~ Pergamon Continental Shelf Research, Copyright ~ 1996 Elsevier Science Vol. 16, No. 12, pp. 1609-1619, 1996 Lid
Printed in Great Britain. All rights reserved 0278--4343(95)00085--2 0278-4343/96 $15.00 + 0.(~
NOTE
Depth distribution of dl3C of dissolved ECO2 in seawater off
eastern Taiwan: effects of the Kuroshio current and its
associated upwelling phenomenon
D. D. SHEU,* W. Y. LEE,* C. H. W A N G , t C. L. WEI$, C. T. A. CHEN,* C. CHERNG* and M. H. HUANG*
(Received 18 August 1993; in revised form 18 May 1995; accepted 5 October 1995)
Abstract--This paper presents depth variations of dissolved 02, ECO2, alkalinity, and d13C of ECO 2 in seawater off eastern Taiwan. 02 decreases from an averaged surface value of approxi- mately 4.6 ml I I to a minimum of about 2.0 ml 1-1 at a depth of 1000 m, and then increases gradually to about 3.5 ml 1- t near the seafloor. Y~CO 2 concentrations increase from typical values of 190(O2050/~mol kg -1 at the surface to 2100-2300/~mol kg -1 at depths of more than 1000 m, corresponding to an increase in alkalinity from about 2250/~eq kg- l at the surface to 2450/~eq k g i in the bottom water. 613C of ~,CO 2 at the surface averages about +0.25% and becomes lighter with increasing depth to approximately - 1 . 0 % . The observed depth distributions of 02, EC02, alkalinity and 613C of ]2CO 2 are thus similar to those reported in the open ocean and can be attributed to 0 2 utilization and CO 2 production during organic decomposition. On a close examination, however, rates of 0 2 decrease and ECO 2 and alkalinity increases are less profound than those observed in the open ocean, particularly in the Pacific. Furthermore, values of 613C measured are invariably lighter than those reported in the Pacific. The discrepancy is attributed largely to the presence of the Kuroshio current and its associated upwelling phenomenon in the continental margin off eastern Taiwan. Copyright © 1996 Elsevier Science Ltd.
1. INTRODUCTION
In spite of its non-conservative behavior and dynamic nature, d13C of dissolved ECO: ira seawater has been used in combination with other parameters as an additional tracer to study the fate and pathway of carbon in the oceans. For instance, Deuser and Hunter (1969) found that 613C minimum was correlated to dissolved 02 minimum at all depths below the top 200 m of the water column in the Atlantic, and the phenomenon can be attributed to the oxidation of organic matter that is depleted in 13C. They further postulated that the observed isotopic shift in the dissolved inorganic carbon could be used to assess the contribution of organic carbon that was oxidized. Craig (1970) showed that
*Institute of Marine Geology, National Sun Yat-Sun University, Kaohsiung, Taiwan, Republic of China. -Hnstitute of Earth Sciences, Academia Sinica, P.O. Box 1-53, Nankang, Taiwan, Republic of China. $Institute of Oceanography, National Taiwan University, P.O. Box 23-13, Taipei, Taiwan, Republic of China.
1610 D . D . Sheu etal.
the 6J3C of dissolved inorganic carbon in the South Pacific decreased from +2.2% at the surface to +0.5% in the bottom water, and there was a 613C minimum corresponding to the £CO2 maximum and the dissolved 02 minimum at a depth of approximately 2500 m. He then estimated that the increase in deep-water £CO2 from particulate flux would be derived about 30% from planktonic carbonate shells and 70% from organic matter. Soon after these findings, similar depth profiles of 613C and estimates of carbonate vs organic contributions have been reported in both the Atlantic and the Pacific (Kroopnick et al.,
1970, 1972; Kroopnick, 1974a,b, 1980, 1985).
However, these studies had been mainly conducted in the open oceans, with scant attention to the marginal sea thus far. This paper presents systematic measurements of 613C in the water column off eastern Taiwan, a unique marginal sea area characteristic of the existence of the Kuroshio current and its associated upwelling phenomenon.
