metalloproteinase expression during improvement in the
wound-healing process
Ching-Hua Su,1Shu-Hui Liu,1Shi-Yau Yu,1Yi-Ling Hsieh,2Hsiu-O Ho,3Chung-Hong Hu,4 Ming-Thau Sheu3
1Graduate Institute of Biomedical Material Taipei Medical University, Taipei, Taiwan, ROC 2Graduate Institute of Medical Sciences, Taipei Medical University, Taipei, Taiwan, ROC 3Graduate Institute of Pharmaceutical Science, Taipei Medical University, Taipei, Taiwan, ROC 4School of Medicine, Taipei Medical University, Taipei, Taiwan, ROC
Received 25 March 2004; accepted 19 October 2004
Published online 10 December 2004 in Wiley InterScience (www.interscience.wiley.com). DOI: 10.1002/jbm.a.30235
Abstract: SACCHACHITIN membranes, prepared from the waste residue of the fruiting body of Ganoderma taugae, were used in our previous study to enhance skin wound healing in animal models. In the present study, the effects of the membrane on the growth of keratinocytes and the activ-ity of matrix metalloproteinases (MMPs), as well as on the healing of skin wounds in humans, were estimated. Fresh human foreskin was employed as the source of the keratin-ocyte culture, and a modified keratinkeratin-ocyte-SFM medium supplemented with 0.2 ng/mL of recombinant epidermal growth factor and 30g/mL bovine pituitary extract was used to enhance the successful growth of keratinocytes un-der an atmosphere of 5% CO2, at 37°C. The results indicated
that 0.01% SACCHACHITIN enhanced the proliferation of keratinocytes in the culture on the fourth and fifth days, and cells showed neither morphological alteration nor disor-dered proliferation. This evidence clearly indicated that SACCHACHITIN was not cytotoxic to and was safe for the growth of keratinocytes. Thus, SACCHACHITIN might play a positive role in the proliferation and differentiation of keratinocytes around wounds and in accelerated wound
healing of epidermal tissue. In addition, microscopic obser-vations during the growth of keratinocytes showed that normal proliferation and differentiation took place along the margin of the SACCHACHITIN membrane. This indicates that SACCHACHITIN is possibly cytocompatible with ker-atinocytes. Electrophoretic analysis and inhibition tests for the binding effect of SACCHACHITIN on MMPs showed that SACCHACHITIN reduced MMPs in extracellular ma-trix degradation and facilitated establishment of an extracel-lular matrix around wounds; these effects resulted in rapid wound healing. SACCHACHITIN was used as a skin dress-ing for patients who had skin chronicle ulcer, which had not healed for over 7 months. Preliminary clinical observations showed that the wound improved and began to heal. An analysis of MMPs by ELISA in tissue of the wound indicated a significant decrease in MMP levels. © 2004 Wiley Period-icals, Inc. J Biomed Mater Res 72A: 220 –227, 2005
Key words:Ganoderma tsugae; fungal mycelia; wound
heal-ing; MMP; keratinocyte; SACCHACHITIN
INTRODUCTION
The skin wound-healing process is known to be regulated by cells/extracellular matrix (ECM) interac-tions and by cytokines and/or growth factors. In skin cells, injury is likely to initiate a series of changes in
gene expression that mediate downstream events re-quired for tissue repair, including cell proliferation, migration, and remodeling of the ECM.1Wound heal-ing is often segregated into three stages: (1) inflamma-tion (early and late); (2) re-epithelializainflamma-tion and gran-ulation tissue formation; and (3) matrix formation and remodeling.2 During wound healing, proteinases, in-cluding serine proteinases such as the urokinase–plas-minogen activator and plasmin, and matrix metallo-proteinases (MMPs) are implicated in epidermal repair.3–5 These proteinases primarily facilitate kera-tinocyte movement by remodeling extracellular matrix proteins. In addition, proteinases modulate intracellular signaling, secretion, bioactivation, and
Correspondence to: M-T. Sheu; e-mail: mingsheu@tmu.
