Abstract: 297 words; Text: 6509 words; 176 references; 0 Tables; 1 Figure
NMDA neurotransmission dysfunction in mild cognitive impairment and Alzheimer’s
disease
Running Title: NMDA in MCI and Alzheimer’s disease
Reprints: Guochuan Emil Tsai, MD, PhD, Department ofPsychiatry, Harbor-UCLA Medical Center, 1000 W Carson St, Torrance,CA 90502 (etsai@labiomed.org).
Conflict of interest statement: All authors declare that they have no conflicts of
Abstract
The prevalence of Alzheimer’s disease (AD) in the elderly is growing rapidly
worldwide, and the deteriorating clinical course of AD places a heavy burden on both
the patients and their families. Early detection and intervention of mild cognitive
impairment in the early phase of AD is vital for the purpose of improving the
cognitive performance of patients and preventing the existing deficits from
worsening. However, the main compounds currently used to treat early AD,
acetylcholinesterase inhibitors (AChEIs), are unsatisfactory in efficacy and safety.
Moreover, evidence indicates that AChEIs are ineffective in treating AD at extremely
early stages, which implies that mechanisms other than those targeted by AChEIs
underlie the pathogenesis of AD. Dysfunctional glutamatergic neurotransmission,
particularly that mediated by the N-methyl-D-aspartate (NMDA) receptor, has been
reported to play a role in the pathophysiology of AD. The NMDA receptor (NMDAR)
regulates synaptic plasticity, memory, and cognition, and the attenuation of
NMDAR-mediated neurotransmission can result in impaired neuroplasticity and cognitive
deficits in the aging brain. Furthermore, NMDARs also interact with amyloid beta
peptide/amyloid precursor protein and tau protein, whose production represents the
main manifestations of AD. In this paper, we review the evidence supporting NMDA
review the literature that suggests that NMDA-enhancing agents such as glycine and
D-cycloserine can improve cognitive functions. The benefits and limitations of
NMDAR antagonists that can diminish the excitatory neurotoxicity triggered by
glutamate are also addressed in relation to AD. We propose that enhancing
glutamatergic neurotransmission by activating the NMDAR may be effective in
treating the cognitive decline that occurs in AD. Prospective studies on AD in which
NMDA-enhancing agents are used are required to verify this hypothesis and confirm
the long-term efficacy and safety of the treatment agents.
Outline:
1. Introduction to Alzheimer’s disease
1.1 Alzheimer’s disease prevalence and course and the importance of early detection and treatment
1.2 Acetylcholine theory and the limitation of AChEIs
2. Pathophysiology of mild cognitive impairment and Alzheimer’s disease 2.1 Amyloid and tau protein theory
2.2 Glutamate theory, focusing on NMDA, synaptic plasticity, memory, and cognition 2.3 Interactions between NMDA receptor, amyloid, and tau proteins
3. Evidence of NMDA hypofunction in mild cognitive impairment and Alzheimer’s disease 3.1 In vitro studies: neurogenesis, anti-apoptosis, and synaptic plasticity
3.2 Animal models
3.3 Clinical studies: D-serine and D-cycloserine in Alzheimer’s disease
4. Efficacy and limitation of NMDA antagonists in treating mild cognitive impairment and Alzheimer’s disease
5. Perspective
5.1 Evidence supporting the use of NMDA-enhancing treatment to improve cognition in schizophrenia
5.2 Enhancing NMDA neurotransmission to treat mild cognitive impairment and Alzheimer’s disease
1. Introduction to Alzheimer’s disease
1.1 Alzheimer’s disease prevalence and course and the importance of early detection and treatment
Dementia is gaining increasing attention because of the high morbidity and mortality it causes in the aging population. According to The Centers for Disease Control and Prevention, more than 16 million American adults are estimated to suffer from cognitive impairment [1]. Worldwide, 24.3 million people are afflicted with dementia, and the number is forecast to double every 20 years and reach 81.1 million by 2040 [2]. Furthermore, the prevalence and incidence of dementia increase with age: the prevalence of dementia is 1%–1.5% in people aged 65 years and this increases to 40%–60% in people aged 95 years or older. Alzheimer’s disease (AD) is the most common type of dementia.
According to the statistical data on disability adjusted life years (DALYs) collected in 2004 by the World Health Organization (WHO), “AD and other dementias” represent the fourth-leading cause of disease burden among people aged over 60 years [3]. Moreover, AD is one of the leading causes of death in the elderly population in USA and the mortality resulting from AD is continuing to increase [4]. In addition to the suffering it causes to patients afflicted with dementia, AD places great burdens on patients’ families, the health-care system, and the entire society. Currently, the total underlying cost of the health care required for AD patients is as high as $385 billion [4].
Although cognitive deterioration is common in old age, the relationship between aging and AD remains unclear. Whereas aging may be an AD risk factor, AD may not necessarily be a normal part of the aging process. A meta-analysis revealed that the relationship between age and the prevalence of senile dementia can be more appropriately described as a “flattened S curve” rather than as an exponential growth pattern [5]. The pathological changes that are detected in the brains of people afflicted with AD, such as the presence of amyloid plaques and neurofibrillary tangles, are
considered to appear several years before the development of clinical symptoms [6]. These pathological changes cause neurodegeneration and eventually lead to the clinical manifestations of the disease. Therefore, early detection and treatment can help prevent or slow the progression of AD.
Mild cognitive impairment (MCI) is a term used to describe a slight impairment in cognitive function, especially in memory, that is accompanied with mostly normal function in processes that control the performance of daily activities [7]. The concept of MCI was developed in an attempt to recognize dementia in its earliest clinically expressed form in people who may be destined to eventually develop progressive dementia [8]. MCI can be classified into 3 clinical subtypes: amnestic MCI, multiple-domain MCI, and MCI affecting a single non-memory domain [9]. Although definitive criteria have not been established to diagnose MCI, the prevalence of MCI is estimated to be between 11% and 17% in people aged 60 years and over in Europe and North America [7].
MCI may represent a transitional stage between normal aging and dementia that includes AD and Lewy body disease [10]. A certain proportion of MCI, particularly amnestic MCI, is an AD risk factor or may be a manifestation of the early symptoms of AD, and early investigations on the relationship between MCI and dementia (or AD) typically focused on amnestic MCI. Cognitive decline is faster in patients suffering from MCI than it is in healthy subjects [11]. In a study conducted by Bowen and colleagues, 48% of patients afflicted with isolated memory loss developed dementia at approximately the 48-month follow-up time [8]. Petersen et al. reported that nearly 50% of the people who had consulted a physician regarding MCI symptoms developed dementia in 3–4 years [11]. The rate at which MCI converts to dementia is 10%–15% annually, which is higher than that in the general population (1%–2%/y) [11]. Therefore, intervention in MCI is critical for delaying its deterioration to dementia and also for developing novel strategies to protect against
memory impairment.
1.2 Acetylcholine theory and the limitations of AChEIs
Acetylcholine, one of the major neurotransmitters in the central nervous system, is crucial for memory and cognition. The acetylcholine theory refers to one of the most widely accepted mechanisms of AD pathophysiology. Cognitive decline in aging and dementia are related to diminished cholinergic function [12, 13], and administering anticholinergic drugs results in memory impairments that resemble AD [14]. The loss of choline acetyltransferase (an enzyme that synthesizes acetylcholine), acetylcholinesterase (an enzyme that hydrolyzes acetylcholine), and cholinergic neurons and reduced synaptic acetylcholine levels are detected in the brains of AD patients[15, 16], and, furthermore, the extent of cholinergic deficits correlates with the severity of AD [17]. Based on the cholinergic hypothesis, acetylcholinesterase inhibitors (AChEIs), which increase synaptic acetylcholine levels by suppressing the degradation of acetylcholine, were developed for treating AD [18].
