This article was downloaded by: [National Chiao Tung University 國立交通大學]
On: 30 April 2014, At: 21:26
Publisher: Taylor & Francis
Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer
House, 37-41 Mortimer Street, London W1T 3JH, UK
Journal of the Air & Waste Management Association
Publication details, including instructions for authors and subscription information:
http://www.tandfonline.com/loi/uawm20
Operational Characteristics of Effective Removal
of H
2
S and NH
3
Waste Gases by Activated Carbon
Biofilter
Ying-Chien Chung
a, Yu-Yen Lin
b& Ching-Ping Tseng
ba
Department of Industrial Engineering and Management , China Institute of Technology ,
Taipei , Taiwan
b
Department of Biological Science and Technology, , National Chiao Tung University ,
Hsin-chu , Taiwan
Published online: 21 Feb 2012.
To cite this article: Ying-Chien Chung , Yu-Yen Lin & Ching-Ping Tseng (2004) Operational Characteristics of Effective
Removal of H
2S and NH
3Waste Gases by Activated Carbon Biofilter, Journal of the Air & Waste Management Association,
54:4, 450-458, DOI:
10.1080/10473289.2004.10470915
To link to this article: http://dx.doi.org/10.1080/10473289.2004.10470915
PLEASE SCROLL DOWN FOR ARTICLE
Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained
in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no
representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of
the Content. Any opinions and views expressed in this publication are the opinions and views of the authors,
and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied
upon and should be independently verified with primary sources of information. Taylor and Francis shall
not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other
liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or
arising out of the use of the Content.
This article may be used for research, teaching, and private study purposes. Any substantial or systematic
reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any
form to anyone is expressly forbidden. Terms & Conditions of access and use can be found at
http://
www.tandfonline.com/page/terms-and-conditions
Operational Characteristics of Effective Removal of H
2
S and
NH
3
Waste Gases by Activated Carbon Biofilter
Ying-Chien Chung
Department of Industrial Engineering and Management, China Institute of Technology,
Taipei, Taiwan
Yu-Yen Lin and Ching-Ping Tseng
Department of Biological Science and Technology, National Chiao Tung University,
Hsin-chu, Taiwan
ABSTRACT
Simultaneous removal of hydrogen sulfide (H2S) and am-monia (NH3) gases from gaseous streams was studied in a biofilter packed with granule activated carbon. Extensive studies, including the effects of carbon (C) source on the growth of inoculated microorganisms and gas removal efficiency, product analysis, bioaerosol emission, pressure drop, and cost evaluation, were conducted. The results indicated that molasses was a potential C source for inoc-ulated cell growth that resulted in removal efficiencies of 99.5% for H2S and 99.2% for NH3. Microbial community observation by scanning electron microscopy indicated that granule activated carbon was an excellent support for microorganism attachment for long-term waste gas treat-ment. No disintegration or breakdown of biofilm was found when the system was operated for 140 days. The low bioaerosol concentration emitted from the biofilter showed that the system effectively avoided the environ-mental risk of bioaerosol emission. Also, the system is suitable to apply in the field because of its low pressure drop and treatment cost. Because NH3 gas was mainly converted to organic nitrogen, and H2S gas was con-verted to elemental sulfur, no acidification or alkalinity phenomena were found because of the metabolite prod-ucts. Thus, the results of this study demonstrate that the
biofilter is a feasible bioreactor in the removal of waste gases.
INTRODUCTION
Hydrogen sulfide (H2S) and ammonia (NH3) are emitted simultaneously into the atmosphere from various facili-ties, including carcass-processing plants, sewage treat-ment plants, composting works, livestock farms, and wastewater treatment plants.1–3These emissions, in addi-tion to their own toxicity, constitute a source of olfactory nuisance. Traditional waste gas treatment technologies, such as carbon (C) adsorption, wet scrubbing, thermal incineration, and catalytic incineration, have been used to remove gaseous pollutants from waste gases,4 but the technologies suffer from high treatment costs and secondary waste stream problems.5 As regulatory mea-sures move toward more stringent control of gaseous pollutants (especially for malodorous compounds), the demand for cost-efficient air pollution control technology will increase. Currently, biofiltration is regarded as the best available control technology in treating diluted pol-lutants or odorous compounds because it is more cost-effective than other technologies and minimizes genera-tion of secondary contaminated waste streams.6 – 8
Among the air-phase bioreactors, the biofilter has been considered to be one of the most promising tech-nologies for treating waste gases.9,10This basically consists of only a simple packed bed column containing microbial populations and solid supports. The microorganisms or-ganize themselves into a biolayer on the surface of the packing material. In a biofilter, water either is not applied or is applied only intermittently, and the water layer is so thin that it often can be neglected. Therefore, when con-taminated air or water passes through the material, the pollutants are transferred to the biolayer, where they are biodegraded by the microbes residing in it.
IMPLICATIONS
The activated C biofilter was applied first in simultaneously removing H2S and NH3mixtures. Some important research
data related to the system’s performance were presented to explain the feasibility of the system. Evidence indicates that the system has the potential to be an effective means of simultaneously removing H2S and NH3for a long period.
In addition, it could find further application in widespread waste gas treatment.
Previous studies have shown that various inoculated bacteria11,12 and packing material13 have been applied to the removal of gaseous pollutants. Several reports have examined the air-phase bioreactor for the treatment of H2S and NH3,3,10,14,15which is emitted from leather man-ufacturing, wastewater treatment, asphalt production, and the pulping process. Although screening methods for appropriate bacteria as well as selected guidelines for packing material have been established,9,16,17this search only focused on evaluating removal efficiency, re-moval capacity, rere-moval kinetics, or reaction mecha-nisms. Biofiltration treatment of H2S can achieve a 97% removal efficiency,18 –21and 85% of NH
3emissions were eliminated using different types of bioreactors,12,22–24 but few studies focus on or present data on the risks of bioaerosol emission, pressure drop, or treatment cost.