edu.tw
Contract grant sponsor: National Health Research Insti-tute; contract grant number: NHRI-EX90-8815EP
Contract grant sponsor: National Science Council of the ROC; contract grant number: NSC 91-2314-B-038-010
stability of cytokinases and growth factors important for epidermal healing.6 –10
Matrix metalloproteinases (MMPs) were first demon-strated to be involved in wound healing in guinea pigs.11 MMPs are subdivided into five main classes by structure and substrate specificity: collagenases (MMP-1, MMP-8, and MMP-13), gelatinases (MMP-2 and MMP-9), strome-lysins (MMP-3, MMP-10, and MMP-11), membrane-type MMPs (MT-MMPs, MMP-14, MMP-15, MMP-16, and MMP-17), and an undefined set.12It has been shown that MMP-1 is closely related to epithelial migration and is essential for wound re-epithelialization,13–14that MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are important during angiogenesis15 and prolonged matrix remodel-ing,14and that MMP-3 is crucial for normal wound con-traction.16
The current use of wound-dressing materials is based mostly on empirical knowledge rather than on an actual understanding of the healing process. Balassa showed that pulverized chitin aids wound healing based on observations relating to the mechan-ical resistance of scar tissue.17 It was claimed that a process for facilitating wound healing indicated that chitin acted as a wound-healing accelerator.18 –19 It was also reported that chitin is physiologically soluble as a consequence of the effect of lysozyme, suggesting that N-acetylglucosamine is important for the orienta-tion and crosslinking of collagen, and that uridine diphosphate N-acetylglucosamine is a key compound in the biosynthesis of hyaluronic acid. Widra20 only described associations of chitosan and keratin or col-lagen, but discovered no biological or histological ef-fect of chitosan on tissue components. Yano21 demon-strated that chitin increases traction resistance compared to controls, but did not increase the quan-tity of collagen in the healing tissues. Malette and Quigley,22 when using chitosan acetate salt to heal wounds, speculated that if fibrin clot formation is avoided, fibroblasts will not be stimulated, and cells can replicate the lost tissue and reduce the thickness of scar tissue. Ricardo18delineated the high susceptibil-ity of a chitosan derivative (5-methylpyrrolidone) to depolymerization by lysozyme when applied in vivo to a wound, providing an efficient way to stimulate macrophages and spleen cells, and favoring the or-dered deposition of collagen, while providing glu-cosamine and N-acetylgluglu-cosamine monomers to the biosynthetic route of hyaluronic acid and glycosami-noglycans.
In an endeavor to develop an ideal skin substitute, the performance of SACCHACHITIN membranes, prepared from residues of the fruiting body of the medicinal fungus, Ganoderma tsugae, as an effective skin prosthesis has been examined.23 That previous study evaluated the effectiveness of SACCHACHITIN membranes in the management of excised wounds in guinea pigs and compared their performance with
gauze and BESCHITIN威. However, the underlying mechanisms responsible for the accelerated wound healing by SACCHACHITIN were not disclosed. SACCHACHITIN membranes were demonstrated to accelerate wound healing by inducing cell pro-liferation in another study.24 With a covering of SACCHACHITIN, it was found that a mild acute in-flammatory reaction attracted a large number of poly-morphonuclear leukocytes and some macrophages to clean away debris and blood clots during the healing process. It was also speculated that the secretion of cell cytokines and growth factors by these cells provided an excellent environment for wound healing. The migration of fibroblast cells, which is promoted by SACCHACHITIN, was also suspected to play another important role in the acceleration of wound healing. However, all those facts are not sufficient to demon-strate the effect of SACCHACHITIN on re-epithelial-ization of keratinocytes and how it improves the heal-ing of chronic wounds, which is a result of imbalance between MMPs and TIMPs (tissue inhibitors of metalloproteinases). In this study, the influence of SACCHACHITIN membranes on the proliferation of keratinocytes and the activity of MMPs was char-acterized.