AChEIs are effective in slowing the cognitive decline that occurs in mild to moderate AD[19, 20]. A meta-analysis showed that AChEI treatment exhibited potent therapeutic effects in AD, but the heterogeneity among the various studies examined was high [21]. Another study indicated that the proportion of responders to AChEIs decreased when the treatment duration was lengthened, dropping from 17.8% at 3 months to 14.6%–15.7% (based on distinct definitions used in distinct assessments) after 9 months of treatment [22]. Furthermore, AChEIs are not recommended for use in treating MCI patients because of the weak beneficial effects and the high risk of side effects associated with using these compounds [23]. Cochrane Database concludes that no evidence is available to support the use of donepezil in treating patients with MCI because, in one study, the drug caused more adverse effects than the placebo did and was moreover ineffective in delaying the
onset of AD [24]; in another study, MCI patients who received AChEIs exhibited a more severe impairment of function and a faster cognitive decline than did patients who didn’t received AChEIs [25]. Rivastigmine and galantamine were also ineffective in slowing the progression from MCI to AD [26, 27]. The consensus statement of the British Association for Psychopharmacology concludes that MCI is not effectively treated by either AChEIs or memantine and that no treatment has been developed to date that can stop the progression of dementia [28].
2. Pathophysiology of mild cognitive impairment and Alzheimer’s disease 2.1 Amyloid and tau protein theory
The pathological hallmark of AD is the presence of amyloid plaques and neurofibrillary tangles, both of which are involved in the pathogenesis of AD [29]. Amyloid plaques are formed as a result of β-amyloid peptides (Aβ) being deposited in the extracellular space, whereas neurofibrillary tangles develop because of the intracellular accumulation of phosphorylated-tau protein. Both amyloid plagues and neurofibrillary tangles can induce inflammatory responses and cause neurotoxicity [6].
Aβ is derived from amyloid precursor protein (APP), a trans-membrane protein. APP can be processed by distinct secretases, which generate distinct end products. When APP is cleaved by β-secretase and γ-β-secretase, a peptide called Aβ is formed that contains 40–42 amino acids. By contrast, when APP is processed by α-secretase and γ-secretase, a shorter but non-pathogenic peptide is generated. Therefore, the regulation of APP processing plays a key role in the formation of Aβ.
Tau is a microtubule-associated protein whose physiological function is to stabilize microtubules in axons and dendrites. Tau protein is involved in the outgrowth of neurites, the development of neuronal polarity, and the maintenance of normal neuronal morphology [30].
Hyperphosphorylated-tau protein is prone to aggregate and form insoluble filaments, which represent the major component of neurofibrillary tangles [31, 32]. The extent of tau pathology in the brain correlates with the severity of AD [33]. However, tau pathology is not restricted to AD, and it can be detected in other types of dementia such as frontal temporal dementia.
The interaction between tau protein and Aβ exacerbates neurotoxicity and cognitive impairment. Aβ may exert its neurotoxic effect by modulating the phosphorylation of tau [34]. Conversely, reducing tau protein levels prevents the synaptic-plasticity deficits that develop in human-APP transgenic mice overexpressing Aβ [35].
2.2 Glutamate theory, focusing on NMDA, synaptic plasticity, memory, and cognition
Glutamate is the most abundant amino acid and the main excitatory neurotransmitter in the mammalian brain. In addition to functioning as a neurotransmitter at synapses, glutamate plays a critical role in regulating neurogenesis, neurite outgrowth and synaptogenesis, neuronal survival, and synaptic plasticity [36], and glutamate signaling also underlies complex neuronal activities such as learning and memory [37]. Glutamate binds to 2 types of glutamate receptors: metabotropic receptors and ionotropic receptors. Most of the evidence supporting the involvement of the glutamatergic system in AD is focused on the NMDA-type ionotropic receptors (NMDARs), which have been demonstrated to play a critical role in cognition and neurotoxicity [38, 39]. To date, only NMDAR-containing synapses have been confirmed to be effective therapeutic targets of clinical treatments in which glutamate neurotransmission is regulated. Thus, in this review, we focus on the glutamatergic neurotransmission that is mediated by NMDARs.
NMDARs regulate not only neurotransmission, but also synaptic plasticity, learning, memory, and cognition [40]. The calcium influx that occurs after NMDAR activation triggers the phosphorylation of calmodulin-dependent kinase (CaMK) and cAMP response element-binding
protein (CREB) and stimulates the downstream gene transcription that is required for long-term potentiation (LTP), which is considered to be the molecular basis of neural plasticity and memory formation, and calcium signaling also regulates long-term depression (LTD) [41, 42]. Glutamate-mediated excitotoxicity has been hypothesized to be involved in the pathogenesis of AD, with a core assumption being that the tonic overactivation of neurons mediated by NMDA-type glutamate receptors ultimately leads to neuronal damage and death [43]. However, completely blocking NMDARs also impairs neuronal plasticity, which has given rise to the long-standing paradox that dysfunction of neuronal activity results from both hyper- and hypo-activation of the glutamatergic system [43]. Attenuation of NMDAR-mediated neurotransmission can lead to a loss of neuronal plasticity and cognitive deficits. Olney and Farber [44] proposed that hypo-NMDA function induced by NMDAR antagonists is neurotoxic and may account for neuronal deterioration and brain atrophy. Recently, the stimulation of extrasynaptic NMDARs has been reported to promote cell death, whereas the activation of synaptic NMDARs has been shown to exert neuroprotective effects by promoting non-amyloidogenic α-secretase processing of APP, which is followed by a reduction in Aβ production [45-48].
The memory loss and cognitive decline associated with aging were shown to be related to the reduction in NMDAR-dependent LTP in the hippocampus of rats [49]. Age-dependent decline in motor functions in rats was also reported to be associated with the loss of NMDARs [50]. Different effects of aging were noted on the expression of NMDAR subunits in memory-associated brain structures in rats. In aged rats, the expression levels of NMDAR Subunit 1 (NR1) was significantly decreased in the ventral hippocampus and the entorhinal and postrhinal cortices, but the Subunit 2A (NR2A) expression were substantially lower in aged parahippocampal region, not in the hippocampus [51]. Furthermore, circuit-specific alterations of NR1 in the dentate gyrus of aged monkeys suggested that aging also impairs NMDAR function [52]. Whereas the excitotoxicity
resulting from the excessive activation of NMDARs triggered by endogenous glutamate has been hypothesized to be involved in AD, the hypoactivity of NMDARs associated with aging has also been postulated to contribute to the development of AD and other neurodegenerative diseases; this is based on the evidence that the NMDA system becomes markedly hypoactive with advancing age in mice, rats, and monkeys [53].
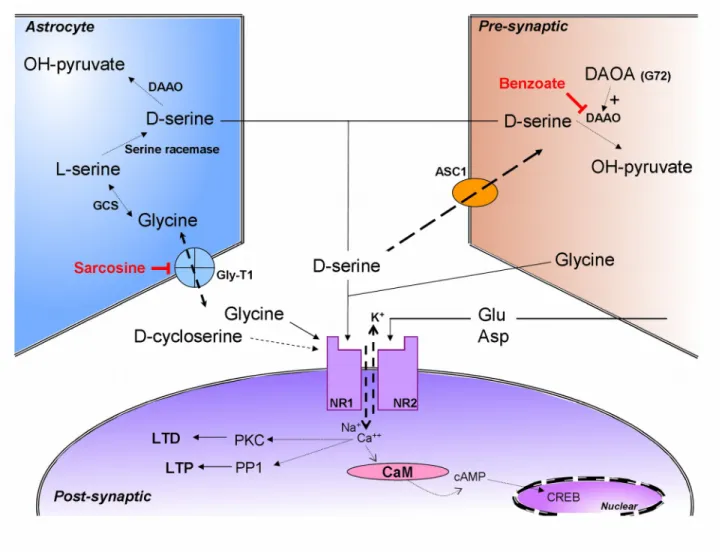
In humans, glutamate neurotransmission has been widely reported to undergo changes during the normal process of aging, such as the age-related reduction in glutamate content and synthesis in the cerebral cortex and hippocampus [54, 55]. The most notable and consistent finding has been that the density of NMDARs decreases with age [54]; thus, exploring the regulation mediated by NMDAR signaling in the aging brain is critical. In AD patients, glutamate levels were diminished in the cerebrospinal fluid (CSF) [56] and in ante mortem brain tissue [57], the number of glutamate terminals in the hippocampus were decreased [58], and low levels of D-serine and high levels of L-serine were measured in the serum [59]. Moreover, in the frontal cortex of AD patients, reduced binding of MK-801, an NMDAR-channel ligand, was observed [60]. Therefore, a dysfunction in the neurotransmission mediated by NMDA and related excitatory amino acids may also contribute substantially to the pathophysiology of AD.