2. E X P E R I M E N T A L M E T H O D S AND P R O C E D U R E S
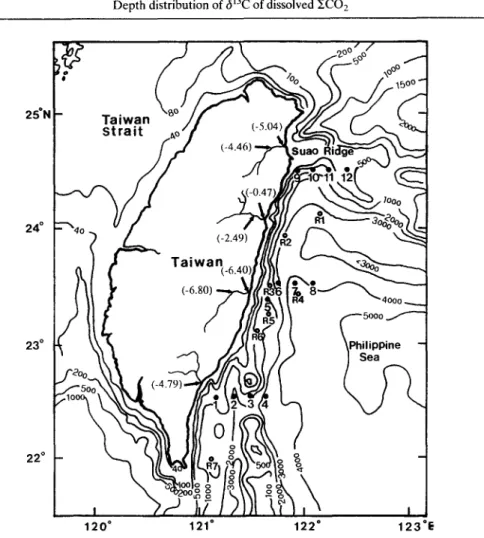
A total of 19 hydrographic stations off eastern Taiwan was investigated during cruises in April 1989 (stations R1-R7 for O2) and September 1990 (stations 1-12 for £CO2, alkalinity and 613C), respectively (Fig. 1). All water samples were collected at various depths with 2.5-1 Niskin bottles using a rosette sampler aboard the RV Ocean Research I.
Except for 02 measurements, samples used for alkalinity (500 ml), ECO2 (500 ml) and carbon isotope analyses (300 ml) were added with 1 ml of HgCI2 immediately after sampling and stored at 5°C in darkness to prevent biological production of CO2 (Sackett and Moore, 1966; Kroopnick et al., 1972).
Measurement of dissolved 02 concentration was performed aboard the ship using the Winkler titration method with minor modifications (Carpenter, 1965). Upon return to the laboratory, concentrations of £CO2 and alkalinity were determined by the conventional titration techniques (Bradshaw et al., 1981). Precision estimated from replicates was +0.1 ml 1-1, _+5/~mol kg -1 and _+3 peq kg -1 for O2, ~1CO2 and alkalinity, respectively. In-situ measurements of chlorophyll a fluorescence in seawater were performed with a fluometer (Sea Tech Inc.) mounted on CTD and Rosette assemblies (Gong et al., 1993). The fluorescence output from the fluometer had been calibrated with the known chlorophyll a concentrations and a proportional factor of 5 was applied to the fluometer for the seawater concentration range of chlorophyll a in the region (<10pg 1-~; Chen, 1992). The averaged standard error of measurements was better than _+0.1 pg 1 -~.
Procedures of extracting £CO2 from seawater for isotopic measurement consist of acidifying the seawater, then collecting and purifying the evolved CO2 from samples under vacuum. Approximately 3 ml of 100% H3PO4 was introduced into the side arm of a custom-made reaction vessel, which could be closed with a high vacuum valve. Next, approximately 60 ml of water sample was transferred into the vessel without protection from contact with air. The procedure took only a few seconds and had been shown previously by Presley and Claypool (1971) to result in an insignificant atmospheric contamination. The vessel was then assembled on the vacuum line to remove the remaining air from the spare space of the vessel.
Because of the difficulty of freezing the larger-size sample vessels in the vacuum line and the frequent crack of glass vessels during the thawing of water after being deep frozen, the removal of air from the reaction vessel in this study was performed without freezing the water samples. There was an attempt to carefully evacuate the air out from the head space,
Depth distribution of 613C of dissolved XCO 2 1611 2 5 ° N 2 4 ° 2 3 ° 22 ° 120" 121" 122" 123"E
Fig. 1. Bathymetric map showing the sampling locations (stations R1-R6 for dissolved 02 measurement only; stations 1-12 for ECO2, alkalinity and 613C analyses). Data of additional 613C
measurements for river water samples are shown in parenthesis.
but a vigorous bubbling was often observed and thus made the direct pumping method unsatisfactory. Consequently, the removal of air was furnished by opening repeatedly (usually three times) the vessel to a large space on the line until an incipient degassing is barely observed. After the air was expelled, the vessel was closed and removed from the line. The vessel was tilted to allow the reaction of the water with the acid and placed in a water bath at 50°C with agitation for 20 min. The vessel was then re-assembled on the vacuum line. The CO2 gas was collected with a liquid nitrogen trap after complete removal of the water vapor by a slurry of a mixture of dry ice and alcohol. In this study, the extraction of CO 2 from water samples usually gave a yield of better than 92%, with the exception of a few samples. The percentage of yield was shown by Sackett and Moore (1966) not to affect the 613C value from a given water sample.