Aerosolization of pathogenic or nonpathogenic mi-crobes is an inevitable consequence of the generation and handling of a bioreactor. Based on safety considerations, it is necessary to determine bioaerosol concentrations. Ottengraf and Konings have examined six full-scale bio-filters located in the Netherlands for bioaerosol dis-charge.25They concluded that the bioaerosol concentra-tion in the outlet gas of the different biofilters is only slightly higher than that encountered in the open air and is of the same order of magnitude as that encountered in indoor air. They further concluded that the concentration of microorganisms of a highly contaminated inlet gas is considerably reduced by the biofilter.25
Because partial peat biofilters emit considerable quan-tities of bioaerosols in a long-term treatment,26it is nec-essary to assess the environmental risk associated with the bacteria released from a new biosystem. A high pressure drop often results from aging packing material (e.g., com-post or peat),27 thus leading to relatively high energy demand and operational costs. Each of these parameters becomes important when the system is required further in the field application.
In illustrating the feasibility and competitiveness of a new biosystem, a consideration of costs and economic issues is indispensable. Generally, capital costs include system equipment, medium, and piping costs. Operating costs arise from energy consumption, water consumption and disposal, maintenance, and medium replacement. However, making more specific capital and operating cost assessments is difficult because of differences in waste gases, performance requirements, and system designs.28
In general, capital costs for small designs (⬍100 m3) have been estimated at $1000 to $3500 per m3 of filter bed. As bioreactors increase in volume, costs fall by about one-third.29Operating costs vary from system to system, but generalized costs have been reported to range from
$0.1 to $3/1000 m3/yr of waste gas treated.30 Further-more, metabolite analysis and microbial population ob-servation on the biofilter also can improve a biosystem’s removal efficiency under different conditions. Therefore, in this study, several operational characteristics of the activated carbon biofilter in simultaneously treating H2S and NH3, including the acidification phenomenon, safety considerations, and economic evaluation, were exam-ined. Direct evidence has proved that the microorganisms in this system performed well during the operational period.
MATERIALS AND METHODS
Organism Cultivation and Medium Preparation Pseudomonas putida CH11 for H2S oxidation and
Ar-throbacter oxydans CH8 for NH3 oxidation were isolated from swine wastewater.10 Stock cultures were grown in plate count broth at 26 °C with 120 strokes/min. The broth contained 5 g/L yeast extract, 10 g/L tryptone, and 2 g/L dextrose. In all consecutive experiments, the inflow medium (cycling solutions) was supplied and stored in the nutrient tank. The inflow medium contained glucose 10 g/L (unless otherwise specified), KH2PO44.08 g/L, K2HPO45.22 g/L, NH4Cl 0.4 g/L, MgCl2
.6 Hr 2O 0.2 g/L, and Fe(III)-citrate 0.01 g/L. The final pH of the me-dium was adjusted to neutral by using 2 N some-dium hy-droxide or hydrochloric acid. The buffer capacity in the inflow medium was calculated as 0.033 (mol/L).
Cell Growth at Different Carbon Substrates and Concentrations
A platinum loop of P. putida CH11 or A. oxydans CH8 from the plate count agar was inoculated into 100 mL of a basal medium in shaken flasks and was incubated at 26 °C by reciprocal shaking (120 strokes/min) overnight growth. One-half an mL of overnight cell culture was transferred into 100 mL basal medium with a different C source as substrates. The composition of the basal medium was similar to that of the inflow medium except for the C source and its concentration. In this basal medium, glu-cose, molasses, fructose, and sucrose were added sepa-rately in the range of 0.05–1% to examine the growth characteristics of the strains. Every 2 hr, 0.1 mL of the cell suspension was drawn out, and the cell growth was de-termined by absorptivity at 600 nm in the Beckman spec-trophotometer.
Bioaerosol Analysis
Microorganisms emitted from the activated C biofilter were collected by liquid impingement.10The air escaping from the bottom of the filter was forced through a 250-mL flask containing 100 mL aseptically distilled water for 12
Chung, Lin, and Tseng
hr at 4 °C. One mL of the collected solution was inocu-lated to different media, and the numbers of cells were determined by the serial dilution method. The potato dextrose agar was used for fungi, the Luria-Bertani me-dium was used for heterotrophic bacteria, and the thio-sulfate medium was used for Thiobacilli spp. The cell counts of autotrophic NH3 oxidizer were determined by the amount of nitrite (NO2⫺) produced.14 The selective media, Hagedorn and Holt medium and Acetamide-Cetrimide-Glycerol-Mannitol medium, were used sepa-rately to determine Arthrobacter spp. and Pseudomonas spp. The counts were reported as colony forming units in air (CFU/m3).
