Lancet 2011; 377: 1077–84 Published Online March 23, 2011 DOI:10.1016/S0140-6736(11)60310-3 See Comment page 1049 Christchurch Hospital, Christchurch, New Zealand (M Than MBBS, S Aldous MBChB, Prof P M George MBBS); Royal Brisbane and Women’s Hospital, Herston, QLD, Australia (L Cullen MBBS, W A Parsonage MD); Monash University, Melbourne, VIC, Australia (Prof C M Reid PhD); Singapore General Hospital, Singapore, Singapore (Prof S H Lim MBBS); University of Otago, Christchurch, New Zealand (Prof M W Ardagh PhD, Prof A M Richards MD); Cleveland Clinic Foundation, Cleveland, OH, USA (W F Peacock MD); Queen Elizabeth Hospital, Hong Kong, China (H F Ho MD, H F Ko MD); Medanta—The Medicity, Gurgaon, India (R R Kasliwal MD, M Bansal MD); National Cardiovascular Centre Harapan Kita, Jakarta, Indonesia (S Soerianata MD); People’s Hospital, Beijing, China (Prof D Hu MD, R Ding MD); Xuanwu Hospital, Beijing, China (Q Hua MD); Severence Hospital, Seoul, South Korea (Prof K Seok-Min MD); Ramathibodi Hospital, Bangkok, Thailand (Prof P Sritara MD, Prof R Sae-Lee MD); Chang Gung Memorial Hospital, Keelung, Taiwan (T-F Chiu MD); Far Eastern Memorial Hospital, Taipei, Taiwan (K-C Tsai MD, F-Y Chu MD); China Medical University Hospital, Taichung, Taiwan (W-K Chen MD); MacKay Memorial Hospital, Taipei, Taiwan
(W-H Chang DrPH); Queensland University of Technology, Brisbane, QLD, Australia (L Cullen); and University of
A 2-h diagnostic protocol to assess patients with chest pain
symptoms in the Asia-Pacifi c region (ASPECT): a prospective
observational validation study
Martin Than, Louise Cullen, Christopher M Reid, Swee Han Lim, Sally Aldous, Michael W Ardagh, W Frank Peacock, William A Parsonage, Hiu Fai Ho, Hiu Fai Ko, Ravi R Kasliwal, Manish Bansal, Sunarya Soerianata, Dayi Hu, Rongjing Ding, Qi Hua, Kang Seok-Min, Piyamitr Sritara,
Ratchanee Sae-Lee, Te-Fa Chiu, Kuang-Chau Tsai, Fang-Yeh Chu, Wei-Kung Chen, Wen-Han Chang, Dylan F Flaws, Peter M George, A Mark Richards
Summary
Background Patients with chest pain contribute substantially to emergency department attendances, lengthy
hospital stay, and inpatient admissions. A reliable, reproducible, and fast process to identify patients presenting with chest pain who have a low short-term risk of a major adverse cardiac event is needed to facilitate early discharge. We aimed to prospectively validate the safety of a predefi ned 2-h accelerated diagnostic protocol (ADP) to assess patients presenting to the emergency department with chest pain symptoms suggestive of acute coronary syndrome.
Methods This observational study was undertaken in 14 emergency departments in nine countries in the
Asia-Pacifi c region, in patients aged 18 years and older with at least 5 min of chest pain. The ADP included use of a structured pre-test probability scoring method (Thrombolysis in Myocardial Infarction [TIMI] score), electrocardiograph, and point-of-care biomarker panel of troponin, creatine kinase MB, and myoglobin. The primary endpoint was major adverse cardiac events within 30 days after initial presentation (including initial hospital attendance). This trial is registered with the Australia-New Zealand Clinical Trials Registry, number ACTRN12609000283279.
Findings 3582 consecutive patients were recruited and completed 30-day follow-up. 421 (11·8%) patients had a major
adverse cardiac event. The ADP classifi ed 352 (9·8%) patients as low risk and potentially suitable for early discharge. A major adverse cardiac event occurred in three (0·9%) of these patients, giving the ADP a sensitivity of 99·3% (95% CI 97·9–99·8), a negative predictive value of 99·1% (97·3–99·8), and a specifi city of 11·0% (10·0–12·2).
Interpretation This novel ADP identifi es patients at very low risk of a short-term major adverse cardiac event who
might be suitable for early discharge. Such an approach could be used to decrease the overall observation periods and admissions for chest pain. The components needed for the implementation of this strategy are widely available. The ADP has the potential to aff ect health-service delivery worldwide.
Funding Alere Medical (all countries), Queensland Emergency Medicine Research Foundation and National Health
and Medical Research Council (Australia), Christchurch Cardio-Endocrine Research Group (New Zealand), Medquest Jaya Global (Indonesia), Science International (Hong Kong), Bio Laboratories Pte (Singapore), National Heart Foundation of New Zealand, and Progressive Group (Taiwan).
Introduction
Every year, an estimated 5–10% of presentations to emergency departments, and up to a quarter of hospital admissions are attributable to symptoms suggestive of acute coronary syndromes.1 Patients with a missed
diagnosis of acute myocardial infarction are at increased risk of a major adverse cardiac event. The need for safe discharge without a substantial risk of a major adverse cardiac event is a priority and a driver of clinician behaviour. Consequently, most patients with symptoms suggestive of acute coronary syndromes undergo lengthy assessment, either in the emergency department or as hospital inpatients, even though 75–85% of these patients ultimately do not have a fi nal diagnosis of acute coronary syndromes.2–4 The assessment processes vary
between institutions, with no one process being ideal.