2.3 Interactions between NMDARs and amyloid and tau proteins
The relationship between NMDA-dependent neurotransmission and Aβ is complicated and remains debated, with certain studies reporting that these interact mutually and lead to a neurotoxic vicious cycle: NMDA activation can regulate APP processing [61] and increase Aβ production by inhibiting α-secretase and shifting the balance toward β-secretase-mediated APP processing [62]; Aβ, in turn, can increase NMDA activity [63] and trigger NMDAR-dependent cell death by inducing a massive inward Ca2+ current and mitochondrial dysfunction [64]. Furthermore,
Aβ-induced synaptic loss may be mediated through NMDAR activation [65].
Although the aforementioned studies revealed that the underlying mechanism of glutamate excitotoxicity in AD may be related to Aβ deposition, other studies have reported opposite findings on the modulatory relationship between NMDA neurotransmission and Aβ formation. NMDAR activation can promote non-amyloidogenic APP cleavage by modulating α-secretase trafficking [66] and increasing α-secretase activity; conversely, Aβ aggregation can interfere with NMDA neurotransmission, which may lead to the impairment of cognitive functions [67] [68]. Aβ can inhibit LTP formation and lower NMDAR-mediated synaptic currents [69], and the mechanism underlying this inhibitory effect may involve the disruption of protein synthesis [70], activation of microglia [71], induction of inducible nitric oxide synthase (iNOS) [71], activation of c-Jun N-terminal kinase (JNK), cyclin-dependent kinase 5 (Cdk5), and p38 mitogen-activated protein kinase (MAPK) [72], and tumor necrosis factor α (TNFα)-mediated responses [73]. Aβ also promotes the endocytosis of NMDARs [74], and the pretreatment of neuronal cultures with Aβ lowers the expression of the NR1 subunit on the cell surface and reduces glutamate excitotoxicity [75]. Moreover, Aβ interferes with the signal transduction initiated by NMDARs, including the signaling by the Ca2+-dependent protein phosphatase calcineurin [70], Ca2+/calmodulin-dependent protein kinase II [76], protein phosphatase 1 [77], and CREB [70], and Aβ also induces synapse loss by blocking NMDAR activity [78].
Bidirectional interaction also exists between tau protein and NMDAR function. Overexpression of tau in primary neuronal cultures led to cell death that was mediated by extrasynaptic NMDARs [79], and neuronal degeneration induced by NMDA was associated with tau phosphorylation [80]. Reducing tau levels normalized imbalanced NMDAR-mediated currents in human-APP transgenic mice overexpressing Aβ [35], whereas the overexpression of tau-tubulin kinase in mice, which led to Tau aggregation, diminished the expression of NR2B receptors and
impaired spatial-learning ability in mice [81]. Moreover, tau protein can interact with Fyn, a Src-family kinase that phosphorylates NMDARs and facilitates NMDA downstream signaling; in transgenic mice in which the interaction between tau and Fyn was disrupted, NMDA-mediated excitotoxicity and Aβ toxicity were attenuated [82].
Conversely, hyperactivity of NMDAR function may lead to tau pathology in AD [83]. Glutamate increases tau mRNA expression by means of an NMDAR-related mechanism [84], and the induction of tau hyperphosphorylation may depend on NMDARs [85]. Under physiological conditions, NMDARs are likely to influence tau phosphorylation in the hippocampal area. In rat brain slices, NMDA treatment under depolarizing conditions results in tau dephosphorylation [86]. Memantine, a partial antagonist of NMDA, inhibits the hyperphosphorylation and accumulation of tau in rat hippocampal cultures [87]. However, NMDA antagonists have also been reported to potentially increase tau phosphorylation: inhibition of NR2A-containing NMDARs by using NMDA antagonists increased tau phosphorylation in rat hippocampal slices [88], and chronic administration of ketamine, an NMDA antagonist, in mice and monkeys for 6 months increased the levels of hyperphosphorylated tau and this was associated with apoptosis [89].
NMDARs are also involved in the interaction between Aβ and tau protein: Aβ-related tau modification may be mediated by NMDARs [34], and memantine can block Aβ-induced activation of tau kinase in primary mouse cortical neurons [90].
3. Evidence of NMDA hypofunction in mild cognitive impairment and Alzheimer’s disease 3.1 In vitro studies: neurogenesis, anti-apoptosis, and synaptic plasticity
NMDAR activation was shown to promote cell proliferation in the subventricular zone of the neonatal rat brain, which indicates that NMDA can promote neurogenesis [91]. By contrast, treatment with MK-801, a noncompetitive NMDAR antagonist, reduced the diameter and number
of the neurospheres derived from embryonic rat brain, which resembles an inhibition of the proliferation of neural progenitor cells [92]. Treating the cells of the suprachiasmatic nucleus with glutamate can result in the activation of the anti-apoptotic pERK/MAPK pathway without affecting the pro-apoptotic p-p38/MAPK pathway, which indicates a role of glutamate in preventing neurodegenerative processes [93]. A recent study [94] showed that apoptosis caused a reduction in D-serine immunoreactivity in cerebellar granule neurons. Conversely, D-serine treatment reduced neuronal death by 45%–55% relative to that in neuronal cultures not exposed to D-serine, which suggested that D-serine protects neurons against apoptosis. Moreover, treating neurons with both NMDA and D-serine showed that these molecules produced an additive effect in suppressing neuronal death [94]. In another study, immunohistochemical analysis was used to demonstrate that neural stem cells in primary cultures can synthesize D-serine and also serine racemase and thereby promote their own proliferation and neuronal differentiation [95]. An array-based study revealed that in the forebrain of F-344 rats, D-serine exposure affected the expression of genes in a wide range of signaling pathways that are implicated in neurodegenerative disorders [96]. Glycine-dependent radioligand binding to the NMDAR complex was previously shown to be diminished in cerebral cortex samples obtained from postmortem and neurosurgical procedures conducted on AD patients [60, 97]. High levels of glycine induced LTD of excitatory postsynaptic currents in hippocampal CA1 pyramidal neurons, which indicates a critical function of glycine in modulating synaptic plasticity [98]. D-cycloserine, a partial agonist that targets the glycine site of the NMDAR, was also reported to facilitate the activation of the NMDAR complex in the brain of AD patients [99].