Apparatus and H2S/NH3Simultaneous Removal
for Continuous Operation
A setup and design of the pilot-scale experimental acti-vated carbon biofilter is shown in Figure 1 and illustrated as follows. Two glass columns (12 cm ⫻ 40 cm working height) connected in series were packed with cell-laden granular activated carbon (GAC; 6 ⫻ 6 mesh), and a perforated sieve plate was fitted at the bottom of the column to allow the circulating liquid to flow out. The cell-laden GAC was produced during an immobilization process. The initial cell numbers of P. putida CH11 and A.
oxydans CH8 were⬃8 ⫻ 1010and 1.13⫻ 1010CFU/g dry GAC, respectively. The packed volume and GAC dry weight in the activated C biofilter were 9.05 L and 4.34 kg. The pure H2S and NH3 gases, supplied from separate gas cylinders, were first diluted with compressed air,
which passed through an air filter (pore size 0.2m, LIDA 3000 – 06), and then flowed downward through the bio-filter at the top. An inflow medium (see medium prepa-ration) stored in the nutrient tank was intermittently recirculated by a peristaltic pump at 10 L/min for 6 min every 4 hr and by a spray nozzle at the top of the filter, which uniformly sprayed the medium to maintain the moisture of the filter and supplied nutrient to the at-tached cells. Generally, 10 g/L of glucose was supplied once every 2 weeks. To evaluate the effect of the C source in the continuous operation, molasses with the same con-centration was added to the nutrient tank when glucose was exhausted. In the 180-day treatment period, various H2S and NH3concentrations ranging from 10 to 120 ppm were introduced to the activated C biofilter at various flow rates (180 –1080 L/hr) or empty bed detention times (3– 0.5 min) at 26⫾ 2 °C to evaluate the operational charac-teristics of the system. In the treatment periods, removal efficiencies greater than 99% for H2S and NH3 were achieved.
Microorganism and Biofilter Observation by Scanning Electron Microscopy
For the scanning electron microscopic (SEM) study, the cell-laden GAC was separately drawn from one-half or three-fourths filter depth of the activated C biofilter dur-ing the different operatdur-ing periods. Samples were treated with glutaraldehyde 3% solution buffered with 0.1 M sodium phosphate to fix the cells and then serially de-hydrated in ethanol, critical point dried with a critical point dryer, mounted on aluminum stubs using double-sided tapes, and then sputter-coated with gold. SEMs were taken using a Hitachi S4500 scanning electron microscope.
Analytical Methods
Concentrations of inlet H2S and NH3gases in the reactor were measured periodically by gas detector tubes (Kita-gawa) in the range of 1–150 ppm (the max error is⫾5%). Outlet concentrations were continuously measured using a Single Point Monitor (MDA Scientific) in the range of 50 –1500 ppb or periodically measured by gas detector tubes (Kitagawa) in the range of 1– 60 ppm (the max error is⫾5%). In all consecutive experiments, the variation in H2S and NH3concentrations at steady state was within ⫾5%. Therefore, the 12 values obtained at steady states were averaged as the H2S and NH3outlet concentrations. To determine the pressure drop across the filter under different flow rates, u-tube water manometers were ap-plied during the 100th–125th days, and the unit was expressed as mm H2O/m filter height. Because the inlet site was affected strongly by the inflow medium from a spray nozzle at the top of the filter, the production Figure 1. The pilot-scale BAC biofilter. 1. glass column; 2. flow meter;
3. NH3gas cylinder; 4. H2S gas cylinder; 5. air compressor; 6. nutrient
tank; 7. pump; 8. regulator; 9. air filter; 10. 4-way connector.
analysis resulted in a larger error. Hence, GAC particles were withdrawn from the middle zone of the reactor instead of from the inlet of the bioreactor for product analysis. To analyze the metabolic product, 1 g GAC was withdrawn from the middle zone of the biofilter on the 150th day and mixed with 10 mL of distilled water. After the sample was vortexed for 3 min, the chemical compo-sitions of the liquid solutions were analyzed, except for the elemental sulfur (S). The residual GAC particle was withdrawn independently to analyze for elemental S. Sul-fate (SO4
2⫺), nitrate (NO
3⫺), and NO2⫺concentrations in the solution were measured by ion chromatography (Dionex 4500i). Ammonium (NH4⫹) and sulfide were de-termined using an ion-specific electrode. Sulfite (SO32⫺) was determined by titration using a standard potassium iodide-iodate titrant and a starch indicator.31Elemental S was determined by reacting with cyanide to produce thio-cyanate, which was then quantified as Fe(SCN)63⫺.32 Organic nitrogen (N) was determined by the Kjeldahl method.
RESULTS AND DISCUSSION
Effect of Carbon Substrate and Concentration on Bacterial Growth and Consecutive
H2S/NH3Removal
Organic compounds act as a C and energy source for heterotrophic bacterial growth. Hence, the supply of or-ganic compounds for heterotrophic bacteria is essential. In view of engineering, if microbial activity can be ele-vated and the cost of organic compounds reduced, it will be completed in both respects. Therefore, finding an ap-propriate C source and its optimal concentration is nec-essary for further application in the industrial scale-up system. When A. oxydans CH8, which can remove NH3 gas from waste gas, was cultivated in basal media contain-ing glucose, sucrose, fructose, or molasses in the range of 0.05–1%, different growth rates were observed. The opti-mal C source for the growth of A. oxydans CH8 was mo-lasses and then glucose, whereas sucrose and fructose were not good for bacterial growth. Similar results were found when P. putida CH11, which can eliminate H2S gas from waste gas, was cultivated in these basal media. Fig-ures 2a and 2b indicate the growth of P. putida CH11 cultivated in glucose and molasses basal medium. Figures 2c and 2d indicate the growth of A. oxydans CH8 culti-vated in glucose and molasses basal medium. Apparently, the growth rates of isolated strains were affected by the molasses concentration (see Figure 2b and 2d). Molasses at high concentration would favor the growth of isolated strains, especially that of A. oxydans CH8. In this study, the specific growth rates () of A. oxydans CH8 were calculated as 0.45, 0.38, and 0.1 hr⫺1, at 1, 0.2, and
0.05% molasses added, respectively (see Figure 2d). How-ever, the growth rates of isolated strains were indepen-dent of the concentration of other C sources (e.g., Figure 2a and 2c).
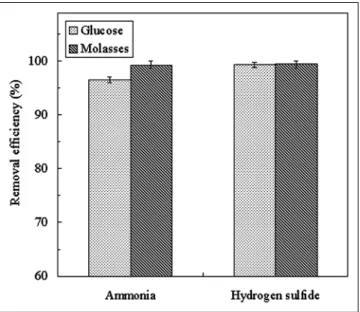
Because P. putida CH11 and A. oxydans CH8 are het-erotrophic bacteria, the supply of extra C will favor mi-crobial activity. According to the results of Figure 2, mo-lasses was the optimal C source for the growth of isolated strains in the batch culture. In this study, a consecutive experiment applying an activated C biofilter inoculated with P. putida CH11 and A. oxydans CH8 for H2S and NH3 removal was conducted. Figure 3 indicates the effect of C source on H2S and NH3removal with the addition of 1% molasses or glucose when 60 ppm of H2S and NH3were simultaneously introduced at 720 L/hr for 2 weeks. The results indicated that greater than 99.3% of H2S gas re-moval efficiency was achieved when glucose or molasses was provided as the C source. When 60 ppm of NH3was introduced to the filter, the outlet concentrations were 0.6 ppm at 99% removal efficiency, but they were in-creased 4-fold (2.4 ppm) at 96% removal efficiency. Hence, high NH3 removal efficiency was found when molasses was used instead of glucose. Molasses originates from agricultural waste and often is reutilized for the production of ethanol and monosodium glutamate. Hence, it is both cheap and easy to acquire. According to analysis, 1 g of molasses contained⬃0.21 g of glucose and 0.79 g of other nutritional components (data not shown). Figure 2. Effect of C substrate on P. putida CH11 and A. oxydans CH8
growth. (a) P. putida CH11 was grown in glucose basal medium; (b) P.
putida CH11 was grown in molasses basal medium; (c) A. oxydans CH8
was grown in glucose basal medium; (d) A. oxydans CH8 was grown in molasses basal medium.
Chung, Lin, and Tseng
Additionally, A. oxydans CH8 especially preferred utilizing molasses for growth (Figure 2c and 2d). In fact, the cell number of A. oxydans CH8 on 1% molasses addition was twice that of 1% glucose addition in the studies (data not shown). Therefore, molasses should be an appropriate C source in a field-scale bioreactor by virtue of its removal efficiency, operational cost, and microbial activity.
Product Analysis
To understand the metabolic products and characteristics of NH3and H2S by A. oxydans CH8 and P. putida CH11 during the long-term operation, 1 g of GAC bed was withdrawn for analysis from the middle zone of the filter on the 150th day of continuous operation. Because the concentrations of metabolic products in the recycling solution are 1.5% less than their concentrations in the GAC bed, only the data of GAC are presented. Table 1 indicates various N- and S-containing compounds make up the lion’s share of the metabolic products in the GAC bed. The results indicated the distribution of the total N and S amounts in the GAC bed into four species at min-imum. The main products of NH3degradation were or-ganic N (84.4%), NH4⫹/NH3 (15.57%), NO2⫺ (0.03%), and NO3⫺(0.002%). These species in the GAC bed took up 98.7% of the total N accumulated in the system ac-cording to the mass balance between inlet NH3loading and accumulated N in the system. Partial NH4⫹/NH3in the leachate was neglected. These data indicated that as-similation, not nitrification, was responsible for the NH3 metabolism. In addition, slightly concentrated NH4⫹and NH3(316.7 g N/kg GAC) were observed because they were adsorbed by GAC. NH3 could partially neutralize the
acidity from SO4
2⫺(360 g S/kg GAC) derived from H 2S oxidization, and consequently keep the pH of the GAC at 7 ⫾ 0.6 throughout the experimental period (data not shown). The main products of H2S degradation were ele-mental S (90.85%), SO42⫺ (9.09%), SO32⫺ (0.05%), and HS⫺ (0.01%). These species in the GAC bed contained 99.2% of the total S accumulated in the system (data not shown). Because the major degradation byproduct was neutral elemental S, accounting for more than 90% of total S compound accumulated in the system, the system easily could operate at a neutral range for a long time. The pH of the liquid effluent over the course of the experi-ment was in the range of 6 – 8. This is a benefit of using the biofilter. Thus, the results demonstrated that appropriate metabolic products or ratios would stabilize pH in the system and effectively prevent the occurrence of acidifi-cation and alkalinity.
Bioaerosol Emission Analysis
Although attempts to deodorize the biotreatment pro-cess have proven very promising,9 a bioreactor often contains tremendous amounts of microorganisms, and the environmental risk associated with the bacteria re-leased from the system should be assessed, especially when large quantities of waste gases are treated. The key factor affecting bioaerosol emission is often im-mobilization efficiency. Table 2 shows the number of microorganisms in the outlet exhaust when the acti-vated C biofilter was continuously operated for 90 days. The bioaerosol amount increased with increasing flow rate, but it was insignificant (p⬎ 0.05). Because GAC is a porous, rigid, and highly specific area carrier, it is suitable for cell attachment or immobilization. The re-sults of Table 2 reveal that the exhaust contained Figure 3. Effect of C source on H2S and NH3removal. The activated C
biofilter was supplied with 1% molasses or glucose when 60 ppm of H2S
and NH3were introduced simultaneously at 720 L/hr for 2 weeks.