Present recommendations are for serial sampling of cardiac troponin over at least 6 h from the onset of symptoms.5–7 Concerns about accuracy of patients’ recall
of events has led many centres to time troponin sampling from the moment of presentation to the emergency department.8 Prolonged assessment
contributes to overcrowding in the hospital or department, physician duplication of eff ort, and clinical risk as patients are treated by diff erent clinical staff .1
Emergency department overcrowding is associated with increased costs and adverse patient outcomes, including increased mortality.9
A reliable, reproducible, and more timely process for the identifi cation of chest pain presentations that have a low short-term risk of a major adverse cardiac event is needed to facilitate earlier discharge.4 Accelerated diagnostic
Queensland, Brisbane, Australia (D F Flaws MSc) Correspondence to: Dr Martin Than, Department of Emergency Medicine, Canterbury District Health Board, Private Bag 4710, Christchurch, New Zealand martinthan@xtra.co.nz
protocols (ADPs), clinical decision rules, and prediction rules are terms for processes or methods intended to help clinicians to make bedside diagnostic and therapeutic decisions. They involve variables from the patient’s history and examination, and often incorporate the results of diagnostic tests.6 ADPs for chest pain are well established
but emphasise the need to assess the patient for at least 6 h after the onset of symptoms.6,10 Some studies have
safely investigated patients with serial biomarkers during 1·5–3 h in a low-risk patient group, but have not defi ned a reproducible method to identify this low-risk group.11
For an assessment of possible acute coronary syndromes, a maximum of 60 min is recommended for the availability of troponin results.12 Many central
laboratories have diffi culty in meeting this standard. Point-of-care bio markers represent a possible solution to meeting this target. The Thrombolysis In Myocardial Infarction (TIMI) score for unstable angina or non-ST elevation myocardial infarction is an externally validated and widely used structured risk assessment method.3,13,14
Its use in conjunction with serial 0–2 h biomarker testing Panel 1: The TIMI score for unstable angina or non-ST
elevation myocardial infarction15
(1) Age 65 years or older
(2) Three or more risk factors for coronary artery disease (family history of coronary artery disease, hypertension, hypercholesterolaemia, diabetes, or being a current smoker)
(3) Use of aspirin in the past 7 days
(4) Signifi cant coronary stenosis (eg, previous coronary stenosis ≥50%)
(5) Severe angina (eg, two or more angina events in past 24 h or persisting discomfort)
(6) ST-segment deviation of 0·05 mV or more on fi rst electrocardiograph
(7) Increased troponin and/or creatine kinase MB on initial blood tests*
The TIMI score had to be zero for the sum of its seven parameters to be categorised as 0. TIMI=Thrombolysis In Myocardial Infarction. *Point-of-care values were used for TIMI score calculation.
3853 eligible patients
202 declined consent
21 excluded because TIMI score incomplete
0 inconclusive result
18 lost to follow-up 3651 consenting eligible
patients
3630 had ADP index test
349 did not have 30-day MACE 3 had 30-day MACE
370 were ADP negative: low risk
352 completed 30-day follow-up 30 lost to follow-up
2812 did not have 30-day MACE 418 had 30-day MACE
3260 were ADP positive: not low risk
3230 completed 30-day follow-up
Figure 1: Trial profi le of participant recruitment and outcomes according to ADP classifi cation
30-day follow-up includes initial hospital attendance. Patients lost to follow-up did not have a MACE during initial hospital attendance. TIMI=Thrombolysis In Myocardial Infarction score for unstable angina or non-ST-elevation myocardial infarction. ADP=accelerated diagnostic protocol. MACE=major adverse cardiac event.
(either via central laboratory or point-of-care systems) and electrocardiograph (ECG) has not been prospectively tested. Importantly, there has been little validation of ADPs based in emergency departments outside North America, or in diverse population groups such as the Asia-Pacifi c population, in whom a mix of ethnic backgrounds and variations in service delivery introduce important diff erences.15
The ASia-Pacifi c Evaluation of Chest pain Trial (ASPECT) was a prospective observational validation study designed to assess whether a predefi ned ADP would identify patients presenting to the emergency department with chest pain, who would be at low risk of harm if they were to be discharged early.
Methods
Participants
Enrolment occurred at 14 urban emergency departments in nine countries in the Asia-Pacifi c region (Australia, China [including Hong Kong], India, Indonesia, New Zealand, Singapore, South Korea, Taiwan, and Thailand). Patients were included if they were at least 18 years old and had at least 5 min of chest pain (or discomfort) suggestive of acute coronary syndromes for whom the attending physician planned to investigate for these syndromes with serial biomarker tests. In accordance with American Heart Association case defi nitions,16
possible cardiac symptoms included acute chest; epigastric, neck, jaw, or arm pain; or discomfort or pressure without an apparent non-cardiac source. Generally, atypical symptoms (fatigue, nausea, vomiting, diaphoresis, faintness, and back pain) were not used as inclusion criteria in the absence of chest pain.
Patients were excluded if they had an ST-segment elevation acute myocardial infarction, there was a clear cause other than acute coronary syndromes for the symptoms (eg, clinical fi ndings of pneumonia), they were unable or unwilling to provide informed consent, staff considered recruitment to be inappropriate (eg, terminal illness), they were transferred from another hospital, they were pregnant, they were recruited on previous presentation, or they were unable to be contacted after discharge. Perceived high risk was not regarded as an exclusion criterion. Recruitment included consecutive eligible cases at each site. Overall enrolment occurred between November, 2007, and July, 2010, but individual sites started and fi nished at diff erent times according to local logistics. Patients were managed according to local protocols.