MATERIALS AND METHODS Reagents
The residue of the fruiting body of Ganoderma tsugae was collected after two hot-water extractions and was a generous gift from a factory in Nantou, Taiwan. Ketamine HCI and xylazine were supplied by Sigma (St. Louis, MO, USA). Female guinea pigs, weighing 380 to 480 g and aged 8 to 10 weeks, were purchased from the Animal Center, National Taiwan University. Analytical-grade reagents were obtained from Merck (Germany). Deacetylated SACCHACHITIN was obtained by dissolving SACCHACHITIN in 45% NaOH with heating. The resulting solution was dialyzed with tap water for 2 days and then with distilled water for 1 day, resulting in a solution with a neutral pH. This solution was then freeze-dried to obtain deacetylated SACCHACHITIN. -Glucan was the alkaline-soluble fraction of the polysac-charides obtained by treating the residue of the fruiting body of Ganoderma tsugae with 1 N NaOH at 90°C for 4 h. SACCHACHITIN was cut into pieces and suspended in deionized phosphate-buffered solution (D-PBS). This sus-pension was ultrasonicated until homogeneous and subse-quently steam sterilized. A SACCHACHITIN stock suspen-sion was obtained by dispensing the resulting suspensuspen-sion into medium to a concentration of 0.5% w/v. An MMP-8 substrate solution was prepared by dissolving MMP-8 sub-strate powder in 0.1% trifluoroacetic acid, and then this was diluted with stock solution containing 50 mM tricine, 200 mM NaCl, 10 mM CaCl2, 0.05% BRIJ威, and 50 M ZnSO4,
Preparation of SACCHACHITIN membranes
The purification of fibers to form SACCHACHITIN mem-branes followed a procedure similar to that reported in a previous paper,3
except that the treatment with 1 N NaOH was conducted at 90°C for 4 h. The fibers with lengths in the range of 10 to 50m were collected and dispersed in deion-ized water to form a suspension, which was then filtered. The membrane formed on the nylon filter cloth was freeze-dried (EYELA, model FD-5N) to obtain a porous membrane with a diameter of 7 cm and thickness of 0.1 to 0.2 mm for the following studies. The chemical constituents of the final product were determined to be 40% N-acetyl-d-glucosamine and 60% -1,3-d-glucan. The membranes were autoclaved and kept under aseptic conditions until use.
Isolation and primary culture of human keratinocytes
Human foreskin samples were obtained from Taipei Med-ical University Hospital. Isolation of human keratinocytes followed the procedure described by Ham and colleagues.6
Primary cells were seeded into T-75 flasks at a cell density of 3 ⫻ 105
cells/mL. Cultures were incubated at 37°C in an atmosphere containing 5% CO2.
Assessment of keratinocyte proliferation
Third-passage human keratinocytes were seeded into petri dishes (40 mm) at a cell density of 2⫻ 104
cells/mL to which the SACCHACHITIN stock suspension (0.5% w/v in medium) had been added at a concentration of 0.01% w/v. At each predetermined time point, three culture samples were treated with 0.05% of a trypsin–EDTA solution to dissociate cells for determination of cell numbers. Thirty microliters of each cell suspension was sampled with the addition of an equivalent volume of 0.5% trypan blue. A hemocytometer was used to count the cell numbers under light microscopy, and a growth curve was then drawn for a 5-day period. The morphology of keratinocyte cells was examined by light microscopy to compare the cytotoxicity of the SACCHACHITIN suspension with the controls. A cell culture with no added SACCHACHIYTIN stock suspension was used as the control.
In vitro activity measurements of MMP-1, MMP-8,
and MMP-9
MMP (5.6 mU/mg for MMP-8, 2.07 mU/mg for MMP-9, and 0.45 mU/mg for MMP-1) was added to an MMP sub-strate solution (1.2M for MMP-8 and 0.5 M for MMP-9 and MMP-1). A mixture was prepared in a 10-mm cuvette by thoroughly mixing the above solution with a SACCHA-CHITIN stock solution to produce concentrations of 0.0025%, 0.001%, 0.01%, or 0.025%. The resulting activity of
MMP was monitored by measuring the fluorescence at re-spective excitation and emission wavelengths (MMP-8, 280 and 360 nm; MMP-9 and MMP-1, 365 and 450 nm). A standard sample with no addition of SACCHACHITIN was used as the control for comparison. The same procedure for measuring the activity of MMP was repeated by replacing SACCHACHITIN with N-acetylglucosamine at the same four concentrations and with chitin at the two concentra-tions of 0.001% and 0.0025% only.