Table 1. Metabolic products of NH3and H2S in the GAC bed of activated
carbon biofilter. Amount Ratio (%) Metabolic Products of NH3 a NO2 – 0.54 0.03 NO3 – 0.043 0.002 NH4⫹/NH3 301.6/15.1 15.57 Organic N 1717.1 84.4 Metabolic Products of H2S b SO4 2– 360 9.09 Elemental S 3600 90.85 SO3 2– 1.8 0.05 HS– 0.22 0.01
Note: The biofilter system was provided with glucose as a carbon source once every two
weeks;aAmount⫽ g N/kg GAC;bAmount⫽ g S/kg GAC.
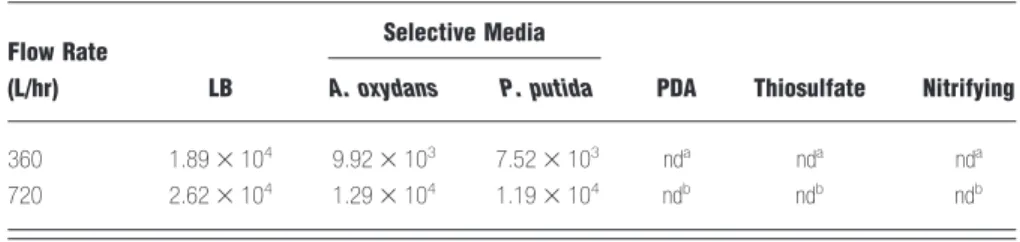
heterotrophic bacteria (1.89 –2.62 ⫻ 104 CFU/m3), A.
oxydans CH8 (0.99 –1.29⫻ 104CFU/m3), and P. putida CH11 (0.75–1.19⫻ 104CFU/m3). Less fungi,
Thiobacil-lus spp., and nitrifying bacteria were determined.
Pre-vious reports have shown that the bioaerosol concen-trations of bacteria in peat plants, refuse collections, composting facilities, and homes were 106, 105, 105, and 103 CFU/m3, respectively.33–36 The bioaerosol re-leased from the peat biofilter and the immobilized cell bioreactor were 2 ⫻ 102 CFU/m3 of fungi26 and 103 CFU/m3 of bacteria.10 In addition, Malmros suggested that an acceptable air quality in terms of total bacteria is 104 CFU/m3.37 In Poland, an OEL (occupational ex-posure limit) for total microorganisms is set at the level of 3⫻ 105CFU/m3.38 Hence, the bioaerosol (104CFU/ m3) emitted from the GAC biofilter is a relatively low and acceptable. In addition, Pseudomonas putida and
Arthrobacter oxydans are not pathogens and exist in the
natural environment, and the risk to the environment is relatively low. Because only A. oxydans CH8, P. putida CH11, and a few heterotrophic bacteria were found in the outlet exhaust, it was supposed that the inoculated species should remain dominant. Thus, using GAC as packing material effectively obviates the environmen-tal risk of bioaerosol emission, and this system might be placed safely close to populated areas.
Pressure Drop
Economic considerations are crucial in designing a practical bioreactor, second only to operational effi-ciency. The pressure drop, a problem common to long-term operations, is an important evaluation parameter in determining the operational cost.15 A high pressure drop will result in higher energy consumption require-ments to maintain the good performance of the biore-actor. Hence, operational cost will increase with in-creasing energy input. Pressure drop formation often is caused by the aging of the packing material.27Easily biodegraded or unrigid packing material, such as compost or peat, suffers from aging. The relationship between gas flow rate and pressure drop is shown in Figure 4. In this experiment, the flow rate was raised gradually from 180 to 1080 L/hr, and the temperature
was maintained at 26 °C. When the variation of outlet H2S/NH3 concen-tration was within⫾5%, a new flow rate was selected. The results indi-cated that pressure drop of the bio-filter increased with increasing gas flow rate (data not shown). When the relationship between pressure drop and the square of velocity was drawn, a linear line through all four points in Figure 4 was observed (R2⫽ 0.997). The pres-sure drop ranged from 8 to 65 mm H2O/m and corre-sponded well with the operational standard for biofil-ters (below 300 mm H2O/m).39 The pressure drop of activated C biofilter is acceptable and suggests that the system possesses excellent dispersion characteristics.17 After the system was operated for 125 days, the results of pressure drop were better than that of other studies that utilized peat (84 mm H2O/m), rock wool (78 mm H2O/m), fuyolite (74 mm H2O/m), and ceramics (73 mm H2O/m) as the packing media under similar oper-ational conditions but shorter operoper-ational times.9,40 Apparently, GAC was a good packing material with respect to the feature of pressure drop. The results were consistent with the previous study reported by Malhau-tier et al.3However, if the pressure drop of the biofilter reached 250 mm H2O/m, the clogging of the bed would be cleaned by a backwashing method to decrease the pressure drop of the biofilter.
Microbial Growth Observation by Scanning Electron Microscopy
Activated C is a promising packing material because of its highly specific surface area, high water-holding capacity, Table 2. Bioaerosol analysis of the outlet exhaust of activated carbon biofilter with different media.
Flow Rate
(L/hr) LB
Selective Media
PDA Thiosulfate Nitrifying A. oxydans P. putida
360 1.89⫻ 104 9.92⫻ 103 7.52⫻ 103 nda nda nda
720 2.62⫻ 104 1.29⫻ 104 1.19⫻ 104 ndb ndb ndb
Note: nd⫽ not determined;a⬍57 CFU/m3;b⬍29 CFU/m3.
Figure 4. Relationship between gas flow rate and pressure drop across
the filter. The data were obtained at 26 °C after continuously operating for 100 days.