3.2 Animal models: Drosophila and rodents
memory, synaptic plasticity, and neurogenesis. Glutamate itself can trigger de novo spine growth from dendrite shafts in Layer 2/3 pyramidal neurons of the mouse cortex [100]. Inhibition of glycine transporter-1 (GlyT1) increases the availability of synaptic glycine, a co-agonist of NMDARs, and consequently enhances NMDA neurotransmission. A novel GlyT1 inhibitor, ASP2535, was shown to reduce cognitive impairment in animal models of AD [101], and blocking NMDARs prevented the induction of hippocampal LTP, which resulted in an impairment of spatial learning [102]. Intriguingly, administration of rapamycin increased NMDA signaling and thereby enhanced learning and memory in mice [103]. NR1-subunit hypomorphic mice exhibited deficits in sensorimotor gating, which was measured using pre-pulse inhibition (PPI) [104], and memory consolidation was impaired in mice in which the NMDAR gene was knocked out specifically in the hippocampal CA1 region [105]. Genetically engineered mice in which the NMDAR gene was ablated specifically in hippocampal CA3 pyramidal cells exhibited impaired associative-memory recall [106], whereas transgenic mice overexpressing the NR2B subunit of NMDARs in the forebrain showed enhanced social-recognition memory [107]. Mice in which the gene encoding GlyT1 was inactivated were similar to mice exposed to GlyT1-inhibitor treatment: both exhibited enhanced hippocampal NMDAR function and memory retention [108]. In the Drosophila ellipsoid body, silencing of 2 NMDAR homologs, dNR1 and dNR2, suggested that the NMDAR is required for consolidating long-term memory [109]. Hypomorphic mutations of the essential dNR1 gene disrupted olfactory learning, and the transient induction of a dNR1 antisense transgene also disrupted Pavlovian learning, indicating that acute involvement of NMDARs is crucial for associative learning and memory [110]. In Caenorhabditis elegans, mutants defective in the nmr-1 gene, which encodes an NMDAR subunit, were shown to fail to form both long-term memory and short-term/middle-term memory [111].
through modulation at the glycine site of the NMDAR. In rats, D-cycloserine treatment reversed MK-801-induced disruption in locomotion, increased behavioral activity, and reduced the time required to complete the radial-arm-maze and water-maze tasks [112]. When administered at the dose of 1.0 mg/kg/d, D-cycloserine could reverse the deficits in spatial learning and working memory observed in a state of central cholinergic hypofunction induced by scopolamine [113]. In another study, acute treatment with D-cycloserine at a dose of 6 mg/kg/d also reversed scopolamine-induced amnesia, but the treatment did not affect the deficit in spatial working memory induced by entorhinal-cortex lesions; these results suggest that the positive effect of D-cycloserine are likely related to cholinergic-glutamatergic interactions [114]. D-D-cycloserine acted synergistically with nicotine in enhancing spatial navigation in the water maze [115], and tetrahydroaminoacridine, a cholinesterase inhibitor, and D-cycloserine synergistically enhanced the acquisition of spatial navigation [116, 117], further supporting cholinergic-glutamatergic interactions.
3.3 Clinical studies: D-serine and D-cycloserine in Alzheimer’s disease
Numerous clinical trials have been conducted to test the efficacy of NMDA-enhancing agents in improving cognition. Tsai et al. reported that patients afflicted with schizophrenia who received D-serine treatment exhibited considerable improvements not only in positive and negative symptoms but also in cognitive functions, and some patients also showed improved performance in the Wisconsin Card Sorting Test (WCST) [118]. D-serine also produced beneficial effects and was tolerated well when used for treating patients with Parkinson’s disease (PD). In a 6-wk double-blind, placebo-controlled, crossover trial conducted on PD patients, treatment with a D-serine adjuvant (at 30 mg/kg/d) was shown to substantially reduce the total scores of Unified Parkinson's Disease Rating Scale, Simpson-Angus Scale for Extrapyramidal Symptoms, and Positive and
Negative Syndrome Scale (PANSS) [119]. Moreover, a Phase III clinical trial organized by The National Institute of Mental Health and Hebrew University tested the efficacy of D-serine, targeting the cognitive impairment associated with schizophrenia and the cognitive impairment in PD [120].
Another NMDA enhancing agent, cycloserine, has been studied more extensively than D-serine in AD treatment. However, conflicting results have been obtained regarding the efficacy of D-cycloserine in the treatment of AD. In a 11-wk double-blind, placebo-controlled, crossover trial conducted on AD patients, short-term use of D-cycloserine at doses ranging from 25 to 500 mg failed to enhance neuropsychological functions [121]. In a multicenter, double-blind, placebo-controlled, randomized trial that included >400 AD patients, 5, 15, or 50 mg of D-cycloserine or placebo were administered twice daily; in this study, D-cycloserine did not show any efficacy in the treatment of AD relative to placebo [122] except for the implicit improvement of memory in AD cases of mild-to-moderate severity at the dose of 15 mg [123]. In another trial, 17 AD patients received a short-term D-cycloserine treatment [124], and the results showed that D-cycloserine improved the score (by 3.0 points) on the cognitive subscale of the Alzheimer's Disease Assessment Scale when administered at a dose of 100 mg/d, which suggests that NMDA-enhancing agents can exert beneficial effects on cognition in AD patients [124]. A systematic review conducted by Cochrane Database that consisted of 2 large and 2 small randomized, controlled trials showed that D-cycloserine was ineffective in the treatment of AD [125].
The efficacy of all NMDA-enhancing agents was examined in a meta-analysis of all the double-blind, placebo-controlled studies conducted on patients afflicted with schizophrenia [126]. The result obtained on 800 subjects from 26 studies showed that NMDA-enhancing agents are effective in improving the cognitive symptoms in patients, with the effect size being 0.28. The above evidence derived from both in vitro and in vivo studies demonstrates that NMDA
neurotransmission is pivotal for regulating diverse domains of brain function, including neurogenesis, synaptic plasticity, and learning and memory, which are especially critical in the case of the aging brain. Enhancing NMDAR function may slow or even reverse age-related memory decline that forms the core and is typically the earliest symptom of AD.
4. Efficacy and limitation of NMDA antagonists in treating mild cognitive impairment and Alzheimer’s disease
In addition to AChEIs, NMDAR antagonists have been developed to treat AD based on the “glutamate excitotoxicity theory.” Memantine is a weak, partial, uncompetitive NMDAR antagonist that binds with a low affinity to the channel and appears to block NMDAR overactivation by preventing excessive calcium influx without affecting physiological NMDAR activity [127-129]. Memantine is currently approved for use as an anti-dementia medication in moderate to severe AD cases [130]. Although certain studies have shown short-term therapeutic benefits of using memantine in mild AD, the drug has limited or no effect on MCI and mild AD. However, a combination therapy involving the use of memantine and rivastigmine exhibited strong treatment effect on mild to moderate AD in a 12-wk, open-label study [131], and a short-term combination treatment of galantamine and memantine was also shown to be cognitively beneficial in the case of patients afflicted with MCI of presumed AD etiology in a double-blind, placebo-controlled study [132]. In a study conducted by Bakchine et al., memantine treatment resulted in statistically significant improvement in cognitive function and global function in mild to moderate AD at 12 and 18 wk, but the treatment efficacy measured was not statistically significant at 24 wk [133]. Other randomized, controlled trials have also shown small but not statistically significant effects of memantine in the treatment of mild to moderate AD [134]. Furthermore, a meta-analysis of randomized, placebo-controlled trials showed no treatment benefit of memantine in mild AD [135],
and the results of another randomized, double-blind, placebo-controlled trial revealed no therapeutic advantage of using memantine as an add-on treatment in patients afflicted with mild to moderate AD who were receiving AChEIs concurrently [136].
NMDAR antagonists other than memantine have failed to show neuroprotective effects in several large-scale Phase III trials [137]. Moreover, the clinical usefulness of NMDAR antagonism is limited because of the severe side effects it produces, such as psychosis, nausea, vomiting, and memory impairment. NMDAR antagonists also induce apoptosis and neurodegeneration. Administration of MK-801 activated caspase-3 and caused neuronal death in rat brains and cortical cultures [138], and Wozniak et al. (1998) determined that MK-801 can induce disseminated neuronal degeneration in corticolimbic regions in rats, a finding that may be relevant to the pathophysiology of AD.
NMDAR antagonists have also been reported to cause cognitive impairment in animal and human studies. Chronic administration of NMDA antagonists suppressed LTP and impaired spatial learning [102]. HA-966, an antagonist that targets the glycine site on NMDARs, impaired visual-recognition memory in rhesus monkeys [139]. MK-801 also impaired cognitive function in mice and rats and, moreover, the dose range at which MK-801 induced cognitive impairment was lower than that at which it caused sensory or locomotor side effects: impairment of cognitive functions were more sensitive to the MK-801 than were sensory and locomotor functions impairment [140]. In a double-blind, randomized, placebo-controlled trial conducted on healthy human subjects, ketamine administration impaired spatial and verbal learning ability [141].
