Control of the Arrangement of Dipolar
Orientation of Pyrimidine Inside the
Conjugated Backbone
Ken-Tsung Wong* and Chun Che Hsu
Department of Chemistry, National Taiwan UniVersity, Taipei 106, Taiwan, R.O.C. kenwong@ccms.ntu.edu.tw
Received November 16, 2000
ABSTRACT
Linear molecules with different arrangement of dipolar pyrimidine moieties in the π-conjugated backbone were synthesized by a Pd-catalyzed Sonogashira coupling reaction. Significant reduction of the HOMO−LUMO energy band gap and improvement of fluorescence quantum yield were observed upon incorporating pyrimidine into the conjugated backbone.
Linear organic molecules with extendedπ-conjugation have received a lot of attention due to their potential use as molecular wires.1Many practical synthetic approaches and testing methods have been developed for constructing and evaluating molecular wires of different lengths.2 More interestingly, there has been significant progress in modifying the primary structure of the conjugated system for developing
functional molecular wires.3 Control of the regioregularity
in polymerization is an important issue that results in the formation of polymers with interesting properties by com-parison with those of their irregular counterparts. Regio-regular poly(pyridine),4ploy(3-alkylthiophene),5and oligo-(thiophene ethynylenes)6 with precise length have been synthesized and have proven to have very interesting optoelectronic, nonlinear properties. We wish to report herein on the synthesis of a new linear π-conjugated system by manipulating the orientation of the dipolar component in their backbone. This approach will ultimately change the dipolar character of a π-conjugated system that may lead to the development of an interesting method to probe the structure-property relationship.
A highly electronegative pyrimidine ring was utilized as the dipolar moiety for incorporation into the conjugated backbone.7The chemoselectivity of 5-bromo-2-iodopyrimi-dine 18 toward the Pd-catalyzed coupling reaction with
(1) Lehn, J.-M. Supramolecular Chemistry: Concepts and PerspectiVes; VCH: Weinheim, 1995. Petty, M. C.; Bryce, M. R.; Bloor, D. Introduction
to Molecular Electronics; Edward Arnold: London, 1995.
(2) Tour, J. M. Acc. Chem. Res. 2000, 33, 791.
(3) Marsella, M. J.; Wang, Z.-Q.; Mitchell, R. H. Org. Lett. 2000, 2, 2979. Matsuda, K.; Irie, M. J. Am. Chem. Soc. 2000, 122, 7195. Fernandez-Acebes, A.; Lehn, J.-M. Chem. Eur. J. 1999, 5, 3285. Jones, N. D.; Wolf, M. O. Organometallics 1997, 16, 1352. Collbert, M. C. B.; Lewis, J.; Long, N. J.; White, A. J. P.; Willams, D. J. J. Chem. Soc., Dalton Trans. 1997, 99. Lebreton, C.; Touchard, D.; Pichon, L. L.; Darido, A.; Toupet, L.; Dixneuf, P. H. Inorg. Chim. Acta 1998, 272, 188. Dembinski, R.; Bartik, T.; Bartik, B.; Jaeger, M.; Gladysz, J. Am. Chem. Soc. 2000, 122, 810. Bruce, M. I.; Low, P. J.; Costuas, K.; Halet, J.-F.; Best, S. P.; Heath, G. A.
J. Am. Chem. Soc. 2000, 122, 1949. Kheradmandan, S.; Heinze, K.;
Schmalle, H. W.; Berke, H. Angew. Chem., In. Ed. 1999, 111, 2412. Guillemot, M.; Toupet, L.; Lapinte, C. Organometallics 1990, 9, 1992. Bruce, M. I.; Ke, M.; Low, P. J. Chem. Commun. 1996, 2405. Alain, V.; Blachard-Desce, M.; Ledoux-Rak, I.; Zyss, Joseph Chem. Commun. 2000, 353. Slama-Schwok, A.; Blanchard-Desce, M.; Lehn, J.-M. J. Phy. Chem.
1990, 94, 3894.
(4) Yamamoto, T.; Maruyama, T.; Zhou, Z. H.; Ito, T.; Fukuda, T.; Yoneda, Y.; Begum, F.; Ikeda, T.; Sasaki, S.; Takezoe, H.; Fukuda, A.; Kubota, K. J. Am. Chem. Soc. 1994, 116, 4832. Gebler, D. D.; Wang, Y. Z.; Blatchford, J. W.; Jessen, S. W.; Lin, L.-B.; Gustafson, Wang, H. L.; Swager, T. M.; MacDiarmid, Esptein, A. J. J. Appl. Phys. 1995, 78, 4264. (5) McCullough, R. D. AdV. Mater. 1998, 10, 93 and references therein. (6) Pearson, D. L.; Tour, J. M.J. Org. Chem. 1997, 62, 1367.