All data collection occurred prospectively and the data dictionary has been published previously.17 Research
nursing staff collected the demographic and risk data from each patient, supervised ECG testing, and drew blood samples for biomarker testing. If a patient was unsure of an answer (eg, family history) a response of no was recorded. Patients were tracked for adverse events at 30 days from initial attendance with hospital records and
telephone follow-up. Data coordination, monitoring and analysis, and source verifi cation was done through an independent university clinical research organisation at a non-recruitment location in Australia (Centre for Clinical Research Excellence, Monash University, Melbourne). Approval from local ethics committees was obtained, and all patients provided written informed consent.
Procedures
The primary endpoint was major adverse cardiac events within 30 days after initial presentation (including initial hospital attendance). The criteria for major adverse cardiac event included any of the following: death (not clearly non-cardiac), cardiac arrest, an emergency revascularisation procedure, cardiogenic shock, ventri cular arrhythmia needing intervention, high-degree atrio ventricular block needing intervention,
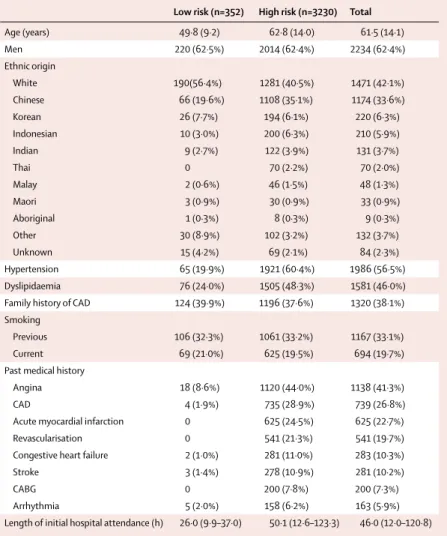
Low risk (n=352) High risk (n=3230) Total
Age (years) 49·8 (9·2) 62·8 (14·0) 61·5 (14·1) Men 220 (62·5%) 2014 (62·4%) 2234 (62·4%) Ethnic origin White 190(56·4%) 1281 (40·5%) 1471 (42·1%) Chinese 66 (19·6%) 1108 (35·1%) 1174 (33·6%) Korean 26 (7·7%) 194 (6·1%) 220 (6·3%) Indonesian 10 (3·0%) 200 (6·3%) 210 (5·9%) Indian 9 (2·7%) 122 (3·9%) 131 (3·7%) Thai 0 70 (2·2%) 70 (2·0%) Malay 2 (0·6%) 46 (1·5%) 48 (1·3%) Maori 3 (0·9%) 30 (0·9%) 33 (0·9%) Aboriginal 1 (0·3%) 8 (0·3%) 9 (0·3%) Other 30 (8·9%) 102 (3·2%) 132 (3·7%) Unknown 15 (4·2%) 69 (2·1%) 84 (2·3%) Hypertension 65 (19·9%) 1921 (60·4%) 1986 (56·5%) Dyslipidaemia 76 (24·0%) 1505 (48·3%) 1581 (46·0%)
Family history of CAD 124 (39·9%) 1196 (37·6%) 1320 (38·1%) Smoking
Previous 106 (32·3%) 1061 (33·2%) 1167 (33·1%)
Current 69 (21·0%) 625 (19·5%) 694 (19·7%)
Past medical history
Angina 18 (8·6%) 1120 (44·0%) 1138 (41·3%)
CAD 4 (1·9%) 735 (28·9%) 739 (26·8%)
Acute myocardial infarction 0 625 (24·5%) 625 (22·7%)
Revascularisation 0 541 (21·3%) 541 (19·7%)
Congestive heart failure 2 (1·0%) 281 (11·0%) 283 (10·3%)
Stroke 3 (1·4%) 278 (10·9%) 281 (10·2%)
CABG 0 200 (7·8%) 200 (7·3%)
Arrhythmia 5 (2·0%) 158 (6·2%) 163 (5·9%)
Length of initial hospital attendance (h) 26·0 (9·9–37·0) 50·1 (12·6–123·3) 46·0 (12·0–120·8)
Data are mean (SD), number (%), or median (IQR). Data were missing for each category as follows: ethnic origin (84), hypertension (75), dyslipidaemia (148), family history of CAD (118), smoking (54), previous medical history (824), and time in hospital (196). ADP=accelerated diagnostic protocol. CAD=coronary artery disease. CABG=coronary artery bypass graft.
Table 1: Characteristics for low-risk (ADP negative) and high-risk (ADP positive) participants in the
and prevalent (ie, being the cause for the patient’s initial presentation) and incident (ie, occurring during the 30-day follow-up) acute myocardial infarction. Out-comes and investigations were reported with minimum subjectivity with predefi ned standardised reporting guidelines (webappendix p 1).16–20 The presence of a
major adverse cardiac event was adjudicated independently by local cardiologists with these reporting guidelines. Cardiologists were masked to results of the index test biomarkers under investigation and derived TIMI score, but had knowledge of the clinical record, ECG, and serial troponin results from usual care.
In accordance with international guidelines, blood troponins at presentation, and then at least 6 h afterwards formed part of the reference standard to establish presence of acute myocardial infarction.7,16 These
measurements were part of normal care and were analysed at the recruitment site central hospital laboratory. Webappendix p 2 provides a summary of the characteristics of the laboratory troponins used at each hospital site. Treating clinicians were masked to the results of the index tests, with only central laboratory troponin results used in patient management. Classifi cation of acute myocardial infarction was based on global taskforce recommendations requiring evidence of myocardial necrosis together with evidence of myocardial ischaemia (ischaemic symptoms, ECG changes, or imaging evidence).7 Necrosis was diagnosed
on the basis of a rising or falling pattern of the laboratory cardiac troponin concentrations, with at least one value above the 99th percentile, at a level of assay imprecision near to 10%. If the troponin concentration was greater than the reference range, but no rise or fall was recorded, other causes of a raised troponin concentration were considered by the adjudicating cardiologist. If no clear alternative cause of the troponin rise was apparent, and if the clinical presentation was suggestive of acute coronary
syndromes, an adjudicated diagnosis of acute myocardial infarction was made.