5. Perspective
5.1 Evidence supporting the use of NMDA-enhancing treatment to improve cognition in schizophrenia
NMDAR hypofunction has drawn attention for its role in the pathophysiology of schizophrenia over the past decades. Deficits in cognitive functions, such as those in sustained attention, working memory, and executive function, have been considered as core symptoms of the disease and have been associated with poor outcome and functioning in schizophrenia more strongly than have other symptoms including the psychotic symptoms of hallucination and delusion [142-144]. NMDA-enhancing treatments have shown clinical benefits (relative to placebo add-on treatment) in chronically ill schizophrenia patients not only on positive, negative, and depressive symptoms, but also on cognitive symptoms [118, 145-148]; however, rigorous and methodical cognitive studies on NMDA-enhancing agents had not yet been published. Certain cognitive improvements, although limited to the PANSS cognitive subscale or to WCST, have been observed in schizophrenia patients following treatment with high-dose glycine [146], D-serine [118, 149], and sarcosine (a GlyT1 inhibitor) [147, 150]. Claiming that NMDA-enhancing agents exert cognition-improving effects will be premature until strong evidence is gathered from long-term trials and neurocognitive studies. However, the potential of using NMDA-enhancing agents to improve cognitive functions in neuropsychiatric disorders is worth investigating further.
Several NMDA-enhancing agents have been developed and these can be classified based on 3 mechanisms by which they act: (1) full agonists that target the co-agonist site: glycine, D-serine, and D-alanine; (2) partial agonists that target the co-agonist site: D-cycloserine; and (3) GlyT1 inhibitors that block the reuptake of glycine: sarcosine. These NMDA-enhancing agents used currently exhibit distinct efficacies in treating cognitive symptoms [151] because they do not belong to a homogenous group. For example, glycine and D-cycloserine were reported to help improve negative symptoms, but not positive and cognitive symptoms [145, 152, 153]. Adjunctive D-serine offered considerable benefits to chronically stable patients in relation to cognitive symptoms and WCST performance, which reflects a prefrontal function of the drug [118, 149]. Another D-amino
acid that is a full agonist of the NMDA-co-agonist site, D-alanine, also showed efficacy similar to that of D-serine in treating the cognitive symptoms in chronically stable schizophrenia patients when D-alanine was added to antipsychotics for use in treating schizophrenia [148].
A distinct approach used to enhance NMDA neurotransmission is to attenuate the reuptake of glycine, which increases the availability of synaptic glycine. A suitable candidate is sarcosine (N-methylglycine), which is an endogenous GlyT1 inhibitor. Adding-on sarcosine in the treatment of chronically ill but stable schizophrenia patients who were receiving risperidone or typical antipsychotics showed that sarcosine was substantially more beneficial than placebo was in improving cognitive and general psychiatric symptoms, and that sarcosine was tolerated well and did not produce major side effects [147]. Notably, sarcosine was considerably more beneficial than D-serine and placebo were in improving cognitive function and quality of life when used in adjuvant therapy combined with risperidone; the benefits of the treatment were noted not only in chronically-ill but stable patients [154], but also in acutely exacerbated patients afflicted with schizophrenia [150]. These results suggest that the GlyT1 inhibitor exerts stronger effects than agonists themselves in enhancing NMDA neurotransmission in schizophrenia.
5.2 Enhancing NMDA neurotransmission to treat mild cognitive impairment and Alzheimer’s disease
The evidence presented in the preceding sections has suggested that glutamate neurotransmission through NMDARs plays a critical role in synaptic plasticity, learning, memory, and cognition, particularly in the aging brain. Declines in the markers of glutamatergic neurotransmission in AD and in normal aging indicate that pharmacological enhancement of the NMDA system could help improve cognitive functions [155]. We have also discussed that agents that enhance NMDA neurotransmission potently improve cognitive symptoms in both schizophrenia patients and AD
patients and, moreover, that they are safe and tolerated well. Based on these findings, we propose that agents that promote NMDA neurotransmission can potentially be used in novel treatments of or even in the early prevention of the declining processes in MCI and AD because of the function of these agents in regulating neurogenesis and neuroplasticity.
NMDARs are widely recognized to play a critical role in neuronal plasticity, but excessive activation of NMDARs results in excitotoxic death. The overactivation of the glutamate system that occurs through the stimulation of NMDARs may cause excitotoxicity and lead to neuronal damage and death; thus, to effectively enhance NMDA-mediated functions without triggering cytotoxicity, modulating NMDA neurotransmission at an optimal magnitude is crucial. NMDAR antagonists such as memantine could reduce the pro-survival activity of NMDAR while concurrently protecting neurons (and brains) against excitotoxicity. Therefore, identifying the switches that control pro-survival versus pro-excitotoxic activity resulting from NMDAR stimulation may lead to the development of a favorable NMDAR-enhancement strategy [156]. This switch can be observed anatomically, because the activation of extrasynaptic NMDARs and that of intrasynaptic NMDARs lead to distinct physiological outcomes.
Stepwise fine-tuning of the dosing strategy might serve as a method suitable for timely monitoring of the risk-benefit balance. Another plausible approach to avoid excitotoxicity relies on developing feasible and reliable biomarkers to assess glutamatergic-system functions for the purpose of prognostic evaluation and therapy optimization. For example, to identify subpopulations of patients in whom NMDA activity is low, glutamate or D-serine levels could be assessed in the plasma and CSF in cases where increased levels of these amino acids have been reported in patients afflicted with stroke, amyotrophic lateral sclerosis, AIDS dementia complex [157], or schizophrenia [158]. In an intriguing study, glutamate carboxypeptidase enzymatic activity was measured in skin tissues obtained from human subjects and was used as a pharmacodynamic marker assay, because
inhibiting glutamate carboxypeptidase is regarded to be beneficial when treating neurodegenerative diseases in which excess glutamate is considered to be pathogenic [159].
Gene expression and other biological signatures could potentially be developed as markers of excitotoxicity induced by excessive glutamate activation. Phencyclidine and MK802, which are both NMDA antagonists, were previously demonstrated to induce microglial activation and the expression of neuronal 70-kDa heat-shock protein (HSP70) in rats, which illustrated that microglial activation and HSP70 induction could serve as markers of neurotoxicity [160]. Another example is of a study in which exposing rat cortical neurons to high and toxic concentrations of glutamate led to an NMDAR-mediated change in the c-fos mRNA expression profile, and the excitotoxicity was paralleled by a sustained, elevated expression of c-fos mRNA; these observations suggested that elevated expression of c-fos mRNA can be used as an early marker of excitotoxicity in rodent models [161]. As mentioned above, evidence suggests that the stimulation of extrasynaptic but not synaptic NMDARs promotes cell death [46]. Moreover, the development of neuronal susceptibility to excitotoxicity has been reported to be mediated through the activation of extrasynaptic glutamate-receptor complexes localized on dendrites [162]. Furthermore, overexpression of the Ser/Thr phosphatase Protein Phosphatase-1 (PP1) in hippocampal neurons limits the NMDAR overactivation and Ca2+ overloading that occurs during excitotoxicity; this protective effect of PP1 was associated with the NMDAR subunit NR2B, which promotes pro-survival signaling and is involved in transcriptional pathways [163]. In another study, both endogenous and ectopically expressed NMDAR NR3A subunits in neurons increased neuroprotection against excitotoxicity induced by excessive Ca2+ influx [164]. Therefore, these early studies may offer insights into neuronal susceptibility to excitotoxicity.