Chung, Lin, and Tseng
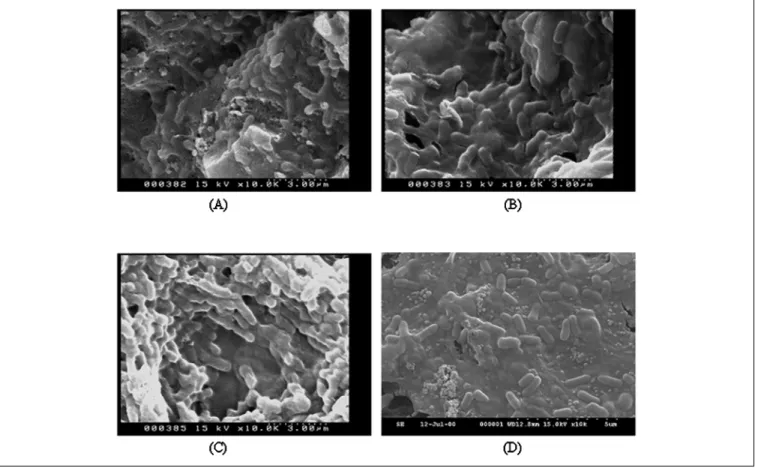
and porosity.9A number of minute holes, distributed over the surfaces, provide for the attachment and growth of microbes as well as the exchange of material, including gases, nutrients, and metabolites. Because activated C is harder and more difficult to biodegrade than other pack-ing material, it often maintained a low pressure drop and a long life span. Figure 5 shows the SEMs of the surface of activated C drawn from one-half filter depth of the bio-filter on the 50th, 70th, 100th, and 140th day. As shown in the SEM on the 50th day (see Figure 5a), cells were directly attached to the support surface, but no biofilm formation by the connection of polysaccharide excreted from cells was observed. After 70 days of operation, the biofilm was distributed throughout the surface of acti-vated C drawn from one-half filter depth (see Figure 5b), but the relatively loose structure in the biofilm was ob-served at the surface of activated C drawn from three-fourths filter depth (data not shown). After 100 days of operation, cells were not only laden in the surface of activated C, but also inside its holes (see Figure 5c). For a long-term operation (e.g., 140 days), the well struc-ture of biofilm and cells were maintained as illustrated in Figure 5d. No disintegration or breakdown was found. In the meantime, H2S and NH3 average removal efficiencies greater than 99% were achieved (data not
shown). In addition, the dominant species, more than 99% in the different filter depths, were analyzed as the original inoculated strains A. oxydans CH8 and P. putida CH11 (data not shown). It all shows GAC to be a very promising packing material in the removal of H2S- and NH3-containing waste gases.
Cost Evaluation
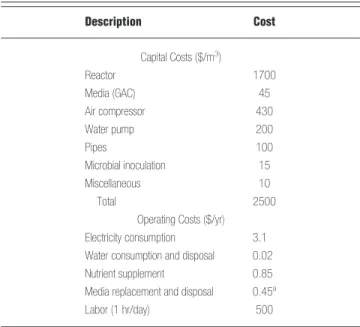
In designing an air-phase bioreactor, the main goal is to meet a performance level while minimizing capital and operating costs.40 Because the biofilter was a pilot-scale system in this study, the capital costs were needed to evaluate or compare with a field-scale system. A detailed cost analysis is listed in Table 3. The capital costs included the reactor, medium, equipment, piping, and microbial inoculation costs, as well as other miscellaneous costs. The total capital cost of the system was estimated as $2500/m3of filter bed, a price which would fall by about one-third if the system was scaled up for field applica-tion.29 Operating costs generally include electricity con-sumption, water consumption and disposal, nutrient sup-plement, and medium replacement and disposal.28 In this study, the operating cost was estimated under the following assumptions:28a gas flow rate of 720 L/hr, inlet
Figure 5. SEMs of microorganisms grown on GAC. The GAC drawn from one-half filter depth of the activated carbon biofilter on the (a) 50th, (b) 70th,
(c) 100th, and (d) 140th day.
H2S and NH3 concentrations of 120 ppm, removal effi-ciency of 98%, and year-round operation. The annual operation or maintenance costs would be $3.1/yr, $0.02/ yr, $0.85/yr, and $0.45/yr for electrical consumption, water consumption and disposal, nutrient supplement, and me-dia replacement and disposal, respectively. Therefore, to-tal yearly operating cost is $4.42/yr. Considering the vol-ume of waste air treated and the weight of H2S and NH3 removed, the operating costs in this study are $0.68/1000 m3/yr, $0.005/g S/yr, and $0.012/g N/yr, respectively. This operating cost ($0.68/1000 m3/yr) is less than the $3/1000 m3/yr and $1.9/1000 m3/yr reported by Fouhy and by Deshusses and Cox for the same eval-uated items.30,41 When the labor cost is estimated at $500/yr, the total operating cost will be $77.6/1000 m3/ yr. This cost was lower than the $91/1000 m3/yr of the airlift bioreactor.42 These relatively low operating costs suggest that the biosystem could be a feasible technique.
CONCLUSIONS
The results of this study demonstrate that the biofilter using GAC as packing material can achieve excellent removal performance in treating H2S- and NH3 -con-taining waste gas. Molasses is the optimal C source for the system based on considerations of cost and removal efficiency. The metabolic products of the systems would not acidify or alkalize the reactor because the major product is neutral S or organic N. Because of the good attachment characteristics of GAC, which reas-sures its safety concerning environmental impact,
the bioaerosol released from the activated carbon bio-filter is low compared with those in the outdoor. Low pressure drop and low operating cost indicate that the activated C biofilter is feasible to remove waste gases. In addition, the observation of good biofilm formation provides direct evidence for high H2S and NH3removal efficiency and low bioaerosol emission. Thus, these re-sults suggest that the activated C biofilter with inocu-lated specific microorganisms has a significant poten-tial in treating NH3 and H2S from mixed waste gases. The results of this study also show that the activated C biofilter is a practical method in removing NH3and H2S gas mixtures and can operate with low costs.