ORGANIC
LETTERS
2001
Vol. 3, No. 2
173-175
10.1021/ol006877k CCC: $20.00 © 2001 American Chemical Society Published on Web 12/22/2000
Downloaded by NATIONAL TAIWAN UNIV on September 2, 2009 | http://pubs.acs.org
terminal alkynes (Sonogashira coupling reaction) provides us with an effective synthetic strategy for controlling the dipolar orientation of pyrimidine in the conjugated backbone. A very convenient one-pot consecutive Sonogashira coupling procedure has been developed to introduce different alkynyl
groups at the 2- and 5-positions of pyrimidine.9 In the
presence of a catalytic amount of Pd(PPh3)4, 2 was synthe-sized in 74% yield. 1,4-Diiodo-2,5-dioctylbezene was al-lowed to react with 2 in the presence of Pd(PPh3)4to provide 3 (54%). This new linear molecule 3 contains five aromatic
rings including two pyrimidine rings with two pairs of nitrogen directed away from the central aryl linker (Scheme 1).
Treatment of 1 with 1,4-diethynyl-2,5-dioctylbezene in the
presence of Pd(PPh3)4afforded dibromo compound 4 in 82%
yield after purification on silica gel. Linear molecule 5,
similar to 3 but with two pairs of pyrimidine nitrogens directed toward the central aromatic linker, can be prepared in 71% yield by coupling 4 with 1,3-di-tert-butyl-5-ethyn-ylbenzene in the presence of the same catalyst at elevated temperature (Scheme 2).
Synthetic pathways toward linear molecules possessing the same arrangement of the dipolar orientation of pyrimidine in the conjugated backbone are more complicated. First,
1-iodo-4-(trimethylsilylethynyl)-2,5-dioctylbenzene10 was
treated with 2 at 80°C in the presence of Pd(PPh3)4to afford intermediate 6 in 94% yield. Removal of the TMS group from 6 followed by coupling with 1 afforded 7 in 90% yield. Further treatment of 7 with 1,3-di-tert-butyl-5-ethynylben-zene in the presence of Pd catalyst gave the desired compound 8 in 79% yield (Scheme 3).
Linear molecule 10 (Scheme 4) prepared in a similar manner as that for 5 was employed as a reference compound.
(7) Gommper, R.; Mari, H.-J.; Polborn, K. Synthesis 1997, 696. Kanbara, T.; Kushida, T.; Saito, N.; Kuwajima, I.; Kubota, K.; Yamamoto, T. Chem. Lett. 1992, 583.
(8) Goodby, J. W.; Hird, M. H.; Lewis, R. A.; Toyne, K. J. Chem. Commun. 1996, 2719.
(9) One-pot consecutive coupling procedure: Under argon, a solution of 5-bromo-2-iodopyrimidine (2.85 g, 10.0 mmol), Pd(PPh3)4(580 mg, 0.5 mmol), CuI (100 mg, 0.5 mmol), andiPr2NH (2.67 mL, 19.0 mmol) in THF (40 mL) was stirred for 3 min, and to this was added dropwisely over a period of 3 mim 1,3-di-tert-butyl-5-ethynylbenzene (2.36 g, 11.0 mmol) in THF (10 mL). The mixture was stirred for 2.5 h at 30°C. Trimethyl-silylacetylene (1.70 mL, 12.0 mmol) andiPr2NH (2.67 mL, 19.0 mmol) were introduced quickly, and the mixture was heated to reflux for 1.5 h. After cooling to room temperature, the ammonium salt formed was removed by filtration through a short column of aluminum oxide and washed with ether (2× 20 mL). The combined filtrate was concentrated to dryness under reduced pressure. The crude product was dissolved in THF (20 mL) and methanol (10 mL), and to this solution was introduced 2.0 N NaOH (5 mL) to remove the silyl group in 20 min at room temperature. After concentrating in vacuo, water (20 mL) was added, and the solution was extracted with ether (2× 25 mL). The combined organic solution was dried (MgSO4) and concentrated. The residue was purified by column chroma-tography on silica gel (EtOAc:hexanes ) 1:12) to afford 2 as pale yellow solid (2.34 g, 74%): mp 202-204°C; IR (KBr) 3274 (s), 2957 (m), 2219 (s), 1572 (m), 1414 (s), 1180 (m), 883 (m), 796 (m), 706 (m), 641 (m) cm-1;1H NMR (CDCl3, 400 MHz) 1.32 (s, 18H), 3.47 (s, 1H), 7.49 (t, J ) 1.8 Hz, 1H), 7.55 (d, J ) 1.8 Hz, 2H), 8.81 (s, 2H);13C NMR (CDCl3, 100 MHz) 31.5, 35.1, 85.8, 92.3, 116.1, 120.2, 124.8, 127.4, 151.3, 151.7, 159.9; MS (m/z, FAB+) 316 (100), 301 (15), 261 (9), 245 (6), 224 (4), 154 (15), 136 (11), 89 (4), 58 (25); HRMS cacld for C22H25N2317.2018, found 317.2028. Anal. Cacld for C22H24N2: C, 83.50; H, 7.64; N, 8.85. Found: C, 83.69; H, 7.38; N, 8.68.