A method to avoid glutamate-induced excitotoxicity that warrants exploration is the adding of neuroprotectants to NMDA-enhancing agents for use in the treatment of MCI and AD. Numerous
mediators of neuroprotective signaling have been reported to promote the pro-survival activity of NMDARs, including the activation of survival-signaling kinases such as ERK and Akt and TrkB receptor [165] and the suppression of a pro-apoptotic kinase, GSK-3beta [156]. Certain mood stabilizers such as lithium and valproic acid have been reported to exert neuroprotective effects [166]. Chronic treatment with lithium, which resulted in a reduction in excitotoxin-induced increase in intracellular Ca2+, prevented excitotoxic loss of motor neurons in a dose-dependent manner [167]. Clinically, long-term lithium treatment in a 12-month trial was associated with a substantial reduction in CSF concentrations of phosphorylated-tau protein and the treatment, which was tolerated well, may slow the progression of cognitive deficits [168]. The effectiveness of valproic acid in slowing the progress of AD is controversial. The neuroprotective effect of valproic acid has been postulated to makes it a potential agent suitable for treating AD [169]. However, valproic-acid treatment did not show any efficacy in delaying the emergence of either behavioral and psychological symptoms or cognitive decline in patients afflicted with moderate AD in a large scale multicenter, randomized, placebo-controlled trial; furthermore, compared with the placebo group, the group that received valproic acid even showed considerable toxic effects including high rates of neurological side effects and a greater loss in brain volumes (revealed using magnetic resonance imaging) [170]. Lovastatin, a drug that is used to lower cholesterol levels in patients, was shown to protect primary cortical neurons against glutamate-mediated excitotoxicity by means of activating TNF-receptor 2-signaling pathways [171]. Ginkgo biloba extract (EGb 761) also protected against glutamate-induced excitotoxic neuronal death in vitro in a dose-dependent manner [172]. Several other substances such as neurotrophic factors [36], aqueous extracts of Ashwagandha leaves [173], hydrogen-rich saline [174], orphenadrine [175], and acidic fibroblast growth factors [176] have been demonstrated to exhibit the potential to prevent excitotoxicity mediated by the activation of glutamate receptors. These potential neuroprotectants, especially the ones that act through
non-glutamate mechanisms, could be studied to determine their preventive effects on the excitotoxicity induced by glutamate overload.
6. Summary
This review has discussed the role of NMDA neurotransmission in the pathophysiology of AD and the related aging process from the viewpoints of pathological findings, in vitro studies and animal models, pharmacological studies, and clinical trials. The interactions between NMDA neurotransmission and Aβ and tau proteins, which are the pathological hallmarks of AD, have also been discussed. In this review, we have postulated that the dysfunction of neurotransmission mediated by NMDA and related excitatory amino acids plays as pivotal a role in the pathophysiology of AD that is as critical as the cholinergic system is.
This review has summarized both the excitotoxicity theory and the neuroprotective effects of glutamate neurotransmission occurring through NMDARs. We have also discussed the efficacy and limitations of memantine, a weak, uncompetitive, partial antagonist of NMDAR, in the treatment of AD, particularly in the early phase of the disease. Because no consistent effect of memantine treatment has been demonstrated in the cases of MCI and mild AD, either in monotherapy or in combination with AChEIs, we hypothesize that glutamate excitotoxicity is not critically involved in the etiology of the early AD process, but instead is likely the result of neuronal death late in the process of disease progression.
Schizophrenia is regarded as a mental disorder associated with neurodevelopmental deficits and neurodegeneration and was termed “dementia praecox” by Emil Krapelin; reviewing the evidence supporting the use of agents that enhance NMDA neurotransmission in the treatment of schizophrenia has shown that these agents are not only effective in treating positive, negative, and depressive symptoms of the disease, but also in treating cognitive symptoms, when compared with
placebo add-on treatment. Moreover, NMDA-enhancing agents have exhibited a favorable safety profile both in add-on treatments together with antipsychotics and in monotherapy, which is particularly critical for the treatment of the elderly population. By both promoting cognition and interrupting the vicious cycle of A and NMDA hypofunction, the NMDA-enhancing agents can potentially serve as novel drugs for use in treating MCI and mild AD.
In addition to proposing the potential treatment of MCI and mild AD by means of enhancing NMDA neurotransmission, this review has discussed possible strategies that can be used to modulate NMDA neurotransmission to an optimal magnitude while concurrently ensuring efficacious enhancement of function in the absence of cell toxicity and also monitoring excitotoxicity. We have moreover identified certain agents and molecules that exert neuroprotective effects that can be investigated further to explore possible methods to avoid excitotoxicity induced by glutamate overload.
Early intervention in MCI and the early phase of AD is a vital step required for improving cognitive performance and preventing existing deficits from progressing; however, no treatment has been demonstrated to be efficacious so far. NMDA-enhancing agents are highly likely to exhibit therapeutic efficacy in tackling MCI and early AD as they do in the case of schizophrenia. If confirmed using well-designed clinical trials, the use of NMDA-enhancing agents could bring hope to the growing population of elderly people afflicted with cognitive deficits.
Acknowledgement
This work was funded by the National Science Council, Taiwan (NSC 99-3114-B-182A-003 and NSC 101-2314-B-182A-073-MY2).
The aforementioned institutes had no further role in the writing of the review and in the decision to submit the paper for publication.
References
[1] Promoting brain health. Centers for Disease Control and Prevention. (http://www.cdc.gov/aging/pdf/cognitive_impairment/cogImp_genAud_final.pdf), 2011. [2] Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus
study. The Lancet, 2005; 366: 2112-2117.
[3] Organization WH. The World Health Report 2003 - Shaping the future. 2003.
[4] Thies W, Bleiler L. 2011 Alzheimer's disease facts and figures. Alzheimers Dement, 2011; 7: 208-44.
[5] Ritchie K, Kildea D. Is senile dementia "age-related" or "ageing-related"?--evidence from meta-analysis of dementia prevalence in the oldest old. Lancet, 1995; 346: 931-4.
[6] Holtzman DM, Morris JC, Goate AM. Alzheimer's Disease: The Challenge of the Second Century. Sci. Transl. Med, 2011; 3.
[7] Levey A, Lah J, Goldstein F, Steenland K, Bliwise D. Mild cognitive impairment: an opportunity to identify patients at high risk for progression to Alzheimer's disease. Clin Ther, 2006; 28: 991-1001.
[8] Bowen J, Teri L, Kukull W, McCormick W, McCurry SM, Larson EB. Progression to dementia in patients with isolated memory loss. Lancet, 1997; 349: 763-5.
[9] Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med, 2004; 256: 240-6.
[10] Boeve BF. Mild cognitive impairment associated with underlying Alzheimer's disease versus Lewy body disease. Parkinsonism Relat Disord, 2012; 18 Suppl 1: S41-4.
[11] Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol, 1999; 56: 303-8.
[12] Deutsch JA. The cholinergic synapse and the site of memory. Science, 1971; 174: 788-94. [13] Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Brain
Res Rev, 1995; 21: 285-300.
[14] Patel S, Tariot PN. Pharmacologic models of Alzheimer's disease. Psychiatr Clin North Am, 1991; 14: 287-308.
[15] Katzman R, Brown T, Fuld P, Thal L, Davies P, Terry R. Significance of neurotransmitter abnormalities in Alzheimer's disease. Res Publ Assoc Res Nerv Ment Dis, 1986; 64: 279-86. [16] Schaeffer EL, Gattaz WF. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme. Psychopharmacology (Berl), 2008; 198: 1-27.
[17] Bierer LM, Haroutunian V, Gabriel S, et al. Neurochemical correlates of dementia severity in Alzheimer's disease: relative importance of the cholinergic deficits. J Neurochem, 1995; 64: 749-60.
[18] Lleo A, Greenberg SM, Growdon JH. Current pharmacotherapy for Alzheimer's disease. Annu Rev Med, 2006; 57: 513-33.
[19] Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev, 2006: CD005593.
[20] Burns A, O'Brien J, Auriacombe S, et al. Clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J Psychopharmacol, 2006; 20: 732-55.
[21] Lanctot KL, Herrmann N, Yau KK, et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: a meta-analysis. CMAJ, 2003; 169: 557-64.