In vitro binding of MMP-1, MMP-8, and MMP-9
After thoroughly mixing seven separate sample solutions, including MMP substrate, MMP⫹ MMP substrate, MMP ⫹ MMP substrate ⫹ 0.1% SACCHACHITIN, MMP ⫹ 0.1% SACCHACHITIN, MMP substrate ⫹ 0.1% SACCHA-CHITIN, MMP, and 0.1% SACCHACHITIN with a half vol-ume of sampling buffer (2⫻), mixtures were heated to 100°C for 5 min. Fifteen microliters of the resulting solution was loaded and subjected to SDS-PAGE on 12% SDS gel along with 8L of protein standard at room temperature and 130 V until the tracking dye reached the bottom of the gel. After electrophoresis, gels were stained with Coomassie brilliant blue R-250, destained, and dried for visualization.
Activity measurement and quantitation of MMP in a human chronic wound
This research project was approved by the University Institutional Review Board. Informed consent was obtained for all procedures. Two patients with impaired wound heal-ing for more than 7 months were recruited. Wounds were cleaned with saline solution and then divided into two areas. One area was covered with a SACCHACHITIN mem-brane and gauze was placed on the top, while the other area was covered with only gauze. At predetermined time inter-vals, the healing of the wounds was observed and recorded, and tissues from both areas were sampled for analysis.
Wound tissue was homogenized in buffer solution (50 mM Tris-HCl, 1 mM monothioglycerol, 1 mM phenylmeth-ane sulfonylfluoride, pH 7.4) and centrifuged (2000 g for 10 min) to obtain the supernatant. The protein content in the supernatant was measured based on the Bradford protein assay method (Bio-Rad reagent) using BSA as a standard.
The activity of MMP in the supernatant was measured as follows: 100L of sample (containing 100 g of protein) and standard were incubated in microtiter wells precoated with anti-MMP antibody at 4°C overnight. After washing and aspiration of unbound components, 50L of 1 mM p-amino-phenylmercuric acetate (APMA) was added and incubated at 37°C for 2 h to activate the bound pro-MMP. Equal volumes of pro-detection enzyme (modified urokinase) and chromogenic substrate (S-2444 peptide) were then added and incubated at 37°C for another 2 h. The absorbance at 450 nm was then read.
Amounts of MMP-8 and MMP-9 in the supernatant ex-tracted from wound tissue were quantified as follows: 100-L aliquots of sample (containing 50 g protein) and
standard were incubated in microtiter wells precoated with anti-MMP antibody at room temperature for 1 h. After washing and aspiration of the unbound components, 100L of anti-MMP horseradish peroxidase was added and incu-bated at room temperature for 2 h. After that, free substrate was cleaned off, and 100 L of 3,3⬘,5,5⬘-tetramethylbenzi-dine (TMB) substrate was added immediately. Twenty min-utes after incubation at room temperature, 100 L of 1 M sulfuric acid was added to stop the reaction, and the absor-bance at 450 nm was read.
RESULTS
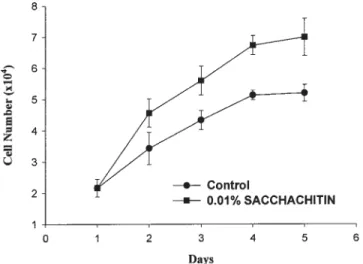
The influence of SACCHACHITIN on the growth of human keratinocytes (HKs) was examined. Figure 1 demonstrates that the promotion of proliferation of HK was slight with the presence of 0.01% SACCHA-CHITIN on the second day but became profound on days 4 and 5. The morphology of HK cells cultured for 72 (A) and 168 h (B) is shown in Figure 2. No obvious change in morphology was observed compared to those cultured with no SACCHACHITIN. It was con-cluded that SACCHACHITIN could accelerate wound healing by promoting proliferation of HKs and by having low cytotoxicity and being biocompatible with HK cells.