The predefi ned ADP under investigation was a combination of TIMI risk score of 0, no new ischaemic changes on the initial ECG, and normal point-of-care biomarker panel (at 0–2 h after arrival). All parameters had to be negative for the ADP to be considered negative (and thus for the patient to be identifi ed as low risk). The TIMI score (panel 1) for unstable angina or non-ST-elevation myocardial infarction had to be zero for the sum of its seven parameters.14
New ECG ischaemic changes, with evidence that these changes were not pre-existing on previous ECGs, had to be absent. They were defi ned as ST-segment depression of at least 0·05 mV in two or more contiguous leads (including reciprocal changes), T-wave inversion of at
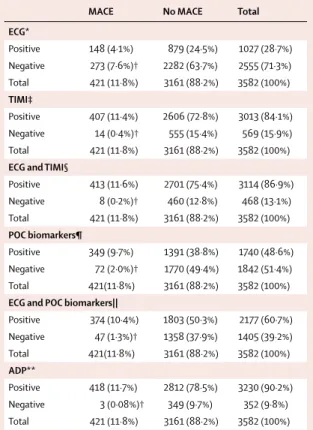
MACE No MACE Total
ECG* Positive 148 (4·1%) 879 (24·5%) 1027 (28·7%) Negative 273 (7·6%)† 2282 (63·7%) 2555 (71·3%) Total 421 (11·8%) 3161 (88·2%) 3582 (100%) TIMI‡ Positive 407 (11·4%) 2606 (72·8%) 3013 (84·1%) Negative 14 (0·4%)† 555 (15·4%) 569 (15·9%) Total 421 (11·8%) 3161 (88·2%) 3582 (100%)
ECG and TIMI§
Positive 413 (11·6%) 2701 (75·4%) 3114 (86·9%) Negative 8 (0·2%)† 460 (12·8%) 468 (13·1%) Total 421 (11·8%) 3161 (88·2%) 3582 (100%) POC biomarkers¶ Positive 349 (9·7%) 1391 (38·8%) 1740 (48·6%) Negative 72 (2·0%)† 1770 (49·4%) 1842 (51·4%) Total 421(11·8%) 3161 (88·2%) 3582 (100%)
ECG and POC biomarkers||
Positive 374 (10·4%) 1803 (50·3%) 2177 (60·7%) Negative 47 (1·3%)† 1358 (37·9%) 1405 (39·2%) Total 421(11·8%) 3161 (88·2%) 3582 (100%) ADP** Positive 418 (11·7%) 2812 (78·5%) 3230 (90·2%) Negative 3 (0·08%)† 349 (9·7%) 352 (9·8%) Total 421 (11·8%) 3161 (88·2%) 3582 (100%)
MACE=major adverse cardiac event. ECG=electrocardiograph. TIMI=Thrombolysis In Myocardial Infarction score for unstable angina or non-ST-elevation myocardial infarction. POC=point of care. ADP=accelerated diagnostic protocol. *ECG alone; any new ischaemia was positive. †Numbers of patients who were identifi ed as low risk by the diagnostic parameter(s) but had a MACE (ie, false-negative cases). ‡TIMI score of ≥1 was positive and TIMI score of 0 was negative. §ECG and TIMI used. Result was positive if TIMI score was ≥1 or ECG was positive. ¶POC biomarkers: troponin I, creatine kinase MB and change, and myoglobin and change. Any positive parameter created a positive result. ||ECG and POC biomarkers used. Any positive parameter created a positive result. **ADP was negative if TIMI score was 0 and if ECG and POC biomarkers were all negative. If TIMI score was ≥1 or any other parameter was positive, then ADP was positive.
Table 3: Occurrence of MACE during initial hospital attendance or
30-day follow-up according to results of individual and combinations of the ADP test parameters
Number of events* Patients (of 421) who had event type (%) Frequency of event type (of 3582 patients in study; %) NSTEMI 363 86·2% 10·1% STEMI 53 12·5% 1·5% Emergency revascularisation 32 7·6% 0·9% Cardiovascular death 19 4·5% 0·5% Ventricular arrhythmia 15 3·5% 0·4% Cardiac arrest 8 1·9% 0·2% Cardiogenic shock 7 1·7% 0·2%
High atrioventricular block 4 1·0% 0·1%
NSTEMI=non-ST-segment elevation myocardial infarction. STEMI=ST-segment myocardial infarction occurring after initial recruitment. *421 of 3582 (11·8%) patients had a total of 501 events during initial hospital attendance or 30-day follow-up.
Table 2: Frequency and type of major adverse cardiac event during initial
hospital attendance or 30-day follow-up
least 0·1 mV, or Q-waves greater than 30 ms in width and 0·1 mV or greater in depth in at least two contiguous leads.17,18,20 Patients with abnormal ECG fi ndings
(eg, pacing, left ventricular hypertrophy, and left bundle branch block) that were proven to be pre-existing on previous ECGs were defi ned as low risk.
Index test point-of-care biomarkers were measured with whole blood drawn at presentation and 2 h afterwards. Blood was immediately tested for troponin I, creatine kinase MB, and myoglobin. Results were available (to research staff only) within 15 min with the TRIAGE platform or CardioProfi lER assay panels (both Alere, San Diego, CA, USA). The following assay results were predefi ned to be positive on either blood draw: troponin I 0·05 μg/L or greater, creatine kinase MB 4·3 μg/L or greater, or an increase of 1·6 μg/L or more within 2 h; and myoglobin concentration of 108 μg/L or greater or an increase of 25% or more within 2 h. The point cutoff s were based on manufacturer recom-mendations, with an elevated troponin defi ned as any detectable concentration of troponin. The levels of change were based on a previous publication21 and
peer-group consensus.
Statistical analysis
Data were collected with the web-based Open-Clinica data capture system. Baseline characteristics of the study population were analysed with conventional group descriptive statistics. χ² analyses were used to generate two-by-two tables for the calculation of sensitivity, specifi city, and positive and negative predictive values. All analyses were done with SPSS (version 18.0.0).
The trial is registered with the Australia-New Zealand Clinical Trials Registry, number ACTRN12609000283279.
Role of the funding source
The sponsors of this study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had fi nal responsibility for the decision to submit for publication.
Results
3651 consenting eligible patients were enrolled, of whom 3582 completed 30-day follow-up (fi gure 1). Web-appendix p 3 shows the countries and hospitals that recruited patients. Study participants were mostly older men, either white or Chinese, and commonly had cardiovascular risk factors and background cardiovascular past medical history (table 1). A major adverse cardiac event occurred within 30 days in 421 (11·8%) patients. Non-ST-segment acute myocardial infarction (NSTEMI) was the most frequently occurring major adverse cardiac event (table 2).
The ADP identifi ed 9·8% (352/3582) of patients as being at low risk of a major adverse cardiac event within 30 days (all ADP parameters were negative). Three (0·9%) of these patients had an event during initial hospital attendance and follow-up (fi gure 1). Webappendix p 4 outlines the clinical details of these false negatives.
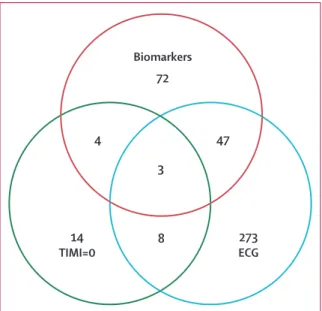
The combinations of parameters of the ADP were more eff ective at identifying patients who had a major adverse cardiac event than were the individual parameters themselves (table 3). The combination of the biomarkers and ECG without the TIMI score did not identify 3 8 47 4 Biomarkers 72 14 TIMI=0 273 ECG
Figure 2: Occurrence of a major adverse cardiac event during initial hospital
attendance or 30-day follow-up in patients with negative results for individual and combinations of diagnostic parameters
Figures refer to numbers of patients. TIMI=Thrombolysis In Myocardial Infarction. ECG=electrocardiograph. ECG* POC biomarkers† TIMI‡ POC biomarkers and ECG§ TIMI and ECG¶ ADP|| Sensitivity 35·2% (30·7–39·8) 82·9% (79·0–86·2) 96·7% (94·5–98·0) 88·8% (85·5–91·5) 98·1% (96·3–99·0) 99·3% (97·9–99·8) Negative predictive value 89·3% (88·0–90·4) 96·1% (95·0–96·9) 97·5% (95·8–98·6) 96·7% (95·5–97·5) 98·3% (96·5–99·2) 99·1% (97·3–99·8) Specifi city 72·2% (70·6–73·7) 56·0% (54·3–57·7) 17·6% (16·3–18·9) 43·0% (41·2–44·7) 14·6% (13·4–15·8) 11·0% (10·0–12·2) Positive predictive value 14·4% (12·3–16·7) 20·1% (18·2–22·0) 13·5% (12·3–14·8) 17·2% (15·6–18·8) 13·3% (12·1–14·5) 12·9% (11·8–14·5) Negative likelihood ratio 0·9 (0·8–1·0) 0·3 (0·3–0·4) 0·2 (0·1–0·3) 0·3 (0·2–0·3) 0·1 (0·1–0·3) 0·1 (0·0–0·2) Positive likelihood ratio 1·3 (1·1–1·5) 1·9 (1·8–2·0) 1·2 (1·1–1·2) 1·6 (1·5–1·6) 1·1 (1·1–1·2) 1·1 (1·1–1·3)
POC=point of care. ECG=electrocardiograph. ADP=accelerated diagnostic protocol. MACE=major adverse cardiac event. TIMI=Thrombolysis In Myocardial Infarction score for unstable angina or non-ST-elevation myocardial infarction. *ECG alone; any new ischaemia was positive. †POC biomarkers: troponin I, creatine kinase MB and change, and myoglobin and change. Any positive parameter created a positive result. ‡TIMI score of ≥1 was positive and TIMI score of 0 was negative. §POC biomarkers and ECG used. Any positive parameter created a positive result. ¶TIMI and ECG used. Result was positive if TIMI score was ≥1 or ECG was positive. ||ADP was negative if TIMI score was 0 and if ECG and POC biomarkers were all negative. If TIMI score was ≥1 or any other parameter was positive, then ADP was positive.
47 patients with a major adverse cardiac event at day 30. With use of the ADP including TIMI score, 44 additional patients were correctly identifi ed, which reduced the number of false negatives to three (fi gure 2).
Table 4 shows the statistical analysis of the ADP and its parameters for the prediction of a major adverse cardiac event by day 30. The ADP had a very high sensitivity and negative predictive value (table 4).