[22] Raschetti R, Maggini M, Sorrentino GC, Martini N, Caffari B, Vanacore N. A cohort study of effectiveness of acetylcholinesterase inhibitors in Alzheimer's disease. Eur J Clin Pharmacol, 2005; 61: 361-8.
[23] Fellgiebel A. [Alzheimer drugs for mild cognitive impairment]. Neuropsychiatr, 2007; 21: 230-3.
[24] Birks J, Flicker L. Donepezil for mild cognitive impairment. Cochrane Database Syst Rev, 2006: CD006104.
[25] Schneider LS, Insel PS, Weiner MW. Treatment with cholinesterase inhibitors and memantine of patients in the Alzheimer's Disease Neuroimaging Initiative. Arch Neurol, 2011; 68: 58-66.
[26] Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol, 2007; 6: 501-12.
[27] Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology, 2008; 70: 2024-35.
[28] O'Brien JT, Burns A. Clinical practice with anti-dementia drugs: a revised (second) consensus statement from the British Association for Psychopharmacology. J Psychopharmacol, 2011; 25: 997-1019.
[29] Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med, 2010; 362: 329-44.
[30] Wang JZ, Liu F. Microtubule-associated protein tau in development, degeneration and protection of neurons. Prog Neurobiol, 2008; 85: 148-75.
[31] Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A, 1988; 85: 4051-5.
[32] Gotz J, Ittner A, Ittner LM. Tau-targeted treatment strategies in Alzheimer's disease. Br J Pharmacol, 2012; 165: 1246-59.
[33] Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging, 1995; 16: 271-8; discussion 278-84.
[34] Nicholson AM, Methner DN, Ferreira A. Membrane cholesterol modulates {beta}-amyloid-dependent tau cleavage by inducing changes in the membrane content and localization of N-methyl-D-aspartic acid receptors. J Biol Chem, 2011; 286: 976-86.
[35] Roberson ED, Halabisky B, Yoo JW, et al. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J Neurosci, 2011; 31: 700-11.
[36] Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann N Y Acad Sci, 2008; 1144: 97-112.
[37] Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res, 2007; 85: 2059-70.
[38] Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med, 1994; 330: 613-22.
[39] Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol, 2008; 7: 742-55.
[40] McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev, 1990; 15: 41-70.
[41] Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci, 2006; 100: 433-42.
[42] Feldman DE. The spike-timing dependence of plasticity. Neuron, 2012; 75: 556-71.
[43] Parsons CG, Stoffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology, 2007; 53: 699-723.
[44] Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry, 1995; 52: 998-1007.
[45] Hu NW, Ondrejcak T, Rowan MJ. Glutamate receptors in preclinical research on Alzheimer's disease: update on recent advances. Pharmacol Biochem Behav, 2012; 100: 855-62.
[46] Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci, 2010; 11: 682-96.
[47] Bordji K, Becerril-Ortega J, Nicole O, Buisson A. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ss production. J Neurosci, 2010; 30: 15927-42.
[48] Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. J Neurosci, 2009; 29: 4442-60.
[49] Boric K, Munoz P, Gallagher M, Kirkwood A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J Neurosci, 2008; 28: 8034-9.
[50] Ossowska K, Wolfarth S, Schulze G, et al. Decline in motor functions in aging is related to the loss of NMDA receptors. Brain Res, 2001; 907: 71-83.
[51] Liu P, Smith PF, Darlington CL. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse, 2008; 62: 834-41.
[52] Yamada K, Nabeshima T. Changes in NMDA receptor/nitric oxide signaling pathway in the brain with aging. Microsc Res Tech, 1998; 43: 68-74.
[53] Olney JW, Wozniak DF, Farber NB. Excitotoxic neurodegeneration in Alzheimer disease. New hypothesis and new therapeutic strategies. Arch Neurol, 1997; 54: 1234-40.
[54] Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev, 2001; 122: 1-29.
[55] Riederer P, Hoyer S. From benefit to damage. Glutamate and advanced glycation end products in Alzheimer brain. J Neural Transm, 2006; 113: 1671-7.
[56] Martinez M, Frank A, Diez-Tejedor E, Hernanz A. Amino acid concentrations in cerebrospinal fluid and serum in Alzheimer's disease and vascular dementia. J Neural Transm Park Dis Dement Sect, 1993; 6: 1-9.
[57] Lowe SL, Bowen DM, Francis PT, Neary D. Ante mortem cerebral amino acid concentrations indicate selective degeneration of glutamate-enriched neurons in Alzheimer's disease. Neuroscience, 1990; 38: 571-7.
[58] Cowburn R, Hardy J, Roberts P, Briggs R. Regional distribution of pre- and postsynaptic glutamatergic function in Alzheimer's disease. Brain Res, 1988; 452: 403-7.
[59] Hashimoto K, Fukushima T, Shimizu E, et al. Possible role of D-serine in the pathophysiology of Alzheimer's disease. Prog Neuropsychopharmacol Biol Psychiatry, 2004; 28: 385-8.
[60] Procter AW, Wong EH, Stratmann GC, Lowe SL, Bowen DM. Reduced glycine stimulation of [3H]MK-801 binding in Alzheimer's disease. J Neurochem, 1989; 53: 698-704.
[61] Gordon-Krajcer W, Salinska E, Lazarewicz JW. N-methyl-d-aspartate receptor-mediated processing of beta-amyloid precursor protein in rat hippocampal slices: in vitro--superfusion study. Folia Neuropathol, 2002; 40: 13-7.
[62] Lesne S, Ali C, Gabriel C, et al. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J Neurosci, 2005; 25: 9367-77.
[63] Uhasz GJ, Barkoczi B, Vass G, et al. Fibrillar Abeta (1-42) enhances NMDA receptor sensitivity via the integrin signaling pathway. J Alzheimers Dis, 2010; 19: 1055-67.
[64] Alberdi E, Sanchez-Gomez MV, Cavaliere F, et al. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium, 2010; 47: 264-72.
[65] Ando K, Uemura K, Kuzuya A, et al. N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease. J Biol Chem, 2011; 286: 7619-28.
[66] Marcello E, Gardoni F, Mauceri D, et al. Synapse-associated protein-97 mediates alpha-secretase ADAM10 trafficking and promotes its activity. J Neurosci, 2007; 27: 1682-91. [67] Kamenetz F, Tomita T, Hsieh H, et al. APP processing and synaptic function. Neuron, 2003;
37: 925-37.
[68] Cisse M, Halabisky B, Harris J, et al. Reversing EphB2 depletion rescues cognitive functions in Alzheimer model. Nature, 2011; 469: 47-52.
[69] Yamin G. NMDA receptor-dependent signaling pathways that underlie amyloid beta-protein disruption of LTP in the hippocampus. Journal of Neuroscience Research, 2009; 87: 1729-36.
[70] Chen QS, Wei WZ, Shimahara T, Xie CW. Alzheimer amyloid beta-peptide inhibits the late phase of long-term potentiation through calcineurin-dependent mechanisms in the hippocampal dentate gyrus. Neurobiol Learn Mem, 2002; 77: 354-71.
[71] Wang Q, Rowan MJ, Anwyl R. Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci, 2004; 24: 6049-56.
[72] Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid beta-peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal kinase, cyclin-dependent kinase 5, and p38 mitogen-activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci, 2004; 24: 3370-8.
[73] Wang Q, Wu J, Rowan MJ, Anwyl R. Beta-amyloid inhibition of long-term potentiation is mediated via tumor necrosis factor. Eur J Neurosci, 2005; 22: 2827-32.
[74] Kurup P, Zhang Y, Xu J, et al. Abeta-mediated NMDA receptor endocytosis in Alzheimer's disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci, 2010; 30: 5948-57.
[75] Goto Y, Niidome T, Akaike A, Kihara T, Sugimoto H. Amyloid beta-peptide preconditioning reduces glutamate-induced neurotoxicity by promoting endocytosis of NMDA receptor. Biochem Biophys Res Commun, 2006; 351: 259-65.