Scheme 1a
a(a) i: 1,3-Di-tert-butyl-5-ethynylbenzene, Pd(PPh
3)4, CuI, iPr
2NH, THF, 30°C, ii: Me3SiCtCH, reflux, iii: 2 N NaOH, THF/ MeOH, 74%; (b) 1,4-diiodo-2,5-dioctylbenzene, Pd(PPh3)4, CuI, iPr
2NH, THF, reflux, 54%.
Scheme 2a
a(a) 1, Pd(PPh
3)4, CuI,iPr2NH, THF, room temperature, 82% (b) 1,3-di-tert-butyl-5-ethynylbenzene, Pd(PPh3)4, CuI, iPr2NH, THF, reflux, 71%.
Scheme 3a
a(a) 1-Ethynyl-4-iodo-2,5-dioctylbenzene, Pd(PPh
3)4, CuI,iPr2NH, THF, room temperature, 94%; (b) i: 2 N NaOH, THF/MeOH, ii: 1, Pd(PPh3)4, CuI,iPr2NH, THF, room temperature, 90%; (c) 1,3-di-tert-butyl-5-ethynylbenzene, Pd(PPh3)4, CuI,iPr2NH, THF, reflux, 79%.
174 Org. Lett., Vol. 3, No. 2, 2001
Downloaded by NATIONAL TAIWAN UNIV on September 2, 2009 | http://pubs.acs.org
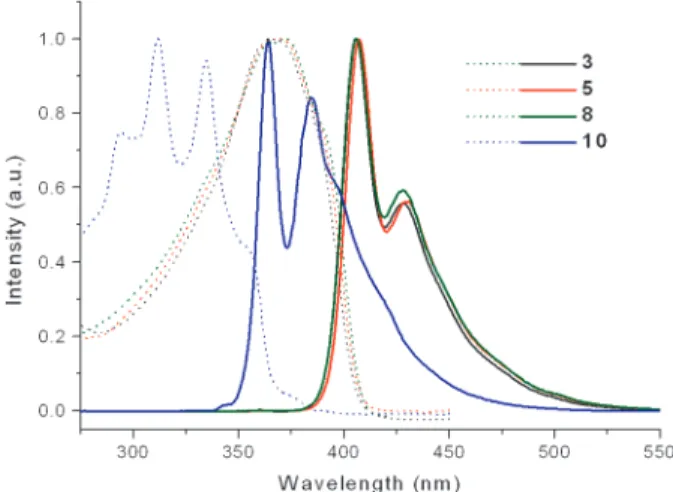
Our synthetic protocol provides a successful control of the dipolar orientation of the pyrimidine moiety in the conjugated backbone. The optical properties are demonstrated in their absorption and photoluminescence (PL) spectra (Figure 1).
Linear molecules 3, 5, and 8 reveal very similar behavior in their photophysical properties. Significant red shifts of 3,
5, and 8 in their absorption maximum (λmax 371 nm) compared with that of the corresponding model compound
10 (λmax311 nm) were observed. By inspecting the edge of
absorption spectra, the energy band gaps for 3, 5, and 8 were estimated to be ca. 0.2 eV smaller than that of 10. All the pyrimidine-containing molecules 3, 5, and 8 exhibit strong blue fluorescence with emission maximum at 406 nm and a weaker band centered on 428 nm. The photoluminescence also indicates substantial red shifts by 38 nm relative to that of 10 (364 nm, 384 nm). The PL efficiency of 3, 5, 8, and
10 are 0.63, 0.61, 0.75, and 0.34, respectively. Apparently,
the incorporation of pyrimidines inside a defined
π-conju-gated system increases the quantum yield of fluorescence. In conclusion, we have demonstrated methods for control-ling the arrangement of dipolar orientation of pyrimidine
inside a defined conjugated backbone. Significant reduction of the HOMO-LUMO energy band gap and improvement of fluorescence quantum yield were observed upon incor-porating pyrimidine into the conjugated backbone. Further studies on the dependence of electochemical properties on the different arrangement of dipolar orientation are in progress.
Acknowledgment. We gratefully thank the National Science Council (NSC 89-2113-M-002-008) for financial support.
Supporting Information Available: 1H NMR spectra of 3, 4, 5, 6, 7, 8, 9, and 10 and detailed experimental
procedures, full characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
OL006877K (10) Prepared by the literature procedure: Davey, A. P.; Elliott, S.;
O’Connor, O.; Blau, W. J. Chem. Soc., Chem. Commun. 1995, 1433.
Scheme 4a
a(a) 1-Bromo-4-iodobenzene, Pd(PPh
3)4, CuI, iPr2NH, THF, room temperature, 91%; (b) 1,3-di-tert-butyl-5-ethynylbenzene, Pd(PPh3)4, CuI,iPr2NH, THF, reflux, 92%.
Figure 1. Normalized absorption (CHCl3, 1 × 10-5 M) and
photoluminescence (CHCl3, 5.5× 10-6M) spectra of
pyrimidine-containing linear compounds 3, 5, and 8 and model compound 10.
Org. Lett., Vol. 3, No. 2, 2001 175
Downloaded by NATIONAL TAIWAN UNIV on September 2, 2009 | http://pubs.acs.org