Secondary analysis showed that patients identifi ed as low risk by negative ADP were associated with a median initial hospital attendance of 26·0 h (IQR 9·9–37·0) and a mean of 43·2 h (95% CI 36·2–51·2), representing 1–2 hospital bed-days.
Discussion
Findings from this large, multinational study have prospectively validated that a 2-h accelerated diagnostic protocol, with use of point-of-care biomarkers, ECG, and TIMI score, can safely identify patients at very low short-term risk of a major adverse cardiac event (panel 2). These patients could potentially be discharged several hours earlier to outpatient follow-up and further investigations than with present practices.
The near 10% possible reduction in patients needing prolonged assessment in this large patient group could reduce overcrowding in hospitals and emergency departments and provide earlier reassurance and greater convenience for patients. The potential reduction in initial length of stay accords with the fi ndings of a six centre study in the UK.22 These fi ndings
together with those from countries included in our study represent 42% of the world’s population. Extrapolation is diffi cult, but on the basis of incidence rates of chest pain in the USA of 2·21%, there might be 64 million presentations of chest pain per year across these study nations. If the true incidence was half of this rate, then earlier discharge of 10% of patients could aff ect 3·2 million presentations. Patients in this study who were identifi ed as low risk had an initial hospital attendance of about 1–2 days; these patients could potentially be discharged within 3–4 h of arrival if follow-up investigations could be arranged as an outpatient. Increasing demand for acute hospital beds is a key challenge for modern health services.
The study shows that each of the components of the ADP is essential when used within such an early timeframe after presentation (fi gure 2, table 3). The use of the TIMI score within the ADP resulted in a lower and more acceptable false negative rate than when only biomarkers and ECG were used for the prediction of 30-day major adverse cardiac event (0·7% vs 11·2%).
Troponin assays with lower and more reliable levels of detection have been developed since this study started, but the assay we used was eff ective in this ADP. The focus of this study was the safety of the ADP when used as a whole; any contemporary troponin could be used either via the central laboratory or point of care as part
of the ADP. Newer assays, which typically have lower detection limits and higher analytical precision, would probably improve the sensitivity of this ADP for the prediction of a major adverse cardiac event. These newer assays might be used with decision rules under development23 for use in a broad risk population. In this
trial, combinations of biomarkers provided cumulative improvement in sensitivity, but a cardiac troponin as a sole biomarker was suffi cient alone to produce a high sensitivity of 98·6% (415/421) once ECG and TIMI were added. Although not an a-priori hypothesis, this fi nding suggests that the ADP might be optimised to include only the cardiac troponin results in conjunction with the ECG and TIMI risk score in the future. Other biomarkers (eg, copeptin and heart fatty acid binding protein) might improve the diagnostic accuracy for acute myocardial infarction; however, their use as part of an ADP has not been reported.24,25
The ADP might be expanded to a broader subset by development of a more specifi c risk score. The TIMI score was developed from a relatively high-risk population with acute coronary syndromes, but it has been externally validated in more general emergency department populations.2,3,26 A modifi ed TIMI risk score has been
derived and validated in an emergency department population previously with laboratory-based troponins,27,28
with a sensitivity of 96·6% reported in the validation Panel 2: Research in context
Systematic review
We searched Medline from March, 1995, to December, 2010, for full reports of original research and review articles with the terms “acute coronary syndrome”, “chest pain”, “emergency department”, “risk stratifi cation tools”, “point of care”, and “clinical decision rule”. We identifi ed 114 articles. Abstracts were downloaded for all titles of potential relevance. Full papers were downloaded when the abstract was also deemed relevant. To be included in the fi nal analysis, studies had to be prospective, have a large population, and have clearly described their methods and results. The methodology must have allowed the conclusions to be generalised to the emergency department population.
Interpretation
Together, the results of these studies show that the identifi cation of patients at low risk for major adverse cardiac events is challenging. Increasing research is emerging into the use of accelerated diagnostic protocols (ADP). These protocols typically include the use of a risk stratifi cation method, serial biomarkers, and electrocardiographs, and usually require an assessment period of 6–12 h. The results of our study indicate that a new ADP incorporating a risk stratifi cation method (TIMI score), electrocardiograph, and point-of-care biomarker testing can identify patients at low risk of 30-day major cardiac event at 2 h.
study. There is no universally accepted defi nition of a low-risk patient for acute coronary syndromes. This lack of consensus is a serious concern, because according to Bayesian decision making, interpretation of post-test probability after a particular test result is dependent on knowledge of the pre-test probability. The use of a structured and reproducible method is important.29–33
Subjective pre-test probability estimation has much lower inter-rater agreement between clinicians than do structured methods.34 Furthermore, patients presenting
to an emergency department are often initially assessed by junior staff , and evidence shows that traditionally taught clinical variables and risk factors are poor predictors of acute coronary syndromes in an un-diff erentiated population in these clinics.35–37
Patients without chest pain but who presented with atypical symptoms (fatigue, nausea, vomiting, diaphoresis, faintness, and back pain) were not included in this trial, and we were unable to quantify the number of patients presenting with these symptoms. Thus the applicability of the ADP is limited to the selected cohort of patients with chest pain (or discomfort) suggestive of acute coronary syndromes for whom the attending physician planned to investigate for these syndromes. Another limitation of this study is that this was an observational, not an intervention study. Ideally, a management study of the diagnostic protocol would now occur; however, in practice, such studies are rare.