ACKNOWLEDGMENTS
The work was partially supported by Grant NSC from the National Science Council, Republic of China.
REFERENCES
1. Yang, Y.; Allen, E.R. Biofiltration Control of Hydrogen Sulfide 1. De-sign and Operational Parameters; J. Air & Waste Manage. Assoc. 1994,
44, 863-868.
2. Chung, Y.C.; Huang, C.; Tseng, C.P. Reduction of H2S/NH3Production From Pig Feces by Controlling Environmental Conditions; J. Environ.
Sci. Health 1996, A31, 139-155.
3. Malhautier, L.; Gracian, C.; Roux, J.C.; Fanlo, J.L.; Cloirec, P.L. Biolog-ical Treatment Process of Air Loaded with an Ammonia and Hydrogen Sulfide Mixture; Chemosphere 2003, 50, 145-153.
4. Chung, Y.C.; Huang,. C.; Tseng, C.P. Advanced Study of H2S Removal by Thiobacillus novellus CH3Biofilter in Autotrophic and Mixotrophic Environments; J. Environ. Eng.-ASCE 1998, 124, 362-367.
5. Deshusses, M.A. Biological Waste Air Treatment in Biofilters; Curr.
Opin. Biotechnol. 1997, 8, 335-339.
6. Gribbins, M.J.; Loehr, R.C. Effect of Media Nitrogen Concentration on Biofilter Performance; J. Air & Waste Manage. Assoc. 1998, 48, 216-226.
7. Martinec, M.; Hartung, E.; Jungbluth, T. Optimizing Biofilters to Reduce Odor and Gas Emissions from Livestock Buildings. In
Pro-ceedings of the 2nd International Conference on Air Pollution from Agricultural Operations, ASAE, St. Joseph: Bloomington, MN, 2000;
pp 391-398.
8. Elias, A.; Barona, A.; Arreguy, A.; Rios, J.; Aranguiza, I.; Penas, J. Evaluation of a Packing Material for the Biodegradation of H2S and Product Analysis; Process Biochem. 2002, 37, 813-820.
9. Leson, G.; Winer, A.M. Biofiltration: An Innovative Air Pollution Con-trol Technology for VOC Emission; J. Air & Waste Manage. Assoc. 1991,
41, 1045-1054.
10. Chung, Y.C.; Huang, C.; Liu, C.H.; Bai, H. Biotreatment of H2S and NH3-Containing Waste Gases by Fluidized Bed Bioreactor; J. Air &
Waste Manage. Assoc. 2001, 51, 163-172.
11. Chung, Y.C.; Huang, C.; Tseng, C.P. Removal of Hydrogen Sulphide by Immobilized Thiobacillus sp. Strain CH11 in a Biofilter; J. Chem.
Technol. Biol. 1997, 68, 58-62.
12. Chung, Y.C.; Huang, C.; Tseng, C.P. Biotreatment of Ammonia from Air by an Immobilized Arthrobacter oxydans CH8 Biofilter; Biotechnol.
Prog. 1997, 13, 794-798.
13. Christen, P.; Domenech, F.; Michelena, G.; Auria, R.; Revah, S. Biofil-tration of Volatile Ethanol Using Sugar Canebagasse Inoculated with
Candida utilis; J. Hazard. Mater. 2002, 89, 253-265.
14. Chung, Y.C.; Huang,. C.; Tseng, C.P.; Pan, J.R. Biotreatment of H2 S-and NH3-Containing Waste Gases by Co-Immobilized Cells Biofilter;
Chemosphere 2000, 41, 329-336.
15. Chung, Y.C.; Huang, C.; Tseng, C.P. Biotreatment of H2S and NH3 Containing Waste Gases by Co-Immobilized Autotrophic Biofilter;
Chemosphere 2001, 43, 1043-1050.
16. Malhautier, L.; Degrange, V.; Guay, R.; Degorce-Dumas, J.R.; Bardin, R.; Le Cloirec, P. Estimating Size and Diversity of Nitrifying Commu-nities in Deodorizing Filters Using PCR and Immunofluorescence;
J. Appl. Microbiol. 1998, 85, 255-262.
Table 3. Cost analysis of the activated carbon biofilter. Description Cost Capital Costs ($/m3) Reactor 1700 Media (GAC) 45 Air compressor 430 Water pump 200 Pipes 100 Microbial inoculation 15 Miscellaneous 10 Total 2500
Operating Costs ($/yr) Electricity consumption 3.1 Water consumption and disposal 0.02
Nutrient supplement 0.85
Media replacement and disposal 0.45a
Labor (1 hr/day) 500
aEvery 5 yr.
Chung, Lin, and Tseng
17. Sorial, G.A.; Smith, F.L.; Suidan, M.T.; Biswas, P.; Brenner, R.C. Eval-uation of Trickle Bed Biofilter Media for Toluene Removal; J. Air &
Waste Manage. Assoc. 1995, 45, 801-810.
18. Webster, T.S.; Devinny, J.S.; Torres, E.M.; Basrai, S.S. Biofiltration of Odors, Toxics and Volatile Organic Compounds from Publicly Owned Treatment Works; Environ. Prog. 1996, 15, 141-147.