The influences of SACCHACHITIN and its possible monomer, N-acetylglucosamine, on the activity of MMP-1 were examined. Figure 3(A) indicates that the extent of inhibition of MMP-1 activity increased with an increasing added amount of both substances. In comparison with the control, a statistically significant inhibition of activity was observed when the concen-tration of N-acetylglucosamine exceeded 0.001%, whereas inhibition was observed when the concentra-tion exceeded 0.01% for SACCHACHITIN. This
indi-cates that inhibition of the activity of MMP-1 was less effective when using SACCHACHITIN than when us-ing N-acetylglucosamine.
Figure 3(B) illustrates that the inhibition on the ac-tivity of MMP-8 increased with an increasing added amount of SACCHACHITIN, and statistically signifi-cant inhibition (p ⬍ 0.01) was observed when the added amount exceeded 0.001% in comparison with the control. On the contrary, inhibition of the activity of MMP-8 by N-acetylglucosamine decreased with an increasing added amount and did not show statisti-cally significant inhibition at the highest added amount (0.025%). Nevertheless, both added amounts of 0.001% and 0.0025% of chitin isolated from crab shell demonstrated significant inhibition on the activ-ity of MMP-8 similar to that of SACCHACHITIN. Inhibition of the activity of MMP-8 for all three sub-stances at these two low added amounts of 0.001% and 0.0025% was determined not to significantly differ.
Figure 1. Growth curve of keratinocytes in medium con-taining 0.01% SACCHACHITIN (values represent the mean⫾ SD, n ⫽ 3).
Figure 2. Morphology of keratinocytes co-cultured in the presence of SACCHACHITIN (0.01%) for 72 (A) and 168 h (B). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3(C) shows that inhibition of the activity of MMP-9 increased with an increasing added amount of SACCHACHITIN and N-acetylglucosamine. This in-hibiting effect was less significant at the two added amounts of 0.01% and 0.025% for chitin in comparison with the control. Because significant inhibition of the activity of MMP-9 was observed at a concentration of as low as 0.001% for SACCHACHITIN, we concluded that SACCHACHITIN was the most effective for in-hibiting MMP-9 activity among these three sub-stances.
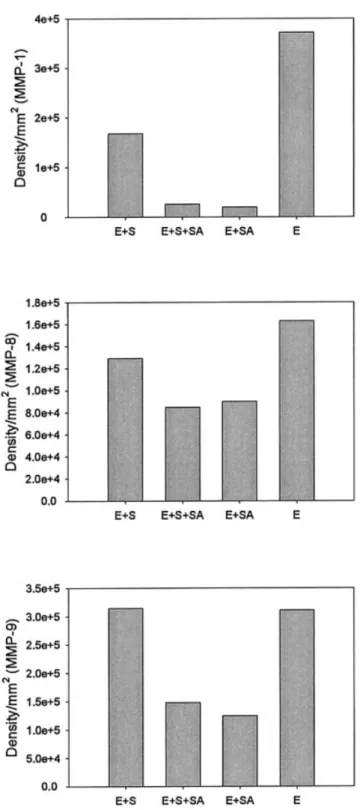
The binding effect of SACCHACHITIN on MMP was examined by determining the migration of MMP proteins during electrophoresis. Figure 4 displays densitographs of electrophoretic migration of the three MMP proteins, respectively. The relative densi-tograms for the corresponding bands at the molecular weights of 44 kDa for MMP-1, of 58 kDa for MMP-8, and of 92 kDa for MMP-9 after electrophoresis were selected for comparison of the extent of free MMP available for migration. All three demonstrated that
the extent of free MMP available for electrophoretic migration decreased in the presence of 0.01% SAC-CHACHITIN. This effect seemed to be more profound for MMP-1 and MMP-9. This demonstrates that the binding effect of MMP by SACCHACHITIN might inhibit the activity of the three MMP proteins.