Isotopic analysis was performed with a VG Sierra 903 mass spectrometer. Results of isotopic measurement were expressed with the conventional 6 notation and reported as per mil (%o) difference relative to the PDB standard (Craig, 1957). A total of more than
1612 D . D . Sheu et al.
150 seawater samples including replicates was measured. Replicates of full procedural analysis showed the precision was variable but generally better than +0.15%o.
As mentioned previously, the procedure for removal of the air from the vessels might result therefore in a slight loss of dissolved CO2 from water samples and yields an enrichment of 613C in the remaining water due to the known isotopic fractionation of carbon between CO2 and HCO3 in the water (e.g. a = 1.0096 at 10°C, Deuser and Degens, 1967; Friedman and O'Neil, 1977). The following two experiments had been carried out to evaluate such a potential problem on the 613C measurements, and necessary corrections were made accordingly. In the first experiment, the CO2 that was outgassed from the water sample was collected, and it was added to that was liberated from the seawater sample after reaction with acid, and then analyzed the combined CO2 for 613C. The second experiment first involved the evacuating of another home-made vessel that had filled with acid and had a rubber septum through which the water samples can be injected into the vessel using a syringe. These two methods ensured a complete recovery of all C O 2 in water. Results from these two experiments showed that the 613C of CO2 prepared by the procedure used in this study consistently exhibited an enrichment of -0.6%0. As a consequence, a corresponding correction of 0.6%0 was applied to the 613C values analyzed in this study.
3. RESULTS AND DISCUSSION
The continental margin off eastern Taiwan (Fig. 1) is characteristic of an abrupt change in seafloor topography due to the island-arc/continent collision, and in many areas the bottom slope drops abruptly from the coast to more than 3500 m within a distance of only 40-50 km. (Fig. 1; Chai, 1972; Wu, 1978; Tsai, 1986; Sheu and Huang, 1989; Huang et al., 1992). The region is also known for the Kuroshio current to develop and intensify to a strong western Pacific boundary current after leaving its source area east of Luzon island (Nitani, 1972). The presence of the Kuroshio water off eastern Taiwan can be readily seen in hydrographic profiles due to its relatively high salinity and temperature compared with the overlying and underlying water (Liu, 1983; Liu et al., 1988; Wong et al., 1991). The depth interval of the main path of Kuroshio flowing northward along the east coast of Taiwan ranges approximately from 100 m to nearly 500 m, with a maximum velocity of about 3 knots.
The abrupt change in bathymetry also causes the upwelling phenomenon in the continental margin east of Taiwan (Bodvarsson, 1976; Fan, 1980). Persistent upflow of deep water below the main stream of the Kuroshio, however, is found mainly in regions off southeastern and northeastern Taiwan, where the Kuroshio encounters the continental shelf. Patches of cold water from depth in southeast Taiwan were first identified in a satellite Gemini X photograph by Emery and Stevenson (1972), and later were confirmed by shoreward rises in isotherms, isohalines and chemical hydrographic data (Hung, 1975; Bodvarsson, 1976; Liu, 1983; Wong et al., 1991; Liu et al., 1992). Recently, the striking difference in these properties between the Kuroshio and its adjacent waters off northeast Taiwan was used to study the upwelling mechanism and its associated exchange processes in the region off northeast Taiwan (Liu and Pai, 1987; Wong et al., 1991; Liu et al., 1992). The extension and intensity of the upwelling are further shown by these authors to vary both spatially and temporally.
D e p t h distribution of 613C of dissolved £ C O 2 1613
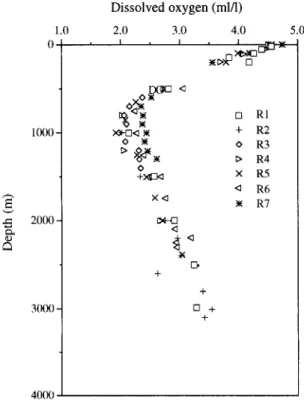
to demonstrate the general distribution of 0 2 in the water column off eastern Taiwan (Fig. 2). As can be seen, 02 averages about 4.6 ml 1-1 at the surface, and then decreases progressively to a minimum of approximately 2.0 m l l - t at 1000 m. Below this depth, 02 increases gradually to about 3.0 ml 1-1 near the seafloor. The observed 02 profiles in this study are consistent with previous observations and are mirror images of the nutrient distributions (Liu et al., 1988; Wen et al., 1989; Wong et al., 1991; Gong et al., 1992). Furthermore, they are similar to those observed in the open ocean despite that dissolve, d 02 minima in waters off eastern Taiwan are less distinct than those in the central Pacific, as reported by Kroopnick et al. (1972) and Kroopnick (1985).