19. Derek, E.C.; Devinny, J.S. Evaluation of a Two-Stage Biofilter for Treat-ment of POTW Waste Air; Environ. Prog. 1999, 18, 212-221. 20. Cook, L.L.; Geostomski, P.A.; Apel, W.A. Biofiltration of Asphalt
Emis-sions: Fill-Scale Operation Treating Off-Gases from Polymer-Modified Asphalt Production; Environ. Prog. 1999, 18, 178-187.
21. Wani, A.H.; Lau, A.K.; Branion, R. Biofiltration Control of Pulping Odors-Hydrogen Sulfide: Performance, Macrokinetics and Coexistence Effects of Organosulfur Species; J. Chem. Technol. Biotechnol. 1999, 74, 9-16.
22. Kapahi, R.; Gross, M. Biofiltration for VOC and Ammonia Emissions Control; BioCycle 1995, 36, 87-88.
23. Yani, M.; Hirai, M.; Shoda, M. Ammonia Gas Removal Characteristics Using Biofilter with Activated Carbon Fiber as a Carrier; Environ.
Tech-nol. 1998, 19, 709-715.
24. Chung, Y.C.; Huang, C. Biotreatment of Ammonia from Air by an Immobilized Nitrosomonas europaea Biofilter; Environ. Prog. 1998, 17, 70-76.
25. Ottengraf, S.P.P.; Konings, J.H.G. Emission of Microorganisms from Biofilters; Bioprocess Eng. 1991, 7, 89-96.
26. Hartikainen, T.; Martikainen, P.J. Removal of Ammonia from Air by a Peat Biofilter; Environ. Technol. 1996, 17, 45-53.
27. Auria, R.; Aycaguer, A.C.; Devinny, J.S. Influence of Water Content on Degradation Rates for Ethanol in Biofiltration; J. Air & Waste Manage.
Assoc. 1998, 48, 65-70.
28. Gerrard, A.M. Economic Design of Biofilter Systems; J. Chem. Technol.
Biotechnol. 1997, 68, 377-384.
29. Van Lith, C.; Leson, G.; Michelson, R. Evaluating Design Options for Biofilters; J. Air & Waste Manage. Assoc. 1997, 47, 37-43.
30. Fouhy, K. Cleaning Waste Gas Naturally; Chem. Eng. 1992, 99, 41-45.
31. Standard Methods for the Examination of Water and Wastewater, 18th ed.; Greenberg, A.E., Clesceri, L.S., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, 1992.
32. Schedel, M.; Truper, H.G. Anaerobic Oxidation of Thiosulfate and Elemental Sulfur in Thiobacillus denitrifans; Arch. Microbiol. 1980, 2–3, 205-210.
33. Anttonen, H.; Itkonen, A.; Kangas, J.; Kalliokoski, P.; Kiiskinen, P.; Alatalo, M. Exposure to Peat Dust and Dust Control in the Peat Industry; Pub. Occup. Health Inst. 1984, 2, 183-195.
34. Neumann, H.D.; Balfanz, J.; Becker, G.; Lohmeyer, M.; Mathys, W.; Raulf-Heimsoth, M. Bioaerosol Exposure during Refuse Collection:
Results of Field Studies in the Real-Life Situation; Sci. Total Environ. 2002, 293, 219-231.
35. Sanchez-Monedero, M.A.; Stentiford, E.I. Generation and Dispersion of Airborne Microorganisms from Composting Facilities; Proc. Safety
Environ. Protect. T I Chem. l Eng.; Part B 2003, 81, 166-170.
36. Pastuszka, J.S.; Paw, U.K.; Lis, D.O.; Wlazlo, A.; Ulfig, K. Bacterial and Fungal Aerosol in Indoor Environment in Upper Silesia, Poland;
At-mos. Environ. 2000, 34, 3833-3842.
37. Malmros, P. Problems with the Working Environment in Solid Waste
Treatment; Report no. 10/1990; The National Labour Inspection of
Denmark: Copenhagen, Denmark, 1990.
38. Dutkiewicz, J. Bacteria and Their Products as Occupational Allergens;
Pneum. Alergol. Pol. 1992, 60, 14-20.
39. Huang, C.C.; Shen, K.P. Technology Review for Vapor Phase Biofiltra-tion Part II: Technological Development and ApplicaBiofiltra-tions; J. Environ.
Eng.-ASCE 1998, 8, 181-196.
40. Lackey, L.W.; Gamble, J.R.; Holt, M.T. Feasibility Testing of Biofil-tration Technology for Remediating Air Contaminated by a Boat Manufacturing Facility; J. Air & Waste Manage. Assoc. 1998, 48, 527-536.
41. Deshusses, M.A.; Cox, H.H.J.A. Cost Benefit Approach to Reactor Siz-ing and Nutrient Supply for BiotricklSiz-ing Filters for Air Pollution Con-trol; Environ. Prog. 1999, 18, 188-195.
42. Zuber, L.; Dunn, I.J.; Deshusses, M.A. Comparative Scale-Up and Cost Estimation of a Biological Trickling Filter and a Three-Phase Airlift Bioreactor for the Removal of Methylene Chloride from Polluted Air;
J. Air & Waste Manage. Assoc. 1997, 47, 969-975.
About the Authors
Ying-Chien Chung is an associate professor in the Depart-ment of Industrial Engineering and ManageDepart-ment at the China Institute of Technology in Taipei, Taiwan. Yu-Yen Lin is an M.S. student and Ching-Ping Tseng is a professor in the Department of Biological Science and Technology at National Chiao Tung University in Hsin-chu, Taiwan. Ad-dress correspondence to: Ching-Ping Tseng; fax: 886-3-5729288; e-mail: cpts@cc.nctu.edu.tw.