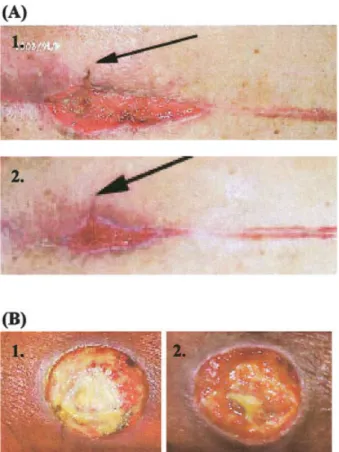
Figure 5(A) shows the accelerated healing of the
Figure 3. MMP (A, MMP1; B, MMP-8; C, MMP-9) activities in the presence of various concentrations of SACCHA-CHITIN (SA) or N-acetyl-glucosamine (NAG) (values rep-resent the mean⫾ SD, n ⫽ 3; *p ⬍ 0.01 vs the control).
Figure 4. Binding effects of SACCHACHITIN on MMPs (A, MMP-1; B, MMP-8; C, MMP-9). E, MMP enzymes; S, MMP substrates; SA, SACCHACHITIN.
infected wound covered with SACCHACHITIN. This wound area originally showed impaired healing for almost 7 months after treatment (by surgery). The debris was then cleaned off by treatment with antibi-otics, and the wound was divided into two areas: one of which was covered with SACCHACHITIN and the other covered with gauze. Two weeks after treatment, healing in the area covered with SACCHACHITIN had improved in comparison to that covered with gauze in terms of shrinkage of the depth and surface area of the wound. Figure 5(B) shows another wound which exhibited impaired healing for up to a year after treatment. However, the half of this area covered with SACCHACHITIN for 1 month showed that granular tissue in the peripheral area of the wound had prolif-erated in an accelprolif-erated rate in comparison to that covered with gauze.
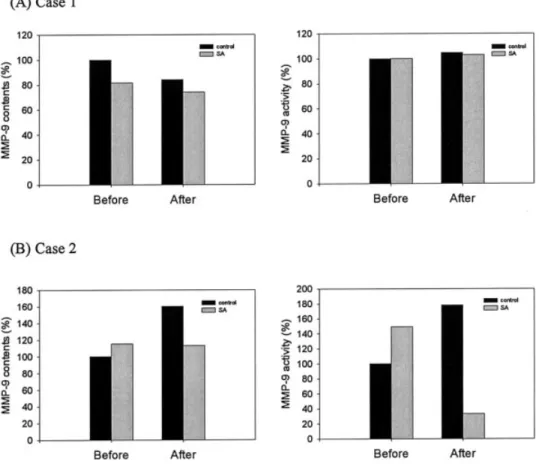
Quantitative analysis of protein levels of MMP-1, MMP-8, and MMP-9 and their activities in these skin wound tissues covered with either SACCHACHITIN or gauze was performed, and sole results for MMP-9 are illustrated in Figure 6. It demonstrates that the content and activity of MMP-1 in those skin wound tissues covered with SACCHACHITIN did not signif-icantly differ from those covered with gauze either
before or after treatments (data not shown). The con-tent of MMP-9 in two cases of skin wound treatment with SACCHACHITIN decreased after treatment compared to that before treatment. There was only one case of skin wound tissue covered with gauze showing a significant change in the content of MMP-9 after treatment. The activities of MMP-9 in skin wound tissue before and after either treatment showed no obvious changes. The content and activity of MMP-8 in skin wound tissue for both treatments were also inconsistent in each individual case (data not shown).
DISCUSSION
SACCHACHITIN is obtained from the residue of the fruiting body of Ganoderma tsugae after extraction of chemical constituents. It contains 40% chitin and 60%-glucan. In 1970, Prudden and colleagues25 con-firmed that chitin is able to accelerate wound healing. Previously, it was demonstrated that SACCHA-CHITIN membranes are able to promote wound healing by inducing cell proliferation. Histological examination of skin wound tissue treated with SACCHACHITIN also revealed that a mild acute in-flammatory reaction attracted a large number of poly-morphonuclear leukocytes and some macrophages to clean away debris and blood clots. Also the secretion of cell cytokines and growth factors by these cells provided an excellent environment for wound heal-ing. The migration of fibroblast cells, which was pro-moted by SACCHACHITIN, plays another important role in the acceleration of wound healing as well.
