Identification of the Chinese box orange (Severinia buxifolia) as an alternative
host of the bacterium causing citrus Huanglongbing
Ting-Hsuan Hung1, Meng-Ling Wu2and Hong-Ji Su1
1Department of Plant Pathology, National Taiwan University, Taipei 106, Taiwan (Fax:+886223636490); 2Division of Forest Protection, Taiwan Forestry Research Institute, Taipei 100, Taiwan
Accepted 25 October 2000
Key words: Severinia buxifolia, Huanglongbing bacterium (HLBB), PCR, graft-inoculation, psyllid-transmission
Abstract
The Chinese box orange (Severinia buxifolia) was shown by graft-inoculation and psyllid-transmission tests to be an alternative host of the bacterium causing citrus Huanglongbing (HLB). A PCR-based assay for detection of the HLB bacterium (HLBB) was used to monitor HLBB. In graft-inoculation tests, the Chinese box orange (CBO) grafted with HLBB-infected scions of Luchen sweet orange (LSO) were positive for HLBB, 2–3 months after grafting. The back-grafting test demonstrated that HLBB-infected CBO scions could transmit HLBB back to LSO hosts via grafting. In psyllid-transmission tests, psyllids (insect vectors) transmitted HLBB to CBO plants, in which HLBB could be detected 3–4 months after inoculation. Acquisition-access tests of psyllids revealed that HLBB-free psyllids can acquire HLBB from diseased CBO hosts and can transmit HLBB back to the LSO plants. A field survey verified the presence of HLBB-infected CBO plants in the vicinity of citrus orchards. In this paper, CBO is shown to be a susceptible host plant in which HLBB can exist and replicate. It is also a donor plant from which HLBB can be transmitted to citrus hosts by grafting or by psyllid vectors.
Introduction
Citrus Huanglongbing (HLB), also commonly called ‘citrus greening’, is a severe disease in Asia. It is caused by a phloem-limited bacterium that inhabits the sieve tubes of host plants (Garnier et al., 1984). This pathogen causes a blotchy mottle symptom on citrus leaves and retards the growth of its host plant (da Graca, 1991). The HLB bacterium (HLBB) has not yet been cultured, but a ‘Candidatus’ genus name of Liberobacter has been proposed (Jagoueix et al., 1994). HLBB is transmitted mainly by vegetative propagation and insect vectors. The Asian citrus psyllid (Diapho-rina citri Kuwayama) is the vector for HLBB in Asia (Capoor et al., 1967), which is one reason why cit-rus HLB has become an epidemic disease. In the past, monitoring of HLBB was not easy because of a lack of efficient detection methods. The diagnosis of HLB by electron microscopy and bioassay is time-consuming and inefficient. Thus, it has been difficult to conduct
epidemiological studies on citrus HLB. Recently, sen-sitive assays based on the polymerase chain reaction (PCR) were developed for HLBB detection (Jagoueix et al., 1994; Hocquellet et al., 1999; Hung et al., 1999b). This assay may prove to be a useful tool for epidemio-logical research.
Like other diseases caused by vascular bacteria, cit-rus HLB cannot be efficiently controlled by chemical methods. Establishment of pathogen-free nurseries and control of the insect vector seem to be the best con-trol strategies. The elimination of inoculum sources, including HLBB-infected citrus trees and alternative hosts, are the most important steps to prevent the spread of HLBB in the field. In addition to hosts of Citrus species, Chinese box orange (Severinia buxifo-lia) is a suitable host for the Asian citrus psyllid in Taiwan. Like all citrus species, Chinese box orange (CBO) belongs to the subfamily Aurrantioideae and the family Rutaceae (Herrero et al., 1996). It is a spiny shrub widely distributed in Taiwan, and in other Asian
countries such as India, Malaysia, Vietnam, southern China, the Philippines and Japan (Reuther et al., 1967; Lin et al., 1973). CBO often appears in the vicinity of citrus orchards, and citrus psyllids like to reside on it. Its fruit are not edible. Graft-inoculation and psyllid-transmission tests were conducted to investi-gate whether CBO is an alternative host for HLBB.
Materials and methods
Plant material
Chinese box orange (Severinia buxifolia) and Luchen sweet orange (Citrus sinensis Osb) were used for this study. Chinese box orange (CBO) was obtained from seed-cultivation, whilst Luchen sweet orange (LSO) was obtained from pathogen-free citrus plants which originated as shoot-tip micrografts (Murashige et al., 1972; Su and Chu, 1984). A HLBB-infected LSO, veri-fied by PCR(Hung et al., 1999b), collected from north-ern Taiwan was used as the inoculum source.
Graft-inoculation
Budwood grafting methods were chosen for the graft-inoculation (Su and Chu, 1984; Yoshida, 1996). For HLBB transmission from LSO to CBO, two HLBB-infected LSO scions were grafted onto a CBO seedling 10 and 40 cm from the top. A total of four CBO seedlings (50 cm tall, one-year-old) were used. Graft-ing was judged successful when the scions survived for more than 3 months. The CBO plant was sampled and DNA extraction was made monthly after grafting. The methods of sampling and DNA extraction are described in a later section. For comparison of HLBB infection between CBO and LSO hosts, two LSO plants (50 cm tall, one-year-old) were included in this grafting test.
To test for HLBB transmission from CBO back to LSO, HLBB-infected CBO scions were grafted onto a LSO seedling. Samples were collected periodically from the grafted LSO seedlings and tested for the pres-ence of HLBB by PCR. Two additional CBO seedlings were graft-inoculated to serve as controls.
HLBB transmission by psyllid
HLBB-free Asian citrus psyllids were bred from eggs and raised on a jasmine orange plant (Murraya pan-iculata) in an insect-proof growth chamber. Jasmine
orange is not a host of HLBB (Hung et al., 2000), but is a host for the Asian citrus psyllid (Lin et al., 1973; Chakraborty et al., 1976; Singh and Nimbalkar, 1977). The psyllid population was sampled (approxi-mately 20% of the colony population) and tested by PCR to confirm the absence of HLBB. HLBB-free psyl-lids were later used in the tests for acquisition-access of HLBB.
For HLBB-acquisition, HLBB-free psyllid adults were transferred to a cage containing an HLBB-infected LSO plant, which was kept in an insect-proof growth chamber. Because psyllid adults tend to feed on very young parts of hosts where the HLBB is not yet present (McClean, 1970; Huang, 1979), young shoots of the HLBB-infected LSO were removed forcing them to feed on mature leaves for better acquisition. After two weeks, the psyllids were transferred from the dis-eased LSO to four healthy CBO seedlings for inoc-ulation access (100 psyllids per plant). Psyllids were removed from the test plants after a two-week inocula-tion period. HLBB replicainocula-tion in the inoculated plants was monitored by PCR detection of HLBB in the same fashion described below. Two healthy LSO plants were also used in this psyllid-transmission test for the com-parison between CBO and LSO hosts.
DNA extraction from citrus tissues and psyllid bodies
DNA extracts from citrus tissues were prepared using the method described by Hung et al. (1999b). In brief, leaf midribs (c. 250 mg collected from 6 to 7 mature leaves) were powdered in liquid nitro-gen and homonitro-genized in DNA extraction buffer (0.1 M Tris–HCl [pH 8.0], 0.05 M EDTA, 0.5 M NaCl, 1% N-Lauroylsarcosine). Samples were incu-bated at 55◦C for 1 h. After low speed (4000×g) centrifugation, the supernatant was treated with 1% CTAB (hexadecyl-trimethyl-ammonium-bromide) at 65◦C for 10 min. DNA was precipitated with iso-propanol after chloroform/isoamyl alcohol (24 : 1) and phenol/chloroform/isoamyl alcohol (25 : 24 : 1) treat-ments. The DNA pellet was resuspended in 150µl TE buffer (10 mM Tris–HCl [pH 8.0], 1 mM EDTA).
For the detection of HLBB in psyllids, a psyllid was put in an eppendorf tube containing 300µl of DNA extraction buffer (same as above), homogenized with plastic rod, and incubated at 55◦C for 1 h. After phenol/ chloroform/isoamyl alcohol extraction, the DNA was precipitated by mixing 200µl of the supernatant and
500µl of 100% ethanol followed by centrifugation at 12000×g at 4◦C for 10 min. The pellet was dried and resuspended in 50µl TE buffer.
Primers and PCR conditions
Two primers for PCR-based detection of HLBB were chosen from the sequence of a cloned HLBB-specific DNA fragment (Hung et al., 1999a). The primer pair, composed of the forward primer 50-CAC CGA AGA TAT GGA CAA CA-30and the reverse primer 50-GAG GTT CTT GTG GTT TTT CTG-30, were designed to amplify a HLBB-specific DNA fragment (226 base pairs) by PCR.
PCR was performed using 25µl of reaction mix-ture (Hung et al., 1999b). The thermal cycle conditions were: one cycle at 94◦C for 3 min; 30 cycles at 94◦C for 1 min, 56◦C for 1 min and 72◦C for 2 min; followed by a 72◦C extension for 10 min. Reactions were car-ried out in a DNA Thermal Cycler 2400 (Perkin Elmer, Norwalk, CT, USA).
Analysis of PCR products by electrophoresis
The PCR products were analyzed by gel electrophore-sis using 1.4% agarose in TAE buffer (40 mM Tris– acetate [pH 8.0], 1 mM EDTA). After electrophoresis, the gel was stained with ethidium bromide (0.5µg/ml), visualized and analyzed by the AlphaImager™ 2000 Documentation & Analysis System (Alpha Innotech Co., San Leandro, CA, USA). A 100 bp DNA Ladder set (Promega, Madison, WI, USA) was included as size markers.
Results
HLBB-transmission from citrus to CBO by grafting
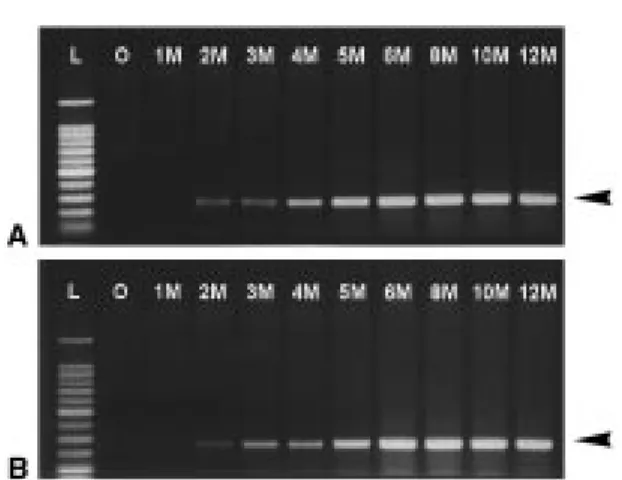
The CBO plant graft-inoculated with HLBB-infected LSO scions was sampled and DNA was extracted monthly after grafting. The multiplication of HLBB in CBO was monitored by a PCR assay with HLBB-specific primers. The results demonstrated that HLBB can survive and replicate in the CBO plant (CBO 1-1) (Figure 1A, Table 1). The infected CBO plant tested positive for HLBB from the 2nd month after graft-ing. The detectable signal derived from the PCR band became stronger each month, reaching a maximum at
Figure 1. PCR detection of the HLBB in a graft-inoculated CBO plant. The CBO plant (A), grafted with HLBB-infected Luchen sweet orange scions, was sampled and tested monthly for HLBB after grafting. The PCR products were analyzed by electrophore-sis in a 1.4% agarose gel, and the positive results were recognized by the appearance of HLBB-specific bands at the 226-bp position (arrows). Lane L, 100-bp DNA ladder for size markers; lane O, a sample taken before graft-inoculation as a negative control; lane 1M–12M, samples collected from 1 month to 12 months after graft-inoculation. A LSO plant grafted with HLBB-infected LSO scions was included in this experiment (B) and tested in the same way for comparison.
approximately the 6th month, and maintained the max-imum over a six month time period. When compared to the LSO host (LSO 1-1), CBO had the same pattern in analysis by electrophoresis as LSO (Figure 1B). This indicated that CBO and LSO have an equal suscepti-bility for infection and replication of HLBB.
An additional three CBO (CBO 1-2, 1-3 and 1-4) and one LSO (LSO 1-2) plants were used for additional experiments in graft-inoculation tests using the same experimental method described above (Table 1). HLBB infected all of the four CBO and two LSO plants. HLBB was detected in three CBO (1-1, 1-2 and 1-3) and two LSO plants from the 2nd to 12th month after grafting, and in CBO 1-4 from the 3rd to 12th month.
HLBB-transmission from CBO back to citrus by grafting
To determine whether CBO is a good donor of HLBB, a back-grafting test was made. Four healthy LSO plants (LSO 2-1, 2-2, 2-3 and 2-4) were inoculated by graft-ing with HLBB-infected CBO scions (from CBO 1-1). Two additional healthy CBO plants (CBO 2-1 and 2-2) were included for comparison. HLBB multiplication was monitored by the PCR assay, and the results are
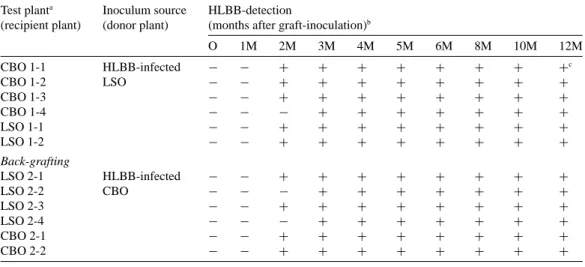
Table 1. Monitoring of the HLBB in graft-inoculated Chinese box orange and Luchen sweet orange plants using PCR Test planta Inoculum source HLBB-detection
(recipient plant) (donor plant) (months after graft-inoculation)b
O 1M 2M 3M 4M 5M 6M 8M 10M 12M CBO 1-1 HLBB-infected − − + + + + + + + +c CBO 1-2 LSO − − + + + + + + + + CBO 1-3 − − + + + + + + + + CBO 1-4 − − − + + + + + + + LSO 1-1 − − + + + + + + + + LSO 1-2 − − + + + + + + + + Back-grafting LSO 2-1 HLBB-infected − − + + + + + + + + LSO 2-2 CBO − − − + + + + + + + LSO 2-3 − − + + + + + + + + LSO 2-4 − − − + + + + + + + CBO 2-1 − − + + + + + + + + CBO 2-2 − − + + + + + + + +
aTest plant: CBO, Chinese box orange (Severinia buxifolia); LSO, Luchen sweet orange (Citrus sinensis Osb). bTest plants were sampled and tested monthly for HLBB after graft-inoculation.
cThe visible HLBB-specific band (226-bp) on agarose gel:+, positive; −, negative.
shown in Table 1. Similar to the results of grafting with diseased LSO scions, LSO and CBO plants graft-inoculated by diseased CBO showed positive results after grafting. In the back-grafting test, HLBB was detected in two LSOs (LSO 2-1 and 2-3) and two CBOs (CBO 2-1 and 2-2) from the 2nd to 12th month after grafting, and in the other two LSOs (LSO 2-2 and 2-4) from the 3rd to 12th month. CBO scions were as effective as LSO scions for HLBB trans-mission, indicating that CBO is also a good donor of HLBB.
HLBB-transmission from LSO to CBO by psyllids
A total of 120 HLBB-free psyllid adults grown on jas-mine orange, were used for the psyllid-transmission test. Psyllids were transferred to an HLBB-infected one-year-old LSO plant for HLBB-acquisition. Ten psyllids from the HLBB-exposed psyllid population were tested by PCR after a two-week acquisition period. Eight out of ten sampled psyllids acquired HLBB from the diseased LSO plant after a two-week feeding period (Figure 2, lane 11–20). The remain-ing psyllids (about 100 psyllids) were transferred to a healthy one-year-old CBO (CBO 3-1) for an inoculation-access period of two weeks. The midribs of plants were individually sampled (c. 250 mg per tested plant) and tested monthly to monitor HLBB replication.
Figure 2. PCR detection of the HLBB in psyllids (Diaphorina citri Kuwayama). The PCR products were analyzed by elec-trophoresis in a 1.4% agarose gel, and the positive results were recognized by the appearance of the HLBB-specific bands at the 226-bp position (arrows). Lane L, 100-bp DNA ladder as size markers; lane 1–10, ten randomly-sampled psyllids from the HLBB-free psyllid population raised on common jasmin orange (Murraya panicualta) for the negative control; lane 11–20, ten randomly-sampled psyllids from the psyllid population on the HLBB-infected Luchen sweet orange after a two-week acquisi-tion period; lane 21–30, ten randomly-sampled psyllids from the psyllid population on the HLBB-infected Chinese box orange after a two-week acquisition period; lane D, a HLBB-infected LSO sample for a positive control.
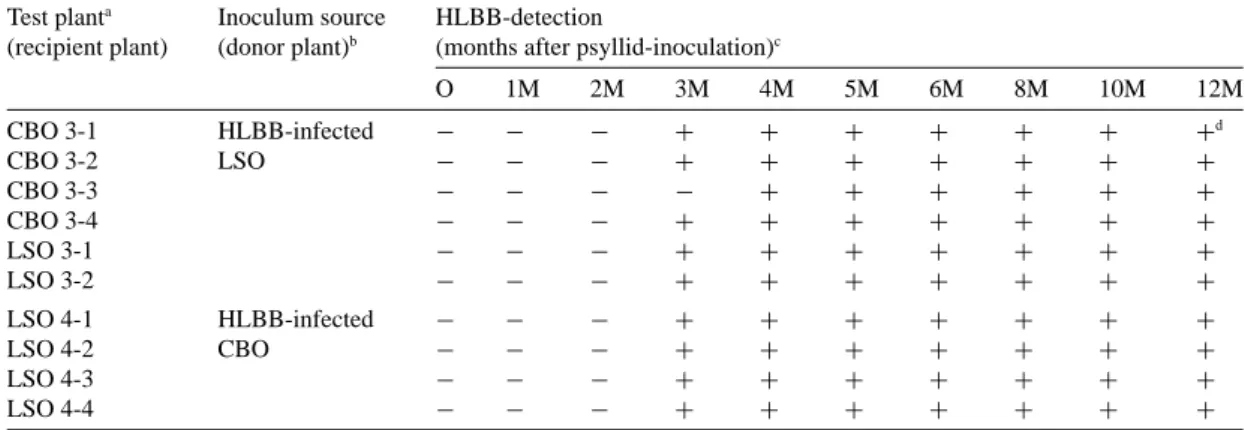
Table 2. Monitoring of the HLBB in Chinese box orange and Luchen sweet orange plants inoculated by psyllids using PCR Test planta Inoculum source HLBB-detection
(recipient plant) (donor plant)b (months after psyllid-inoculation)c
O 1M 2M 3M 4M 5M 6M 8M 10M 12M CBO 3-1 HLBB-infected − − − + + + + + + +d CBO 3-2 LSO − − − + + + + + + + CBO 3-3 − − − − + + + + + + CBO 3-4 − − − + + + + + + + LSO 3-1 − − − + + + + + + + LSO 3-2 − − − + + + + + + + LSO 4-1 HLBB-infected − − − + + + + + + + LSO 4-2 CBO − − − + + + + + + + LSO 4-3 − − − + + + + + + + LSO 4-4 − − − + + + + + + +
aTest plant: CBO, Chinese box orange (Severinia buxifolia); LSO, Luchen sweet orange (Citrus sinensis Osb).
bThe test plants were inoculated by 100 psyllids that acquired HLBB from the HLBB-infected plants (donor plants) through a two-week acquisition period.
cTest plants were sampled and tested monthly for HLBB after inoculation. dThe visible HLBB-specific band (226-bp) on agarose gel:+, positive; −, negative.
An LSO plant (LSO 3-1) was included in the experi-ment for comparison. For further data collection and comparison, an additional three CBO (CBO 3-2, 3-3 and 3-4) and one LSO (LSO 3-2) plants were treated by the same experimental procedure for psyllid trans-mission. The results, demonstrate that HLBB can be transmitted to CBO via psyllid inoculation (Table 2). HLBB was detected in three CBO (CBO 3-1, 3-2 and 3-4) and two LSO (LSO 3-1 and 3-2) plants from the 3rd to 12th month after psyllid inoculation, and in 1 CBO plant (CBO 3-3) from the 4th to 12th month. This psyllid transmission test showed that, like LSO, CBO is also susceptible to HLBB. CBO was as suitable a host as LSO for both Asian citrus psyllid and HLBB. HLBB-transmission from CBO back to LSO
by psyllids
For HLBB-transmission from CBO back to LSO, 120 free psyllid adults were caged with the HLBB-infected CBO plants for a two-week acquisition period. Following the same method described above, ten psyl-lids were sampled and tested to confirm HLBB acquisi-tion. Seven out of ten sampled psyllids were positive for HLBB (Figure 2, lane 21–30). Psyllids acquired HLBB from the diseased CBO plant in this test. The remain-ing psyllids were transferred to a healthy LSO (LSO 4-1) plant for a two-week inoculation period. Follow-ing the same experimental method, an additional three LSO plants (LSO 4-2, 4-3 and 4-4) were tested to repeat
the experiment. HLBB was detected in all four tested plants (LSO 4-1, 4-2, 4-3 and 4-4) the 3rd month after inoculation and thereafter (Table 2). CBO was estab-lished as a donor plant for HLBB-transmission with the ability to transfer HLBB from CBO back to LSO via vector psyllids.
Symptom expression of the HLBB-infected CBO In addition to PCR detection of HLBB, symptoms of Huanglongbing were recorded for each experiment. In the graft-inoculation tests, three infected CBO plants (CBO 1-1, 1-2 and 1-3) showed mild chlorosis on leaves 6 months after grafting, and developed symp-toms of evident chlorosis, leaf-hardening and vein enation 12 months after grafting. Another CBO (CBO 1-4) showed mild chlorosis at the 8th month and evident chlorosis at the 12th month. Symptoms on LSO were more severe than those on CBO. Both of the infected LSO plants (LSO 1-1 and 1-2) showed mild mottle on leaves at the 4th month, evident blotchy mottle at the 8th month, and additional leaf-hardening and severe vein enation at the 12th month.
Development of symptoms in the psyllid-transmission tests was slower than that in grafting tests. Three psyllid-inoculated CBO plants (CBO 3-1, 3-2 and 3-4) showed mild chlorosis at the 9th month and more severe chlorosis at the 12th month. CBO 3-3 only showed mild symptoms of chlorosis at the 12th month. Two psyllid-inoculated LSO plants
Figure 3. PCR detection of the HLBB in samples of CBO collected from fields in southern Taiwan. Lanes 1–10, ten CBO samples collected from fields; lane D, a diseased citrus sample for positive control; lane H, a healthy citrus sample for negative control; lane L, 100-bp DNA ladder for size markers. Two test samples of CBO (lanes 2 and 10) show a positive result for the HLBB-specific bands at the 226-bp position (arrow).
(LSO 3-1 and 3-2) showed mild mottle on leaves at the 8th month, and blotchy mottle at the 12th month. HLBB detection in CBO samples collected
from the field
Ten CBO samples were collected from the vicinity of citrus orchards in southern Taiwan to test by PCR for HLBB. Two samples (Nos 2 and 10) tested positive for HLBB (Figure 3). Sample 2 showed symptoms of chlorosis and leaf-hardening, sample 10 showed mild chlorosis symptoms, and the others did not show any symptoms. Symptom expression corresponded to the presence of HLBB as detected by PCR. The results indicated that there are HLBB infected CBO plants present in the field.
Discussion
Our data showed that HLBB infects CBO in addition to LSO plants. In graft-inoculation tests, CBO was a susceptible recipient plant in which HLBB survived and replicated. It also served as a donor plant from which HLBB could be transmitted to LSO by grafting. In the grafting tests, CBO and LSO were compatible and CBO is known to be a feasible rootstock for citrus grafting (Reuther et al., 1967; Yoshida, 1996).
Psyllid-transmission tests provided further convinc-ing evidence to verify that CBO is an alternative host for HLBB. Psyllids transmitted HLBB to CBO plants in our experiments, and HLBB-free psyllids efficiently acquired HLBB from the HLBB-infected CBO plant. Furthermore, HLBB was detected in field-collected samples indicating the occurrence of naturally-infected CBO plants. For the psyllid-transmission tests, PCR-based detection methods accurately detected HLBB in
a single psyllid and confirmed HLBB-acquisition of psyllids.
The PCR method used in this study is sensitive for HLBB-monitoring in plant tissues and psyllid bodies. One primer pair, which generates a 226-bp HLBB-specific fragment from DNA templates, was chosen for PCR amplification. The PCR-based assay using this primer pair may prove to be the most efficient method for HLBB detections (Hung et al., 1999b). However, it should be paid more attentions to avoid the false positives due to contamination of samples in the PCR detections. Therefore negative controls from healthy samples are always necessary for the PCR-based assay to achieve the reliable data.
In addition to PCR detection, the symptom record was also included as another evidence of HLBB-infection in this study. Symptom expressions correspond to the results of PCR detection. In graft-inoculation tests, symptoms appeared in CBO plants approximately 4 months after HLBB was first detected by PCR. In psyllid-transmission tests, symptoms showed in CBO plants approximately 5 months after HLBB was first detected by PCR. On the other hand, symptom development in LSO was generally 1–2 months faster than in CBO, and LSO had severe symp-toms than CBO. It indicates that CBO is probably more tolerant to HLBB than LSO though CBO is an alternative host.