The low specifi city (11%) of our approach might be regarded as a limitation, but the ADP was used as an exclusion method to predict safety of early discharge of patients and not to establish inpatient management. These patients would otherwise have had extended observation or admission. The low specifi city accords with other diagnostic instruments to exclude acute coronary syndromes.10 The goal of a more specifi c test is
to rule-in a diagnosis if positive with suffi cient certainty to initiate a change in management. In the setting that we studied, a positive protocol result merely classifi ed patients as requiring management as usual. The optimum balance between specifi city and sensitivity is diffi cult to defi ne. A process yielding a higher specifi city is likely to discharge a larger number of patients; however, we believe that the main focus should be on safety and therefore sensitivity. Future research should focus on methods to identify a greater proportion of patients who can be discharged earlier without signifi cant adverse events.
Contributors
MT had overall responsibility for the trial. MT, LC, CMR, SA, DFF, SHL, WAP, and AMR contributed to the study design. MT, SA, and PMG (New Zealand); LC, WAP, and DFF (Australia); HFH and HFK (Hong Kong); RRK and MB (India); SS (Indonesia); DH, RD, and QH (China); KS-M (Korea); SHL (Singapore); PS and RS-L (Thailand); and T-FC, K-CT, F-YC, W-KC, and W-HC (Taiwan) collected data. MT, LC, CMR, SA, WAP, and MWA analysed data. MT, LC, CMR, MWA, WFP, and AMR wrote the report, which was reviewed by all authors. MT and LC did the literature search.
Confl icts of interest
MT, MB, SHL, RRK, and LC received grants and supplies by Alere Medical. MT, AMR, and LC received honoraria for previous speaking and lecturing from Alere Medical. MT, MB, AMR, SHL, RRK, LC, and W-KC received support for travel to meetings from Alere Medical. MT received provision of administrative support funds from Alere Medical. HFH and HFK received grants from Science International Corporation. HFH received support for travel from Science International
Corporation. MWA received unrelated grants from HRCNZ. LC received grants from the Queensland Emergency research Foundation (QEMRF). SA received grants from the National Heart Foundation of New Zealand, and support for travel to meetings from the Christchurch Cardio-Endocrine Research Group. CMR received grants from the National Health and Medical Research Council. WAP has received grants from the QEMRF. He is a board member of Sanofi -Aventis, is a consultant for Hospira, and has been paid to give lectures for Sanofi -Aventis and Roche, all unrelated to this project. WFP has received consultancy payments from Alere for unrelated projects. SS received grants, support for travel to meetings, and fees for participation in review activities from Medquest Jaya Global. DH, RD, QH, KS-M, DFF, RS-L, SS, and PS received support from Alere to travel to meetings. T-FC, K-CT, F-YC, and W-HC received grants for nurses and support for travel from Progressive Group (Taiwan). PMG has received unrelated grants from the Health Research Council New Zealand, National Heart Foundation New Zealand, and National Health and Medical Research Council; and unrelated honoraria from Roche, AstraZenica, and Abbott Laboratories.
Acknowledgments
We thank the patients who participated in the trial; Angela Brennan, Carl Costolloe, and Philippa Loane for independent third party oversight of the study and source data verifi cation at the Centre for Clinical Research Excellence, Monash University, Melbourne; Queensland Emergency Medicine Research Foundation and National Health and Medical Research Council (Australia), Christchurch Cardio-Endocrine Research Group (New Zealand), Alere Medical (all countries), Medquest Jaya Global (Indonesia), Science International (Hong Kong), Bio Laboratories Pte (Singapore), National Heart Foundation of New Zealand, and Progressive Group (Taiwan) for helping to subsidise the costs of the research infrastructure at study sites; Allan S Jaff e, Jeff rey A Kline, Sarah Lord, Deborah Diercks, Steven Goodacre, Anthony F T Brown, Fred Apple, and Alan Maisel for reviewing the manuscript; Naresh Trehan for administrative support and patient recruitment in India; Rahul Mehrotra for patient recruitment and data collection and verifi cation in India; Darren M Beam for assistance with the data dictionary; Christopher M A Frampton for initial statistical advice; and Joanne M Deely for medical writing and editing.
References
1 Goodacre S, Cross E, Arnold J, Angelini K, Capewell S, Nicholl J. The health care burden of acute chest pain. Heart 2005; 91: 229–30. 2 Pollack CV Jr, Sites FD, Shofer FS, Sease KL, Hollander JE.
Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population. Acad Emerg Med 2006; 13: 13–18.
3 Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE. Prospective validation of the Thrombolysis in Myocardial Infarction risk score in the emergency department chest pain population. Ann Emerg Med 2006; 48: 252–59.
4 Hollander JE. The continuing search to identify the very-low-risk chest pain patient. Acad Emerg Med 1999; 6: 979–81.
5 Pollack CV Jr, Antman EM, Hollander JE. 2007 focused update to the ACC/AHA guidelines for the management of patients with ST-segment elevation myocardial infarction: implications for emergency department practice. Ann Emerg Med 2008 52: 344–55 e1. 6 Amsterdam EA, Kirk JD, Bluemke DA, et al. Testing of low-risk
patients presenting to the emergency department with chest pain: a scientifi c statement from the American Heart Association. Circulation 2010; 122: 1756–76.
7 Thygesen K, Alpert JS, White HD, et al. Universal defi nition of myocardial infarction. Circulation 2007; 116: 2634–53.
8 Aroney CN, Dunlevie HL, Bett JHN. Use of an accelerated chest pain assessment protocol in patients at intermediate risk of adverse cardiac events. Med J Aust 2003; 178: 370–74.
9 Bernstein SL, Aronsky D, Duseja R, et al. The eff ect of emergency department crowding on clinically oriented outcomes.
