz), 4.82 (s, 5 H), 2.72 (s, 3 H) and 2.32 (s, 3 H). Spectral data for 6: MS, mlz 1204 (M+). IR (CC14) v(C0)lcm-l 2061vs, 2024vs, 1 9 8 5 ~ s ~ 1964vw and 1937m; lH NMR (400 MHz, CD2C12, 294 K) 6 7.79 (d, 1 H, JH-H 8.1 H),6.80(t, 1 H,JHVH7.6Hz),4.85 (s, 5 H),2.63 (s,3 H) a n d 2 . 3 6 ( ~ , 3 H). Satisfactory elemental analyses were obtained for 5 and 6.t t Cryst& data for 5 : C26HI5O90s3W1, M = 1225.85, triclinic, space group P1, a = 8.158(3), b = 11.080(2), c = 15.519(3)
A,
a = 106.29(1), fi = 92.44(2), y = 109.96(3)", U = 1251(1) A3, 2 = 2, D, = 3.255 g ~ m - ~ , F(OO0) = 1089, h = 0.70930A,
p(Mo-Ka) = 19.95 mm-1. R = 0.037, R, = 0.033 for total 4596 reflections and 3806 reflections with I > 2.09(Z). 6: C2sH1s080~3W1, M = 1197.84, triclinic, s ace group P1, a = 9.465(2), b = 15.963(5), c = 17.161(3)1,
a = 97.45(2), fi = 103.07(2), y = 90.37(2)", U = 2503(1) A3, 2 = 4, D, = 3.179 g ~ m - ~ , F(OO0) = 2123, h = 0.70930A,
p(Mo-Ka) = 19.93 mm-1. R = 0.081, R, = 0.076 for total 6505 reflections and 5781 reflections with I > 2.04Z).Hz), 7.74 (t, 1 H, JH-H 7.3 Hz), 7.04 (d, 1 H, JH-H 7.9 Hz), 6.82 (s, 1
Fig. 3 The molecular drawing of 6. Bond lengths
(A):
Os(lA)-Os(2A) 2.791(2), OS( lA)-Os(3A) 2.847(2), OS( lA)-W( 1A) 2.826(2), OS(~A)-OS( 3A) 2.827(2) , Os(2A)-W( 1A) 2.75 1(2), Os(3A)-W( 1A) 2.735(2), Os(lA)-C(lSA) 2.07(4), Os(3A)-C(lOA) 2.28(3), Os(3A)- C(11A) 2.26(3), 0~(3A)-C(12A) 2.24(4), 0~(3A)-C(13A) 2.17(3), W ( 1 A)-C( 10A) 2.17 (3), W ( 1 A)-C( 13A) 2.13 (4), W ( 1 A)-C( 14A) 2.45(4), C(lOA)-C(11A) 1.39(5), C(l lA)-C( 12A) 1.43(5), C( 12A)- C( 13A) 1.34(5), C( 13A)-C( 14A) 1.43(4), C( 14A)-C( 15A) 1.49(4), C(14A)-C(19A) 1.49(5), C(15A)-C(16A) 1.44(5), C(16A)-C(17A) 1.35(6), C( 17A)-C( 18A) 1.43(6) and C( 18A)-C(19A) 1.38(6).able metal-metal and metal-substrate bonding interactions for chemisorbed hydrocarbons on catalytic surfaces.
We thank the National Science Council of the Republic of China for support of this research project (Grant No.
NSC80-0208-M007-60).
Received, 6th March 1991; Corn. 1101066B
References 1 2 3 4 5 6 7 8 9 10
R. D. Adams, in The Chemistry of Metal Cluster Complexes, eds. D. F. Shriver, H. D. Kaesz and R . D. Adams, VCH, New York, 1990, ch. 3.
Y. Chi, D.-K. Hwang, S.-F. Chen and L.-K. Liu, J. G e m . SOC., Chem. Commun., 1989, 1540; D.-K. Hwang, Y. Chi, S.-M. Peng and G.-H. Lee, Organometallics, 1990, 9, 2709; Y. Chi, G.-H. Lee, S.-M. Peng and C.-H. Wu, Organometallics, 1989, 8, 1574; D.-K. Hwang, Y. Chi, S.-M. Peng, and G.-H. Lee, J. Organomet. Chem., 1990, 389, C7; Y. Chi. L.-K. Liu, G. Huttner and L. Zsolnai, J . Organomet. Chem., 1990, 390, C50.
Y. Chi, C.-Y. Cheng and S.-L. Wang, J. Organomet. Chem., 1989, 378,45; Y. Chi, F.-J. Wu, B.-J. Liu, C . - C . Wang and S.-L. Wang,
J. Chem. SOC., Chem. Commun., 1989, 873; Y. Chi, S.-H. Chuang, B.-F. Chen, S.-M. Peng and G.-H. Lee, J. Chem. SOC., Dalton Trans., 1990, 3033.
M. I. Bruce, M. G. Humphrey, J. G. Matisons, S. K. Roy and
A. G. Swincer, Aust. J. Chem., 1984, 37, 1955.
B. F. G . Johnson, R. Khattar, J. Lewis, P. R. Raithby and D. N. Smit, J. Chem. SOC., Dalton Trans., 1988, 1421.
Y. Chi, C.-H. Wu, S.-M. Peng and C.-H. Lee, Organometallics, 1990, 9, 2305.
Y. Chi, H.-F. Hsu, S.-M. Peng and G.-H. Lee, J . Chem. SOC., Chem. Commun., the preceding paper.
A. J. Deeming, Adv. Organomet. Chem., 1986,26, 1; G. Ferraris and G. Gervasio, J. Chem. SOC., Dalton Trans., 1972, 1057. A. J. Deeming, S. E. Kabir, N. I. Powell, P. A . Bates and M. B. Hursthouse, J. Chem. SOC., Dalton Trans., 1987, 1529; M. I. Bruce, P. A. Humphery, 0. B. Shawkataly, M. R. Snow, E. R. T. Tiekink and W. R. Cullen, Organometallics, 1990, 9, 2910. P. A. Jackson, B. F. G. Johnson, J. Lewis, A. D. Massey D. Braga, C. Gradella and F. Grepioni, J. Organomet. Chem., 1990, 391, 225.