Figure 3a and b shows the vertical distributions of dissolved ECO2 concentrations measured at stations 1-12. £CO 2 increases gradually from a consistent surface value of about 1950 #mol l-l to nearly 2300/~mol l-I at a depth of 2000 m with minor variation. Thus, the increase in ~CO2 from the surface to 1000 m is accompanied by a decrease in the total dissolved 02. Below this depth, both dissolved £CO2 and 0 2 increase with depth. Ranges of £CO2 variations with depth are comparable to those observed in the north Pacific (cf. Kroopnick, 1985), yet their concentrations are much higher than those reported in the north Atlantic (e.g. 2300 vs 2160 #mol kg -1 at a water depth of 2000 m; Kroopnick et al., 1972).
Alkalinity increases rapidly from an averaged surface value of about 2250/~eg kg i 1:o about 2300kteq kg- 1 at 100 m, remains constant between 100 and 500 m, and then increases gradually to approximately 2450 #eq kg-1 towards the seafloor (Fig. 3c and d). The depth
Fig. 2. ¢o 1.0 4.0 5.0 0 ' , I : 1 0 0 0 - 2000- 3(X)O -
Dissolved oxygen (mid)
2.0 3.0 I , I Xll-r'l<l 1~ 0 +>~g2<l X ~ ~t-E] D + + [3 + + [] RI + R2 o R3 t> R4 x R5 <~ R6 R7 40(~)
D e p t h distributions of dissolved oxygen in s e a w a t e r off eastern Taiwan. Note that the 0 2 m i n i m u m locates at the depth of a p p r o x i m a t e l y 1000 m.
1614 D . D . Sheu et al. 5 0 0 -
g
1000- o 1500 -Total dissolved CO 2 (pmole/kg) 1900 2000 2100 2200 2300 2400
o ~ ~ ~
n I i(a )
+ ~ > V + 0 s t l V I> s t 2 t ~ + V s t 3 V ~ + ~, s t 4 [] s t 5 + + s t 6 2000 I -. 500- E v 1000- 1500- 2000Total dissolved CO 2 (pmole/kg) 1900 2000 2100 2200 2300 2400 O- ~ ' ' ( b ) o st7 st8 & st9 x stlO .~ stl l [] stl2 )0
Total Alkalinity (laeq/kg) 2200 2250 2300 2350 2400 2450
o
t t ~
' '(c)
5(11 - + ~ V "~ 1000- ~ ' ~ 1500 - o + 2000 Ig
oTotal Alkalinity (laeq/kg) 2200 2250 2300 2350 2400 2450 0 o 500- 1000- 15(11- 2000 ,. I I I
(d)
¢OK N O X -2 0 5(11- 1000- 15(11 - 2000 813C ( %,, ) -1 0 1 2 + V 0 + -2 0 5 0 0 - ~ . 1000- 1500- 2000 gl 3C ( %0 ) -I 0 & A [] o 1 2 I ( f )Depth distribution of 613C of dissolved E C O 2 1615
distribution of constant alkalinity thus appears to coincide with the depth interval of the main path of the Kuroshio. Carbon isotopic composition of ~CO2 in the water columns generally decreases from 0 to +0.5%o at the surface to - 1 . 5 to -1.0%o near the seafloor with minor variations (Fig. 3e and 30. The change in 613C distribution with increasing depth is similar to that observed in open oceans, yet the gradient is less distinct than that in the Pacific (Kroopnick, 1985). For instance, Kroopnick et al. (1970) reported a decrease in 613C from 2.2%0 to -5%0 from the surface to 1000 m, while 613C in waters off eastern Taiwan varies from 0.5%o at the surface to approximately -1.0%o at 1000 m (Fig. 3e and f). Furthermore, values of 613C measured in the surface waters off eastern Taiwan are invariably lower than those reported in the north Pacific by a maximal difference of approximately 1%o. The discrepancy can be attributed to the addition of 13C-depleted biogenic CO2 via upwelling and/or advection of water from depth.