Acad Emerg Med 2009; 16: 1–10.
10 Steurer J, Held U, Schmid D, Ruckstuhl J, Bachmann LM. Clinical value of diagnostic instruments for ruling out acute coronary syndrome in patients with chest pain: a systematic review. Emerg Med J 2010; 27: 896–902.
11 Newby LK, Storrow AB, Gibler WB, et al. Bedside multimarker testing for risk stratifi cation in chest pain units: the chest pain evaluation by creatine kinase-MB, myoglobin, and troponin (CHECKMATE) study. Circulation 2001; 103: 1832–37.
12 Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Circulation 2007;
115: e352–55.
13 Conway Morris A, Caesar D, Gray S, Gray A. TIMI risk score accurately risk stratifi es patients with undiff erentiated chest pain presenting to an emergency department. Heart 2006; 92: 1333–34. 14 Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score
for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;
284: 835–42.
15 Miller CD, Lindsell CJ, Anantharaman V, et al. Performance of a population-based cardiac risk stratifi cation tool in Asian patients with chest pain. Acad Emerg Med 2005; 12: 423–30.
16 Luepker RV, Apple FS, Christenson RH, et al. Case defi nitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003; 108: 2543–49. 17 Cullen L, Than M, Brown AF, et al. Comprehensive standardized
data defi nitions for acute coronary syndrome research in emergency departments in Australasia. Emerg Med Australas 2010; 22: 35–55. 18 Hollander JE, Blomkalns AL, Brogan GX, et al. Standardized
reporting guidelines for studies evaluating risk stratifi cation of ED patients with potential acute coronary syndromes. Acad Emerg Med 2004; 11: 1331–40.
19 Forest RS, Shofer FS, Sease KL, Hollander JE. Assessment of the standardized reporting guidelines ECG classifi cation system: the presenting ECG predicts 30-day outcomes. Ann Emerg Med 2004;
44: 206–12.
20 Cannon CP, Battler A, Brindis RG, et al. American College of Cardiology key data elements and defi nitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. A report of the American College of Cardiology Task Force on Clinical Data Standards (Acute Coronary Syndromes Writing Committee). J Am Coll Cardiol 2001; 38: 2114–30. 21 Fesmire FM, Percy RF, Bardoner JB, Wharton DR, Calhoun FB.
Serial creatinine kinase (CK) MB testing during the emergency department evaluation of chest pain: utility of a 2-hour deltaCK-MB of +1.6ng/ml. Am Heart J 1998; 136: 237–44.
22 Goodacre SW, Bradburn M, Cross E, et al. The Randomised Assessment of Treatment using Panel Assay of Cardiac Markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart 2011;
97: 190–96.
23 Hess EP, Wells GA, Jaff e A, Stiell IG. A study to derive a clinical decision rule for triage of emergency department patients with chest pain: design and methodology. BMC Emerg Med 2008; 8: 3. 24 Reichlin T, Hochholzer W, Stelzig C, et al. Incremental value of
copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol 2009; 54: 60–68.
25 Valle HA, Riesgo LG, Bel MS, et al. Clinical assessment of heart-type fatty acid binding protein in early diagnosis of acute coronary syndrome. Eur J Emerg Med 2008; 15: 140–44.
26 Campbell CF, Chang AM, Sease KL, et al. Combining Thrombolysis in Myocardial Infarction risk score and clear-cut alternative diagnosis for chest pain risk stratifi cation. Am J Emerg Med 2009;
27: 37–42.
27 Body R, Carley S, McDowell G, et al. Can a modifi ed thrombolysis in myocardial infarction risk score outperform the original for risk stratifying emergency department patients with chest pain? Emerg Med J 2009; 26: 95–99.
28 Hess EP, Perry JJ, Calder LA, et al. Prospective validation of a modifi ed thrombolysis in myocardial infarction risk score in emergency department patients with chest pain and possible acute coronary syndrome. Acad Emerg Med 2010; 17: 368–75.
29 Dolan JG, Bordley DR, Mushlin AI. An evaluation of clinicians’ subjective prior probability estimates. Med Decis Making 1986;
6: 216–23.
30 Kassirer JP, Kopelman RI. Cognitive errors in diagnosis: instantiation, classifi cation, and consequences. Am J Med 1989;
86: 433–41.
31 Cahan A, Gilon D, Manor O, Paltiel O. Probabilistic reasoning and clinical decision-making: do doctors overestimate diagnostic probabilities? Q JM 2003; 96: 763–69.
32 Attia JR, Nair BR, Sibbritt DW, et al. Generating pre-test probabilities: a neglected area in clinical decision making. Med J Aust 2004; 180: 449–54.
33 Phelps MA, Levitt MA. Pretest probability estimates: a pitfall to the clinical utility of evidence-based medicine? Acad Emerg Med 2004;
11: 692–94.
34 Iles S, Hodges AM, Darley JR, et al. Clinical experience and pre-test probability scores in the diagnosis of pulmonary embolism. Q JM 2003; 96: 211–15.
35 Goodacre S, Locker T, Morris F, Campbell S. How useful are clinical features in the diagnosis of acute, undiff erentiated chest pain? Acad Emerg Med 2002; 9: 203–08.
36 Body R, Carley S, Wibberley C, McDowell G, Ferguson J, Mackway-Jones K. The value of symptoms and signs in the emergent diagnosis of acute coronary syndromes. Resuscitation 2010; 81: 281–86.
37 Han JH, Lindsell CJ, Storrow AB, et al. The role of cardiac risk factor burden in diagnosing acute coronary syndromes in the emergency department setting. Ann Emerg Med 2007; 49: 145–52.