[76] Zhao D, Watson JB, Xie CW. Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J Neurophysiol, 2004; 92: 2853-8.
[77] Knobloch M, Farinelli M, Konietzko U, Nitsch RM, Mansuy IM. Abeta oligomer-mediated long-term potentiation impairment involves protein phosphatase 1-dependent mechanisms. J Neurosci, 2007; 27: 7648-53.
[78] Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. Journal of Neuroscience, 2007; 27: 2866-75.
[79] Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci U S A, 2006; 103: 2892-7.
[80] Couratier P, Lesort M, Sindou P, Esclaire F, Yardin C, Hugon J. Modifications of neuronal phosphorylated tau immunoreactivity induced by NMDA toxicity. Mol Chem Neuropathol, 1996; 27: 259-73.
[81] Sato S, Xu J, Okuyama S, et al. Spatial learning impairment, enhanced CDK5/p35 activity, and downregulation of NMDA receptor expression in transgenic mice expressing tau-tubulin kinase 1. J Neurosci, 2008; 28: 14511-21.
[82] Ittner LM, Ke YD, Delerue F, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell, 2010; 142: 387-97.
[83] Chohan MO, Iqbal K. From tau to toxicity: emerging roles of NMDA receptor in Alzheimer's disease. J Alzheimers Dis, 2006; 10: 81-7.
[84] Esclaire F, Lesort M, Blanchard C, Hugon J. Glutamate toxicity enhances tau gene expression in neuronal cultures. J Neurosci Res, 1997; 49: 309-18.
[85] Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One, 2009; 4: e6344. [86] Fleming LM, Johnson GV. Modulation of the phosphorylation state of tau in situ: the roles
of calcium and cyclic AMP. Biochem J, 1995; 309 ( Pt 1): 41-7.
[87] Li L, Sengupta A, Haque N, Grundke-Iqbal I, Iqbal K. Memantine inhibits and reverses the Alzheimer type abnormal hyperphosphorylation of tau and associated neurodegeneration. FEBS Lett, 2004; 566: 261-9.
[88] Allyson J, Dontigny E, Auberson Y, Cyr M, Massicotte G. Blockade of NR2A-containing NMDA receptors induces Tau phosphorylation in rat hippocampal slices. Neural Plast, 2010; 2010: 340168.
[89] Yeung LY, Wai MS, Fan M, et al. Hyperphosphorylated tau in the brains of mice and monkeys with long-term administration of ketamine. Toxicol Lett, 2010; 193: 189-93. [90] Thornton C, Bright NJ, Sastre M, Muckett PJ, Carling D. AMP-activated protein kinase
(AMPK) is a tau kinase, activated in response to amyloid beta-peptide exposure. Biochem J, 2011; 434: 503-12.
[91] Fan H, Gao J, Wang W, Li X, Xu T, Yin X. Expression of NMDA receptor and its effect on cell proliferation in the subventricular zone of neonatal rat brain. Cell Biochem Biophys, 2012; 62: 305-16.
[92] Mochizuki N, Takagi N, Kurokawa K, et al. Effect of NMDA receptor antagonist on proliferation of neurospheres from embryonic brain. Neurosci Lett, 2007; 417: 143-8.
[93] Karmarkar SW, Bottum KM, Krager SL, Tischkau SA. ERK/MAPK is essential for endogenous neuroprotection in SCN2.2 cells. PLoS One, 2011; 6: e23493.
[94] Esposito S, Pristera A, Maresca G, et al. Contribution of serine racemase/d-serine pathway to neuronal apoptosis. Aging Cell, 2012.
[95] Huang X, Kong H, Tang M, Lu M, Ding JH, Hu G. D-Serine regulates proliferation and neuronal differentiation of neural stem cells from postnatal mouse forebrain. CNS Neurosci Ther, 2012; 18: 4-13.
[96] Davidson ME, Kerepesi LA, Soto A, Chan VT. D-Serine exposure resulted in gene expression changes implicated in neurodegenerative disorders and neuronal dysfunction in male Fischer 344 rats. Arch Toxicol, 2009; 83: 747-62.
[97] Procter AW, Stirling JM, Stratmann GC, Cross AJ, Bowen DM. Loss of glycine-dependent radioligand binding to the N-methyl-D-aspartate-phencyclidine receptor complex in patients with Alzheimer's disease. Neurosci Lett, 1989; 101: 62-6.
[98] Chen RQ, Wang SH, Yao W, et al. Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacology, 2011; 36: 1948-58. [99] Chessell IP, Procter AW, Francis PT, Bowen DM. D-cycloserine, a putative cognitive
enhancer, facilitates activation of the N-methyl-D-aspartate receptor-ionophore complex in Alzheimer brain. Brain Res, 1991; 565: 345-8.
[100] Kwon HB, Sabatini BL. Glutamate induces de novo growth of functional spines in developing cortex. Nature, 2011; 474: 100-4.
[101] Harada K, Nakato K, Yarimizu J, et al. A novel glycine transporter-1 (GlyT1) inhibitor, ASP2535 (4-[3-isopropyl-5-(6-phenyl-3-pyridyl)-4H-1,2,4-triazol-4-yl]-2,1,3-benzoxadiazol e), improves cognition in animal models of cognitive impairment in schizophrenia and Alzheimer's disease. Eur J Pharmacol, 2012; 685: 59-69.
[102] Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature, 1986; 319: 774-6.
[103] Majumder S, Caccamo A, Medina DX, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell, 2012; 11: 326-35.
[104] Fradley RL, O'Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res, 2005; 163: 257-64.
[105] Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science, 2000; 290: 1170-4. [106] Nakazawa K, Quirk MC, Chitwood RA, et al. Requirement for hippocampal CA3 NMDA
receptors in associative memory recall. Science, 2002; 297: 211-8.
[107] Jacobs SA, Tsien JZ. genetic overexpression of NR2B subunit enhances social recognition memory for different strains and species. PLoS One, 2012; 7: e36387.
[108] Tsai G, Ralph-Williams RJ, Martina M, et al. Gene knockout of glycine transporter 1: characterization of the behavioral phenotype. Proc Natl Acad Sci U S A, 2004; 101: 8485-90.
[109] Wu CL, Xia S, Fu TF, et al. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci, 2007; 10: 1578-86. [110] Xia S, Miyashita T, Fu TF, et al. NMDA receptors mediate olfactory learning and memory
in Drosophila. Curr Biol, 2005; 15: 603-15.
[111] Amano H, Maruyama IN. Aversive olfactory learning and associative long-term memory in Caenorhabditis elegans. Learn Mem, 2011; 18: 654-65.
[112] Pitkanen M, Sirvio J, MacDonald E, Niemi S, Ekonsalo T, Riekkinen P, Sr. The effects of D-cycloserine and MK-801 on the performance of rats in two spatial learning and memory tasks. Eur Neuropsychopharmacol, 1995; 5: 457-63.
[113] Pitkanen M, Sirvio J, MacDonald E, Ekonsalo T, Riekkinen P, Sr. The effects of d-cycloserine, a partial agonist at the glycine binding site, on spatial learning and working memory in scopolamine-treated rats. J Neural Transm Park Dis Dement Sect, 1995; 9: 133-44.
[114] Zajaczkowski W, Danysz W. Effects of D-cycloserine and aniracetam on spatial learning in rats with entorhinal cortex lesions. Pharmacol Biochem Behav, 1997; 56: 21-9.
[115] Riekkinen M, Riekkinen P, Jr. Nicotine and D-cycloserine enhance acquisition of water maze spatial navigation in aged rats. Neuroreport, 1997; 8: 699-703.
[116] Aura J, Riekkinen M, Riekkinen P, Jr. Tetrahydroaminoacridine and D-cycloserine stimulate acquisition of water maze spatial navigation in aged rats. Eur J Pharmacol, 1998; 342: