Taiwan J Obstet Gynecol • June 2010 • Vol 49 • No 2 243 ■ RESEARCH LETTER ■
A 33-year-old woman, gravida 2, para 1, was referred to hospital for prenatal imaging evaluation of the fetal brain and limbs at 27 weeks’ gestation. The woman and her husband were healthy and non-consanguineous. Two years previously, she delivered a female baby at 26 weeks’ gestation with Joubert syndrome (JBTS), Dandy-Walker malformation, ventriculomegaly, cerebellar ver-mian hypoplasia and polydactyly of the feet. JBTS was confirmed by magnetic resonance imaging (MRI). The infant’s karyotype was 46,XX. During this pregnancy, cerebellar hypoplasia was noted at 17 weeks’ gestation, and ventriculomegaly and polydactyly were noted at 18 weeks’ gestation. Level II ultrasound examination at 27 weeks’ gestation revealed a male fetus with a femur length and an abdominal circumference equivalent to 27 weeks, polyhydramnios with an amniotic fluid index of 24.07 cm. Macrocephaly was present with an increased biparietal distance of 7.9 cm, an increased head circumference of 28.82 cm, an increased lateral ventricle posterior horn width of 2.64 cm, an increased transcerebellar distance of 3.61 cm, an increased cis-terna magna length of 2.10 cm, an increased head
circumference to femur length ratio of 5.66 (all> 95th centile). Ventriculomegaly, vermian hypoplasia, postax-ial polydactyly of the left hand and preaxpostax-ial polydactyly of the hallux of the left foot were also present. A tentative diagnosis of recurrent JBTS was made. Fetal MRI exami-nation at 27 weeks’ gestation revealed the “molar tooth sign”, Dandy-Walker malformation, ventriculomegaly
F
ETAL
M
AGNETIC
R
ESONANCE
I
MAGING
D
EMONSTRATION OF
C
ENTRAL
N
ERVOUS
S
YSTEM
A
BNORMALITIES AND
P
OLYDACTYLY
A
SSOCIATED
W
ITH
J
OUBERT
S
YNDROME
Chih-Ping Chen1,2,3,4,5,6*, Yi-Ning Su7, Jon-Kway Huang8, Yu-Peng Liu8,9, Fuu-Jen Tsai4,10,11, Chun-Kuang Yang12, Jian-Pei Huang1, Chen-Yu Chen1, Pei-Chen Wu1, Wayseen Wang2,13
Departments of1Obstetrics and Gynecology and 2Medical Research, Mackay Memorial Hospital, Taipei, 3Department of Biotechnology, Asia University, 4School of Chinese Medicine, College of Chinese Medicine,
China Medical University, Taichung, 5Institute of Clinical and Community Health Nursing, National
Yang-Ming University, 6Department of Obstetrics and Gynecology, School of Medicine, National Yang-Ming University, 7Department of Medical Genetics, National Taiwan University Hospital, 8Department of
Radiology, Mackay Memorial Hospital, 9Mackay Medicine, Nursing and Management College, Taipei, Departments of 10Medical Genetics and 11Medical Research, China Medical University
Hospital, Taichung, 12Department of Obstetrics and Gynecology, Tzu-Chi General Hospital, Taipei Branch, and 13Department of Bioengineering, Tatung University, Taipei, Taiwan.
*Correspondence to: Dr Chih-Ping Chen, Department
of Obstetrics and Gynecology, Mackay Memorial Hospital, 92, Section 2, Chung-Shan North Road, Taipei, Taiwan.
E-mail: cpc_mmh@yahoo.com Accepted: January 27, 2010
*
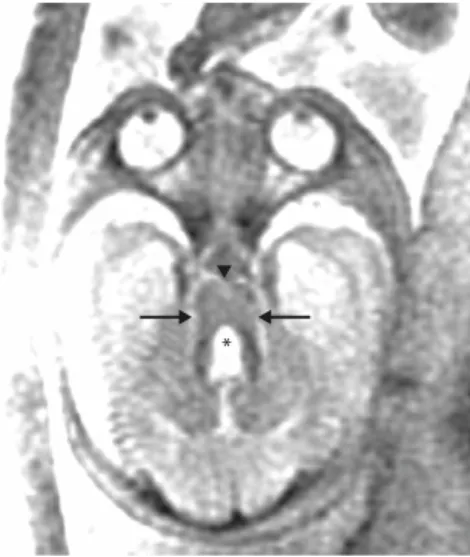
Figure 1.A male fetus at 27 weeks’ gestation with abnormal magnetic resonance imaging findings suggestive of Joubert syndrome. Prenatal axial imaging at the pontomesencephalic junction shows the “molar tooth sign” with cerebellar ver-mian hypoplasia, a dilated fourth ventricle (*), thickened and elongated superior cerebellar peduncles (arrows), and a deep interpeduncular fossa (arrowhead).
Taiwan J Obstet Gynecol • June 2010 • Vol 49 • No 2 244
C.P. Chen, et al
and polydactyly (Figures 1–5). On the transaxial scan, there was a complex brainstem malformation consisting of cerebellar vermian hypoplasia, thickened and elon-gated superior cerebellar peduncles and a deep interpe-duncular fossa, giving the appearance of the molar tooth sign which is pathognomonic for JBTS. The par-ents decided to continue the pregnancy. At 31+5weeks’ gestation, a 2,220 g live male baby was delivered with postaxial polydactyly of both hands, preaxial polydactyly of the left foot, and a 46,XY karyotype. The postnatal central nervous system findings were consistent with JBTS.
JBTS is a genetically heterogeneous disorder char-acterized by hypoplasia of the cerebellar vermis with a characteristic brainstem malformation and neuroradi-ologic molar tooth sign, intellectual disability, hypo-tonia, ataxia, tachypnea/apnea, and abnormal eye
movements [1]. JBTS and related disorders (JSRD) is classic JBTS with variable features such as central nerv-ous system anomalies including occipital encephalocele and corpus callosal agenesis, retinal dystrophy, ocular coloboma, oral frenulae, tongue tumors, polydactyly, cystic renal dysplasia, nephronophthisis, and congenital hepatic fibrosis [1]. JSRD is one type from the wide spec-trum of ciliopathies known to be caused by aberrant function of the primary cilia [1–4].
In the case presented here, JBTS involved an affected female and an affected male from the same sibship and is likely inherited in an autosomal recessive pat-tern. To date, at least nine genes and one additional locus have been identified in patients with JSRD [1]. The nine genes include INPP5E on 9q34.3 (JBTS1), AHI1 on 6q23.3 (JBTS3), NPHP1 on 2q13 (JBTS4), MKS4/
CEP290 on 12q21.32 (JBTS5), MKS3/TMEM67 on
8q21.13–q22.1 (JBTS6), MKS5/RPGRIP1L on 16q12.2 (JBTS7), ARL13B on 3q11.2 (JBTS8), CC2D2A on 4p15.3
Figure 2. Prenatal axial imaging shows a vermian cleft (arrow).
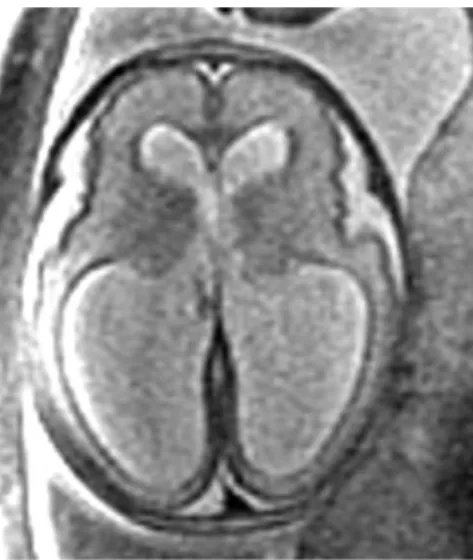
Figure 3. Prenatal axial imaging shows ventriculomegaly with dilation of the lateral ventricles.
*
Figure 4.Prenatal sagittal imaging shows a dilated fourth ventricle (arrowhead) with a round roof, a hypoplastic cere-bellum (arrow), and a large posterior fossa cyst (*).
A B
Figure 5.(A, B) Preaxial polydactyly (arrow) of the hallux of the foot.
Taiwan J Obstet Gynecol • June 2010 • Vol 49 • No 2 245 Fetal MRI and Joubert Syndrome
(JBTS9), and CXORF5 on Xp22.3 (JBTS10). The addi-tional locus of CORS2 has been suggested on 11p12– q13.3 (JBTS2). JBTS1–9 are inherited in an autosomal recessive pattern. JBTS10 is inherited in an X-linked re-cessive pattern [5]. Meckel syndrome (MKS) shares JBTS phenotypes [6,7]. Among the nine JSRD genes, three genes (MKS3/TMEM67, MKS4/CEP290 and MKS5/
RPGRIP1L) are also associated with MKS, indicating
genetic complexity in JSRD. Both of the affected siblings in this case had polydactyly. Polydactyly is present in 8% [8] to 15% [9] of subjects with JSRD. Polydactyly has been shown to be present in cases with JBTS2, JBTS6 and JBTS7 but has rarely been described in other types of JSRD [1]. Polydactyly associated with JSRD is often postaxial, although preaxial polydactyly of the hands or large toes has been observed [1].
JSRD has an estimated prevalence of 1 in 100,000 live births [1]. Prenatal imaging of JSRD has rarely been described in the literature. To date, only 14 reports of prenatal imaging of JSRD have been described [10–23], most of which were examined by ultrasound and only three reports [21–23] were examined by fetal MRI. Pre-natal sonographic findings in fetuses with JSRD are relatively nonspecific and include increased nuchal trans-lucency, enlarged cisterna magna, cerebellar vermian agenesis, occipital encephalocele, ventriculomegaly, hypo-plastic phallus, renal cysts, and polydactyly [20,21]. Prenatal ultrasound lacks sensitivity with regard to posterior fossa malformations. In contrast, fetal MRI is able to identify the characteristic molar tooth sign, which is the cardinal diagnostic imaging sign of JSRD. The molar tooth sign consists of cerebellar vermian hypoplasia, thickened and elongated superior cerebellar peduncles and a deep interpeduncular fossa, and mid-brain dysgenesis is responsible for the molar tooth sign [24–26]. Fluss et al [22] identified the molar tooth sign at 27 weeks of gestation by fetal MRI in a fetus with JBTS. Saleem and Zaki [23] were able to identify the molar tooth sign associated with JSRD as early as 22 weeks of gestation by fetal MRI. We suggest that fetal MRI should be considered in pregnancy with a previous JSRD-affected child and/or documented pos-terior fossa malformations with associated anomalies suspicious of JSRD.
Acknowledgments
This work was supported by research grants NSC-96-2314-B-195-008-MY3 and NSC-97-2314-B-195-006-MY3 from the National Science Council, and MMH-E-99004 from Mackay Memorial Hospital, Taipei, Taiwan.
References
1. Parisi MA. Clinical and molecular features of Joubert syn-drome and related disorders. Am J Med Genet C Semin Med
Genet 2009;151C:326–40.
2. Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genet-ics of ciliopathies. J Med Genet 2008;45:257–67.
3. Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet 2009; 151C:281–95.
4. Cardenas-Rodriguez M, Badano JL. Ciliary biology: under-standing the cellular and genetic basis of human ciliopathies.
Am J Med Genet C Semin Med Genet 2009;151C:263–80.
5. Coene KLM, Roepman R, Doherty D, et al. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet 2009;85:465–81. 6. Chen CP. Meckel syndrome: genetics, perinatal findings, and
differential diagnosis. Taiwan J Obstet Gynecol 2007;46:9–14. 7. Chen CP. Syndromes, disorders and maternal risk factors associated with neural tube defects (III). Taiwan J Obstet
Gynecol 2008;47:131–40.
8. Chance PF, Cavalier L, Satran D, Pellegrino JE, Koenig M, Dobyns WB. Clinical nosologic and genetic aspects of Joubert and related syndromes. J Child Neurol 1999;14:660–6. 9. Saraiva JM, Baraitser M. Joubert syndrome: a review. Am J
Med Genet 1992;43:726–31.
10. Campbell S, Tsannatos C, Pearce JM. The prenatal diagnosis of Joubert’s syndrome of familial agenesis of the cerebellar vermis. Prenat Diagn 1984;4:391–5.
11. van Drop DB, Palan A, Kwee ML, Barth PG, van der Harten JJ. Joubert syndrome: clinical and pathological description of an affected male and a female fetus from the sibship.
Am J Med Genet 1991;40:100–4.
12. Ivarsson SA, Bjerre I, Brun A, Ljungberg O, Maly E, Taylor I. Joubert syndrome associated with Leber amaurosis and multicystic kidneys. Am J Med Genet 1993;45:542–7. 13. Van Zalen-Sprock RM, van Vugt JM, van Geijn HP.
First-trimester sonographic detection of neurodevelopmental abnormalities in some single-gene disorders. Prenat Diagn 1996;16:199–202.
14. Reynders CS, Pauker SP, Benacerraf BR. First trimester isolated fetal nuchal lucency: significance and outcome. J Ultrasound
Med 1997;16:101–5.
15. Souka AP, Snijders RJM, Novakov A, Soares W, Nicolaides KH. Defects and syndromes in chromosomally normal fetuses with increased nuchal translucency thickness at 10–14 weeks of gestation. Ultrasound Obstet Gynecol 1998;11:391–400. 16. Anderson JS, Gorey MT, Pasternak JF, Trommer BL. Joubert’s syndrome and prenatal hydrocephalus. Pediatr Neurol 1999; 20:403–5.
17. Ni Scanaill S, Crowley P, Hogan M, Stuart B. Abnormal pre-natal sonographic findings in the posterior cranial fossa: a case of Joubert’s syndrome. Ultrasound Obstet Gynecol 1999; 13:71–4.
18. Wang P, Chang FM, Chang CH, Yu CH, Jung YC, Huang CC. Prenatal diagnosis of Joubert syndrome complicated with encephalocele using two-dimensional and three-dimensional ultrasound. Ultrasound Obstet Gynecol 1999;14:360–2.
Taiwan J Obstet Gynecol • June 2010 • Vol 49 • No 2 246
C.P. Chen, et al
19. Aslan H, Ulker V, Gulcan EM, Numanoglu C, Gul A, Agar M, Ark HC. Prenatal diagnosis of Joubert syndrome: a case report. Prenat Diagn 2002;22:13–6.
20. Aslan H, Gungorduk K, Yildirim G, Olgac Y, Ceylan Y. Prenatal ultrasonographic features of Joubert syndrome.
J Clin Ultrasound 2008;36:576–80.
21. Doherty D, Glass IA, Siebert JR, et al. Prenatal diagnosis in pregnancies at risk for Joubert syndrome by ultrasound and MRI. Prenat Diagn 2005;25:442–7.
22. Fluss J, Blaser S, Chitayat D, Akoury H, Glanc P, Skidmore M, Raybaud C. Molar tooth sign in fetal brain magnetic reso-nance imaging leading to the prenatal diagnosis of Joubert syndrome and related disorders. J Child Neurol 2006;21:320–4.
23. Saleem SN, Zaki MS. Role of MR imaging in prenatal diag-nosis of pregnancies at risk for Joubert syndrome and related cerebellar disorders. Am J Neuroradiol 2010;31:424–9. 24. Maria BL, Hoang KB, Tusa RJ, et al. “Joubert syndrome”
revisited: key ocular motor signs with magnetic resonance imaging correlation. J Child Neurol 1997;12:423–30. 25. Maria BL, Quisling RG, Rosainz LC, Yachnis AT, Gitten J,
Dede D, Fennell E. Molar tooth sign in Joubert syndrome: clinical, radiologic, and pathologic significance. J Child Neurol 1999;14:368–76.
26. Maria BL, Boltshauser E, Palmer SC, Tran TX. Clinical fea-tures and revised diagnostic criteria in Joubert syndrome.