Elimination of CBO plants in and near citrus orchards may reduce the possible inoculum sources of citrus Huanglongbing. Alternative hosts usually play an important role in an epidemic disease, but they are often neglected in epidemiological studies especially when they cannot be recognized. Alternative hosts are concealed havens for pathogen survival. CBO is con-sidered as a weed and is often seen in fields. In a newly-planting citrus orchard based on healthy citrus trees from a pathogen-free nursery foundation, the HLBB-infected CBO plants are probably the primary inoculum sources in this orchard. In our recent epidemiologi-cal researches, primary data reveal that citrus seedlings cultivated in the surrounding of HLBB-infected CBO plants are easy to be infected by HLBB. Besides, we also found that, like citrus, CBO is very attractive to psyllids.
References
Capoor SP, Rao DG and Viswanath SM (1967) Diaphorina citri Kuwayama, a vector of the greening disease of citrus in India. Indian J Agric Sci 37: 572–576
Chakraborty NK, Pandey DK, Chatterjee SN and Singh AB (1976) Host preference in Diaphorina citri Kuwayama, vec-tor of greening disease of citrus in India. Indian J Entomol 38: 196–197
da Graca JV (1991) Citrus greening disease. Annu Rev Phy-topathol 29: 109–136
Garnier M, Danel N and Bove’ JM (1984) Aetiology of citrus greening disease. Ann Microbiol (Inst Pasteur) 135A: 169–179 Herrero R, Asins MJ, Pina JA, Carbonell EA and Navarro L (1996) Genetic diversity in the orange subfamily Aurantioideae II. Genetic relationships among genera and species. Theor Appl Genet 93: 1327–1334
Hocquellet A, Toorawa P, Bove’ JM and Garnier M (1999) Detec-tion and identificaDetec-tion of the two Candidatus Liberobacter species associated with citrus huanglongbing by PCR amplifi-cation of ribosomal protein genes of the beta operon. Mol Cell Probes 13: 373–379
Huang CH (1979) Distribution of likubin pathogen in likubin-affected citrus plant. J Agric Res China 28: 29–33
Hung TH, Wu ML and Su HJ (1999a) Detection of fastidious bac-teria causing citrus greening disease by nonradioactive DNA probes. Ann Phytopath Soc Japan 65: 140–146
Hung TH, Wu ML and Su HJ (1999b) Development of a rapid method for the diagnosis of citrus greening disease using the polymerase chain reaction. J Phytopathol 147: 599–604 Hung TH, Wu ML and Su HJ (2000) Identification of alternative
hosts of the fastidious bacterium causing citrus greening dis-ease. J Phytopathol 148: 321–326
Jagoueix, S, Bove’ JM and Garnier M (1994) The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int J Syst Bacteriol 44: 379– 386
Jagoueix, S, Bove’ JM and Garnier M (1996) PCR detection of the two ‘Candidatus’ Liberobacter associated with greening disease of citrus. Mol Cell Probes 10: 43–50
Lin SJ, Ke YF and Tao CC (1973) Bionomics observation and integrated control of citrus psylla, Diaphorina citri Kuwayama. J Chinese Soc Hort Sci 19: 234–242
McClean APD (1970) Greening disease of sweet orange: its trans-mission in propagative parts and distribution in partially dis-eased trees. Phytophylactica 2: 263–268
Murashige T, Bitters WP, Naver EM, Roistacher CN and Holiday PB (1972) A technique of shoot tip grafting and its uti-lization towards recovering virus-free citrus clones. Hort Sci-ence 7: 118–119
Reuther W, Webber HJ and Batchelor LD (1967) The Citrus Industry, Vol 1: History, world distribution, botany, and vari-eties. University of California, Berkeley, Berkeley
Singh AB and Nimbalkar MR (1977) Murraya Koenigii L. and Murraya paniculata L. (citrus pests)-preferable hosts of Diaphorina citri Kuwayama (vector of greening mycoplasma). Sci Cult 43: 97–98
Su HJ and Chu JY (1984) Modified technique of citrus shoot-tip grafting and rapid propagation method to obtain citrus bud-woods free of citrus viruses and likubin organism. Proc Int Soc Citriculture 2: 332–334
Tsai JH (1998) Development, survivorship, and reproduction of Toxoptera citricida Kirkaldy (Homoptera: Aphididae) on eight host plants. Environ Entomol 27: 1190–1195
Yoshida T (1996) Graft compatibility of Citrus with plants in the Aurantioideae and their susceptibility to citrus tristeza virus. Plant Dis 80: 414–417