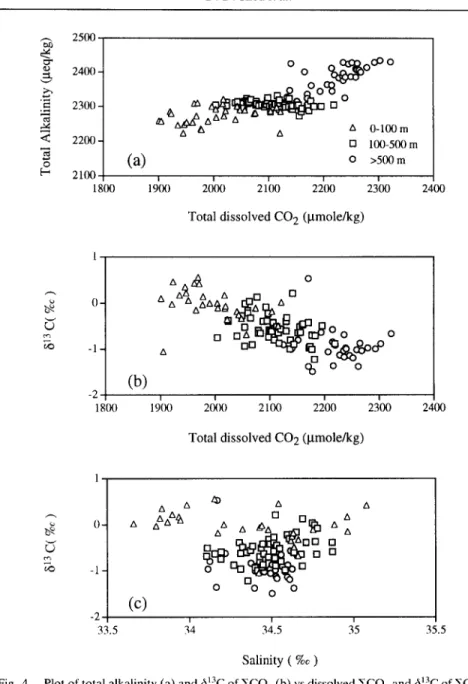
Figure 4a shows a cross-plot of alkalinity vs ~CO2 in the waters off eastern Taiwan. As seen, the lack of an increase in alkalinity with increasing ~CO2 between 100 and 500 m indicates a production of CO2 from organic degradation. Organic detritus in the ocean is readily oxidized and decomposed to form CO2 as it is settling through the water column. This organically derived CO2 is known to deplete in 613C (Williams and Gordon, 1970) and adds to the deep water that then surfaces as upwelling takes place. Figure 4b shows that except for a few data, 613C in waters off eastern Taiwan is correlated fairly well to ECO2. Therefore, the correlation suggests that the depth distribution of 613C observed in these water columns are the results of the addition of 13C-depleted CO2 from in-situ decomposition of organic matter.
Nonetheless, factors affecting the observed depletion of 613C in the surface waters off eastern Taiwan, other than the addition of the 13C-depleted waters from deep, need further discussion. First, effects of latitudinal change in 613C of atmospheric CO2 that may be the cause of the observed depletion of - 1 % in the surface water can be neglected because the present data and those used for comparison are all from stations between 20 and 40°N in the western Pacific and no appreciable change in 613C of atmospheric CO2 is found (Kroopnick, 1985). Furthermore, dissolved CO2 in river water is known to deplete in ~3C relative to seawater and has a range of 613C of approximately - 3 to -10%o (Tan, 1989; and references therein). Therefore, the inflow and mixing of this riverine water with offshore seawater would avert a decrease in the 613C observed. To elucidate this potential effect, an additional seven river water samples from runoffs in eastern Taiwan were collected and analyzed for their 13C compositions. Results (cf. Fig. 1) show that they all fall in the range of typical riverine water previously established in the literature. Two samples of heavier 6~3C values (i.e. -0.47 and -2.49%) may be attributed to the contribution of solutions from the dissolution of carbonate rocks in the drainage basin (Lee and Teng, 1986). A cross-plot of 613C vs salinity in waters between 0 and 100 m (Fig. 4c), howeve~r, reveals that they are not significantly correlated. It should be pointed out that using salinity as a conservative tracer to characterize results of mixing of riverine water with seawater in
Fig. 3. Depth distributions of dissolved ]~CO 2 (a and b), total alkalinity (c and d) and 613C of ]~CO 2 (e and f) in seawater off eastern Taiwan. Note that the depth interval between 100 and 500 m showing consistent values of
1616 D.D. Sheu et al. exl) ..= < O [.. r ~ 2500 2400 - 2300 - 2200 - 2100 1800
(a)
o o ° ~ ° ° ° ° o 0 100-500 m O >500 m 191(K} 2(~00 21~)0 22()0 23~)0 2400Total dissolved CO 2 (I.tmole/kg)
(b)
n A ~ , 0 A A~ A A _17 [ ] o o o o o o o o -2 18(X) 19(K) 20~K) 21100 22100 2;00 2400 Total dissolved CO 2 (I.tmole/kg)O- -1-
(c)
A Akk~
[] th [] A o n [] ~'¢,9 0 0 0 0 -2 I I I 33.5 34 34.5 35 35.5 Salinity ( %o )Fig. 4. Plot of total alkalinity (a) and 613C of ~,CO2 (b) vs dissolved ~CO2 and 613C of ECO2 vs salinity (c) in seawater off eastern Taiwan. Note that waters between 100 and 500 m are characterized by a nearly constant alkalinity with increasing CO2 concentrations, indicating a
biogenic origin of these CO2.
t h e r e g i o n off e a s t e r n T a i w a n is p r a c t i c a l l y i n f e a s i b l e b e c a u s e t h e e m e r g e n c e o f s u b s u r f a c e h i g h - s a l i n i t y K u r o s h i o w a t e r f r o m d e p t h s d u r i n g t h e u p w e l l i n g w o u l d d i s r u p t t h e s a l i n i t y g r a d i e n t t h a t is n o r m a l l y o b s e r v e d in t h e m i x i n g o f r i v e r a n d s e a w a t e r s a l o n e . I n s p i t e o f this, a slightly g r a d u a l i n c r e a s e in s a l i n i t y f r o m 33.8 to 34.5 in t h e s u r f a c e w a t e r s was o b s e r v e d w i t h i n c r e a s i n g d i s t a n c e f r o m t h e c o a s t ( s t a t i o n s 1 - 4 ) , i n d i c a t i n g t h e i n p u t o f r i v e r i n e w a t e r f r o m T a i w a n i s l a n d . A s a c o n s e q u e n c e , a l t h o u g h t h e r e is l a c k o f significant
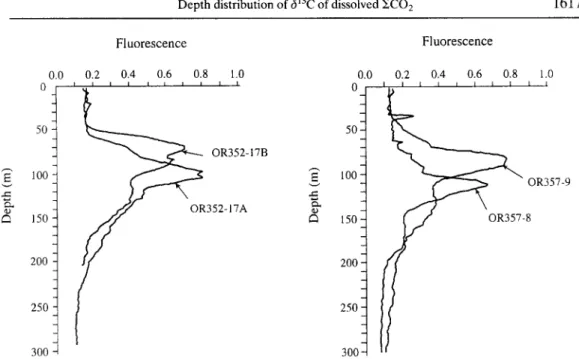
Depth distribution of 613C of dissolved ~CO 2 1617 Fluorescence Fluorescence c-. 0.0 0 50 100 150 200 250 300 0.2 0.4 0.6 0.8 1.0 I I I I I i i I I I OR352-17B 352-17A 50 0.0 0 100 150 200 250 30C 0.2 0.4 0.6 0.8 1.0 I I I I I I I I [ I ~ OR357_9
Fig. 5. Distributions of fluorescence in water columns off eastern Taiwan (station locations: OR352-17A and -17B, 123°10'E 25°00'N; OR357-8, 122°00'E 21°45'N; OR357-9, 122°30'E
21°45'N).
correlation between the 613C and salinity, it is conceivable that the outflow of freshwater from Taiwan island would contribute, to a lesser extent, to the lighter 613C observed near the surface.
Moreover, the isotopic compositions of dissolved 1£CO 2 in surface waters in the ocean can be influenced by the biological activity (Kroopnick, 1974a,b). Marine organisms preferentially utilize the light carbon (12C) isotope and leave behind the heavy carbon (13C) in the ambient water. In this way, the 6t3c of dissolved ~CO2 in seawater will become heavier. As a consequence, high productive water in the surface ocean generally shows more positive 613C values of dissolved ~CO2 than those surrounding waters characteristic of a low productivity. As mentioned previously, the surface water (<100 m) overlying the main path of the Kuroshio in the region off eastern Taiwan is known to be deplete in nutrients. As an indirect measure of biological productivity, values of fluor- escence were recently shown by Gong et al. (1993) to be linearly correlated to tile chlorophyll a in these waters. In the present study, four casts have been carried out of an in-situ fluorescence measurement in the surface 0-300 m waters at three stations (Fig. 5). As shown, the upper 50-100 m water has relatively lower fluorescence values as compared to the underlying Kuroshio water itself. The low fluorescence values measured in this study suggests a low productive surface water in the region off eastern Taiwan and thus may further contribute to the lower 6~3C values measured.
4. CONCLUSIONS
In short, this study presents systematic measurements of 6 ~3C of total dissolved CO2 at various depths of seawater off eastern Taiwan, with pertinent data on alkalinity, 02 and ZCO2. Results show that the low 613C values at the surface (<100 m) and the persistent
1618 D . D . Sheu etal.
depletion of approximately 1%o of 6J3C below 100 m can be largely accounted for by the emergence and mixing of the t3C-depleted, subsurface Kuroshio water from depth. Additional factors that may further act as controls on the observed depletion of 613C at surface are the inflow of freshwater from Taiwan island and the prevailed low productive surface water overlying the Kuroshio in the continental margin off eastern Taiwan. Although the discussion of these processes is constrained by a lack of quantitative estimates, that such mechanisms may operate is neither surprising nor novel. Kroopnick (1985) reported a similar depletion of approximately 1% in the surface water of GEO- SECS stations 222 and 224, where the Kuroshio current flows through the western Pacific off Japan after leaving the eastern coast off Taiwan. Furthermore, it should be mentioned that, unlike in the deep ocean, where various water masses usually are well-defined and vertical settling of organic remains dominates their transports, the present data are from a very unique hydrographic regime where the upwelling and its associated transport and flux may be constantly operating throughout the water column, and organic matter would have been recycled many times before accumulation in sediments, thus making attempts to quantify the fraction of organic contributions and its subsequent changes in 613C from different water sources in this study difficult.
Acknowledgments--The authors are grateful to the Captain and crew aboard RV Ocean Research 1 for water
sampling and technical assistance, S. C. Pai and Y. C. Chung for constructive discussion, and Ms Y. N. Lai and A. E. Sheu for manuscript typing and editing. They also thank three anonymous reviewers for their valuable comments and suggestions on the early version of this manuscript. This work was supported by the National Science Council grants (NSC78-0202-M110-01, NSC79-0202-Ml10-02 and NSC80-0209-Ml10-05) to D. D. Sheu. This research is a contribution to the KEEP (Kuroshio Edge Exchange Process) project, now recognized as the international JGOFS (Joint Global Ocean Flux Study) program of the Republic of China.
R E F E R E N C E S
Bradshaw A. L., P. G. Brewer, D. K. Shafer and R. T. Williams (1981) Measurements of total carbon dioxide and alkalinity by potentiometer titration in the GEOSECS Program. Earth & Planetary Science Letters, 55,
99-115.
Bodvarsson G. M. (1976) On upwelling along the eastern coast of Taiwan: a review of hydrographic and chemical data. Acta Oceanographica Taiwanica, 6, 98-117.
Carpenter J. H. (1965) The accuracy of the Winkler method for dissolved oxygen analysis. Limnology & Oceanography, 10, 135-140.
Chai B. H. T. (1972) Structure and tectonic evolution of Taiwan. American Journal of Science, 272, 389--422.
Chen Y. L. (1992) Summer phytoplankton community structure in Kuroshio current-related upwelling, northeast of Taiwan. Terrestrial, Atmospheric & Oceanic Sciences, 3, 305-319.
Craig H. (1957) Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochimica et Cosmochimica Acta, 12, 133-149.
Craig H. (1970) Abyssal carbon 13 in the south Pacific. Journal of Geophysical Research, 75,691-695.
Deuser W. G. and E. T. Degens (1967) Carbon isotope fractionation in the system CO2(gas)-CO2(aqueous)-HCO3(aqueous ). Nature, 215, 1033-1035.
Deuser W. G. and J. M. Hunter (1969) Stable carbon isotope ratios of dissolved inorganic carbon in the Atlantic.
Deep-Sea Research, 16,221-225.
Emery K. D. and R. E. Stevenson (1972) Taiwan--a ship at sea. Acta Oceanographica Taiwanica, 10, 151-159.
Fan K. L. (1980) On upwelling off northeast shore of Taiwan. Acta Oceanographica Taiwanica, 11,105-117.
Friedman 1. and J. R. O'Neil (1977) Data of geochemistry: Compilation of stable isotope fractionation factors of geochemical interest. Geological Survey Professional Paper, 440-KK, U.S. Government Printing Office,
Washington, DC, 108 pp.
Depth distribution of 613C of dissolved E C O 2 1619
Luzon and a comparison with the west Philippine Sea. Terrestrial, Atmospheric & Oceanic Science. 3,
587~02.
Gong G. C., W. R. Yang and Y. H. Wen (1993) Correlation of chlorophyll a concentration and SEA TECH fluorometer fluorescence in Seawater. Acta Oceanographica Taiwanica, 31, 117-126.
Huang C. Y., C. T. Shyu, S. B. Lin, T. Q. Lee and D. D. Sheu (1992) Marine geology in the arc-continent collision zone off southeastern Taiwan: implications for Neogene evolution of the coastal range. Marine Geology, 107, 183-212.
Hung T. C. (1975) Chemical investigation on upwelling along eastern coast of Taiwan. Acta Oceanographica Taiwanica, 5, 77-94.
Kroopnick P. M. (1974a) Correlation between C-13 and CO 2 in surface waters and atmospheric C O 2 . Earth & Planetary Science Letters, 22, 397-403.
Kroopnick P. M. (1974b) The dissolved O2-CO2-13C system in the eastern equatorial Pacific. Deep-Sea Research, 21, 211-227.
Kroopnick P. M. (1980) The distribution of C-13 in the Atlantic ocean. Earth & Planetary Science Letters, 49, 469-484.
Kroopnick P. M. (1985) The distribution of CO 2 in the world oceans. Deep-Sea Research, 32, 57-84.
Kroopnick P. M. W. G. Deuser and H. Craig (1970) Carbon 13 measurement of dissolved inorganic carbon at the North Pacific (1969) GEOSECS Station. Geophysical Research, 75, 7668-7671.
Kroopnick P. M. R. F. Weiss and H. Craig (1972) Total CO2, C-13, and dissolved oxygen O-18 at Geosecs in the North Atlantic. Earth & Planetary Science Letters, 16,103-110.
Lee C. W. and M. H. Teng (1986) Oxygen and carbon stable isotopes in calcite marbles and calcite-dolomite marbles in the Hoping-tailuko area, eastern Taiwan. Proceedings--Geological Society of China, 29, 35-45. Liu C. T. (1983) As the Kuroshio turns. I. Characteristic of the current. Acta Oceanographica Taiwanica, 14, 88-
95.
Liu C. T. and S. C. Pai (1987) As the Kuroshio turns. II. The oceanic front north ofTaiwan. Acta Oeeanographica Taiwanica, 18, 49~1.
Liu K. K., S. C. Pai and C. T. Liu (1988) Temperature-nutrient relationships in the Kuroshio and adjacent waters near Taiwan. Acta Oceanographica Taiwanica, 21, 1-17.
Liu K. K., G. C. Gong, C. Z. Shyu, S. C. Pai, C. L. Wei and S. Y. Chao (1992) Response of Kuroshio upwelling to the onset of the northeast Monsoon in the sea north of Taiwan: observations and a numerical simulation.
Journal of Geophysical Research, 97, 12511-12526.
Nitani H. (1972) Beginning of the Kuroshio. In: Kuroshio, H. Stommel and K. Yioshda, editors, University of Washington Press, Seattle, WA, pp. 95-128.
Presley B. J. and G. E. Claypool (1971) Determination of total dissolved carbonate and carbon isotopic ratio~'.
Initial Reports DSDP 7, U.S. Government Printing Office, Washington, DC, pp. 1756-1757.
Saekett W. M. and W. S. Moore (1966) Isotopic variations of dissolved inorganic carbon. Chemical Geology, 1,
323-328.
Sheu D. D. and C. Y. Huang (1989) Carbonate and organic carbon sedimentation on the continental margin off southeastern Taiwan. Geo-Marine Letters, 9, 45-51.
Tan F. C. (1989) Stable carbon isotopes in dissolved inorganic carbon in marine and estuarine environments. In:
Handbook of Environmental Isotope Geochemistry, Vol. 3, P. Fritz and J. C. Fontes, editors, Elsevier, Amsterdam, pp. 171-190.
Tsai Y. B. (1986) Seismotectonics in Taiwan. Teetonophysics, 125, 17-39.
Wen L. S., K. K. Liu, S. C. Pai and C. T. Liu (1989) Apparent oxygen utilization in the eastern Philippine Sea and shelf waters near Taiwan. Aeta Oceanographica Taiwanica, 23, 19-32.
Williams P. M. and L. I. Gordon (1970) Carbon 13: carbon 12 ratios in dissolved and particulate organic matter in the sea. Deep-Sea Research, 17, 19-27.
Wong G. T. F., S. C. Pai, K. K. Liu, C. T. Liu and C. T. A. Chen (1991) Variability of the chemical hydrography at the front region between the East China Sea and the Kuroshio north-east of Taiwan. Estuarine, Coastal & Shelf Science, 33, 105-120.