During the process of wound healing, proliferation and differentiation of keratinocytes are an indispens-able factor. Cell culture studies of keratinocytes have revealed that SACCHACHITIN is able to promote its proliferation starting from the second day, and signif-icant proliferation was observed by the fourth and fifth days. This provides further evidence that the acceleration of wound healing by the use of SACCHA-CHITIN is the result of proliferation of keratinocytes leading to the covering of the skin wound area. Fur-ther, the compatibility of SACCHACHITIN with ker-atinocytes with no sign of an immunological response (data not shown) is advantageous for its use as a wound dressing.
The elevated levels and excessive activities of MMPs in chronic wounds have recently been recognized as the principal reason for poor wound healing.28 Activ-ity study results of MMPs demonstrate that SACCHA-CHITIN and its partial monomer, N-acetylglu-cosamine, show a higher extent of inhibition of the activity of MMP-9 than of MMP-1 and MMP-8. Yanger and colleagues reported that 10- and 25-fold elevated
Figure 5. The healing process of chronic wounds (two cases, A and B) covered with SACCHACHITIN membranes. (1) before, (2) after 10 days of treatment. [Color figure can be viewed in the online issue, which is available at www. interscience.wiley.com.]
contents of MMP-1 and MMP-9, respectively, ap-peared in pressure ulcers, and that neutrophil-derived MMP-8 is the predominant collagenase in healing wounds and nonhealing ulcers.26 Therefore, SAC-CHACHITIN might play a role as an inhibitor of the activity of MMPs (MMP-1, MMP-8, and MMP-9) in preventing degradation of the matrix structure around a wound area, leading to accelerated wound healing. However, a standard sample of chitin from crab shells showed no similar effectiveness as that by SACCHA-CHITIN in the inhibition of the activity of MMP-9. The component of-glucan in SACCHACHITIN was sus-pected of being responsible for this difference in inhi-bition.
MMPs represent a family of matrix-degrading pro-teinases with structural similarities. MMPs share com-mon features, including a proenzyme domain (I), a catalytic domain (II), and a C-terminal domain (III), which are thought to define substrate specificity. Cat-alytic Zn interacts with a conserved cysteine (C) in domain I to maintain the proenzyme in an inactive conformation. Coordination of a zinc ion at the active site is required for catalysis,27 and activity is specifi-cally inhibited by tissue inhibitors of matrix metallo-proteinases (TIMPs).28 Binding studies of MMPs re-veal that SACCHCHITIN is able to complex with the three MMPs to significant but different extents.
There-fore, the influence of SACCHACHITIN on the activity of MMPs occurs through its binding effect by masking the catalytic domain of MMPs
SACCHACHITIN was demonstrated to promote wound healing in both animal and cell proliferation studies. Two patients with impaired wound healing for more than 7 months were recruited for treatment with SACCHACHITIN according to protocols ap-proved by our University Institutional Review Board. None of all chronic wounds showed any adverse re-actions to treatment with SACCHACHITIN, and the healing of those wounds was promoted when SAC-CHACHITIN was used as a wound dressing. Even though there was a limited number of samples, the content of MMP-9 in those wound tissues tended to gradually decrease after treatment with SACCHA-CHITIN. This is contrary to the results that elevated levels and activities of MMPs were found in pressure ulcers.26 One might conclude that the enhanced heal-ing of chronic wounds by SACCHACHITIN is due to suppression on the continued increases in the levels and activities of MMPs in chronic wounds.
References
1. Martin P. Wound healing—aiming for perfect skin regenera-tion. Science 1997;276:75– 81.
2. Clark RAF. Wound repair— overview and general consider-ations. In: Clark RA, editor. The molecular and cellular biology of wound repair. New York: Plenum Press; 1996: p 3–50. 3. Rømer J, Bugge TH, Pyke C, Lund LR, Flick MJ, Degen JL,
Danø K. Impaired wound hearing in mice with a disrupted plasminogen gene. Nature Med 1996;2:287–292.
4. Madlener M, Parks WC, Wemer S. Matrix metalloprotein (MMP) and their physiological inhibitors (TIMP) are differen-tially expressed during excisional skin wound repair. Exp Cell Res 1998;242:201–210.
5. Ravanti L, Ka¨ha¨ri VM. Matrix metalloproteinases in wound repair (review). Int J Mol Med 2000;6:391– 407.
6. Gak E, Taylor WG, Chan AM, Rubin JS. Processing of hepato-cyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett 1992;311:17–21.
7. Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y. Degradation of interleukin 1 by matrix metalloproteinases. J Biol Chem 1996;271:14657–14660. 8. Gallea-Robache S, Morand V, Millet S, Bruneau JM, Bhatnagar
N, Chouaib S, Roman-Roman S. A metalloproteinase inhibitor blocks the shedding of soluble cytokine receptors and process-ing of transmembrane cytokine precursors in human mono-cytic cells. Cytokine 1997;9:340 –346.
9. Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: iden-tification of the cleavage sites, kinetic analyses and transform-ing growth factor-beta 1 release. Biochem J 1997;322:809 – 814. 10. Wakita H, Furukawa F, Takigawa M. Thrombin and trypsin induce granulocyte-macrophage colony-stimulating factor and interleukin-6 gene expression in cultured normal human kera-tinocytes. Proc Assoc Am Physicians 1997;109:190 –207. 11. Grillo HC, Gross J. Collagenolytic activity during mammalian
wound repair. Dev Biol 1967;15:300 –317.
12. Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem 1999:274:21491–21494.
13. Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Park WC. The activity of collagenase-1 is required for keratin-ocyte migration on a type I collagen matrix. J Cell Biol 1997; 137:1445–1457.
14. Park WC. Matrix metalloproteinases in repair. Wound Repair Regen 1999;7:423– 432.
15. Itoh T, Tanioka M, Yosida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res 1998;58:1048 –1051.
16. Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Mur-phy B, Ronan J, Werb Z, Banda MJ. Impaired would contrac-tion in stromelysin-1-deficient mice. Ann Surg 1999;230:260 – 265.
17. Balsssa LL. Wound healing composition. GB patent 1,252,373. 1971.
18. Muzzarelli R. Methyl pyrrolidinone chitosan, production pro-cess and uses thereof. US patent 5,378,472. 1995.
19. Balsssa LL. Process for facilitating wound healing with N-acetylated partially depolymerized chitin materials. US patent 3,914,413. 1975.
20. Widra A. Hydrophilic biopolymeric copolyelectrolytes and biodegradable wound dressings comprising same. European patent 0,89,152. 1983.
21. Yano H, Iriyama K, Nishiwaki H, Kifune K. Effects of N-acetyl-d-glucosamine on wound healing in rats. Mie Med J 1985;35: 53–56.
22. Malette WG. Method of achieving hemostasis inhibiting fibro-plasias and promoting tissue regeneration in a tissue wound. US patent 4,532,134. 1985.
23. Su CH, Sun CS, Juan SW, Hu CH, Ke WT, Sheu MT. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitute. Biomaterials 1997;18:1169 – 1174.
24. Su CH, Sun CS, Juan SW, Hu CH, Sheu MT. Development of fungal mycelia as skin substitutes II: effects on the wound healing process. N Taipei J Med 1999;1:31–39.
25. Prudden JF, Migel P, Hanson P, Friedrich L, Balassa L. The discovery of a potent pure chemical wound-healing accelera-tor. Am J Surg 1970;119:560 –564.
26. Yager DR, Zhang LY, Liang HX, Diegelmann RF. Cohen IK. Wound fluid from human pressure ulcers contains elevated matrix metalloproteinase levels and activity compared to sur-gical wound fluids. J Invest Dermatol 1996;107:743–748. 27. Shapiro SD, Senior RM. Matrix metalloproteinases. Matrix
deg-radation and more. Am J Respir Cell Mol Biol 1999;20:1100 – 1102.
28. Bode W, Fernandez-Catalan C, Tschesche H, Grams F, Nagase H, Maskos K. Structural properties of matrix metalloprotein-ases. Cell Mol Life Sci 1999;55:639 – 652.