T
ZU-Y
UH
SIAO,* C
HUNG-L
IW
ANG,
C
HIUNG-N
IENC
HEN,
F
ON-J
OUH
SIEHand
Y
IO-W
HAS
HAU‡Departments of *Otolaryngology and†Diagnostic Ultrasound, National Taiwan University Hospital, Taipei, Taiwan; and‡Institute of Applied Mechanics, National Taiwan University, Taipei, Taiwan
(Received 2 October 2000; in final form 10 April 2001)
Abstract—Color Doppler imaging (CDI) was used to identify the morphology of vocal folds (VF) and to quantify the tissue horizontal displacement velocity (HDV) in the vibrating portion of VF. Mucosal HDV that gives an estimate of the stiffness of the VF cover is very important clinically. The VF and its cover were shown to be very hypoechoic and not adequately visible in B-scan image. However, in this study, we found that the structure of the true VF, especially the mucosa and the superficial layer of the lamina propria, the so-called “cover,” can be easily identified and evaluated using CDI. The mean VF displacement velocity was measured by decoding the pseudocolor codes of the ultrasound (US) image at the vibrating sites. The mucosal mean HDV obtained from 10 normal men of age 34ⴞ 8 years phonating at their most comfortable pitch and intensity (98 ⴞ 12 Hz, 55– 65 dB) was about 68 ⴞ 10 cm/s, which agreed reasonably with the literature. Therefore, the CDI could be used as a potential tool for assessing the phonation function in the larynx nonintrusively. (E-mail: ywshau@spring.iam.ntu.edu.tw) © 2001 World Federation for Ultrasound in Medicine & Biology.
Key Words: Color Doppler ultrasound image, Vocal fold, Phonation function, Mucosal displacement velocity.
INTRODUCTION
Clinical application of ultrasound (US) imaging has not been widely accepted in laryngology (Miller and Neme-chek 1998). The true vocal folds (VF) and the vocal muscles appeared as hypoechoic structures that could not be adequately visualized in US scanning (Raghavendra et al. 1987). Morphologically, the use of B-mode US is not superior to other imaging techniques, such as CT or MRI in visualizing laryngeal structures, especially the true VF (Miller and Nemechek 1998). Therefore, the US studies of phonation function have been limited (Hertz et al. 1970; Schindler et al. 1990).
The vibration of VF periodically interrupts the glot-tal airflow and forms the acoustic signal that is perceived as the voice (Hirano 1975). Due to its unique multilay-ered structure, the VF can be divided into the “cover” and the “body” (Hirano 1974). The cover includes mu-cosa and the superficial layer of lamina propria, called
the Reinke’s space. The body consists of vocal ligament and vocal muscle. Functionally, the vibration of VF is confined mainly to the cover and the mucosal wave is propagated vertically from the lower to the upper margin of the VF (Titze 1988; Titze et al. 1993). The vertical mucosal traveling wave is the summation of sequential horizontal tissue displacement waves from the infraglot-tic to the supraglotinfraglot-tic extent of the VF structure (Fig. 1). Both the vertical and the horizontal waves can be ap-proximated by sine waves.
The stiffness of the VF cover has been found to play an important role in the formation of the mucosal wave and the laryngeal phonation function. Several in vivo methods have been designed to measure human VF stiff-ness or elastic modulus directly by tagging the VF cover (Tran et al. 1993; Tanaka and Hirano 1990). Some au-thors instead measure the displacement velocity, which is the horizontal component of the mucosal wave velocity, using a laryngoscope to estimate the stiffness indirectly (Nasri et al. 1994; Hanson et al. 1995). However, these methods were either invasive or inconvenient, and could not reveal the underlying VF structural properties. There-fore, their application in routine clinical examination was
Address correspondence to: Yio-Wha Shau, Ph.D., Associate Professor, Biophysics and Gasdynamics Lab., Institute of Applied Mechanics, National Taiwan University, 1, Roosevelt Rd. Sec.4, Tai-pei, 106, Taiwan. E-mail: ywshau@spring.iam.ntu.edu.tw
somewhat restricted. It is apparent from previous studies that the mucosal vertical wave velocity (VWV) is posi-tively related to the stiffness of the VF (Titze 1988) and is linearly proportional to the tissue horizontal displace-ment velocity (HDV) (Berke 1992). Therefore, the mea-surement of HDV can be used to estimate the VWV and then to predict the VF stiffness (Nasri et al. 1994).
The aim of this study was to find an in vivo, non-invasive method to quantify the human laryngeal struc-tural function with minimum disturbance using a com-mercially available US system. Color duplex sonography has been widely applied in clinics in recent years. The system uses Doppler effect to reveal the velocity of blood flow or tissue motion toward or away from the scan head. A pseudocolor velocity map is placed on top of grey-scale images to show the mean velocity locally (Ferrara and DeAngelis 1997; Hoskins and McDicken 1997). By using color Doppler imaging, the mean veloc-ity of tissue displacement in the VF cover area can be measured. In this study, we first verified the morphologic appearance of VF in the B-mode image. Then, we ap-plied the color mode to identify the VF vibration patterns and measure the corresponding mucosal HDV.
MATERIALS AND METHODS
The study was performed with a commercially available, high-resolution US scanner (HDI-5000, ATL, Bothell, WA) with a 5–12-MHz linear-array transducer (L12-5 38 mm, ATL). To visualize the VF structure at a depth of up to 4 cm, the frame rate was typically 15–24
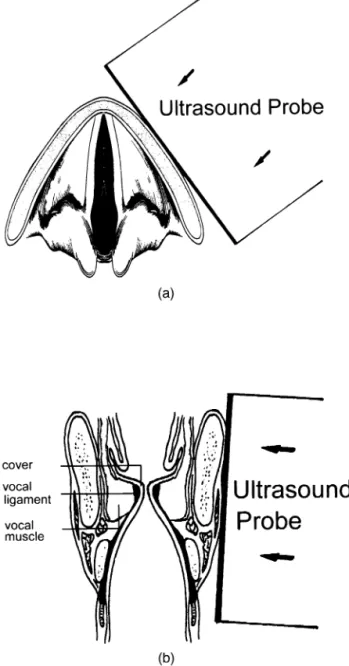
Hz in B-mode. In the color mode, the pulse repetition rate was 10,000 Hz, and the measuring velocity range was set at 0 to 128.3 cm/s with maximum scale and baseline offset that resulted in a frame rate of about 7 Hz. The viewing window of depth 4.8 cm was high enough to detect the vibratory part of the VF in most of the cases. The US scanner was placed both horizontally and coro-nally (Fig. 2a and b, respectively).
In B-mode scanning of the VF, the US transducer was placed on the anterolateral aspect of the neck. For the horizontal view, US scanner was placed in the middle of the thyroid lamina on one side because the true VF lay on the plane at this level. The interface between the true
Fig. 1. Schematic diagram for showing the cover-body and vertical-horizontal wave of vocal fold vibration.
Fig. 2. Schematic diagrams of the US transducer’s position and direction. (a) Horizontal view of the vocal folds; (b) coronal
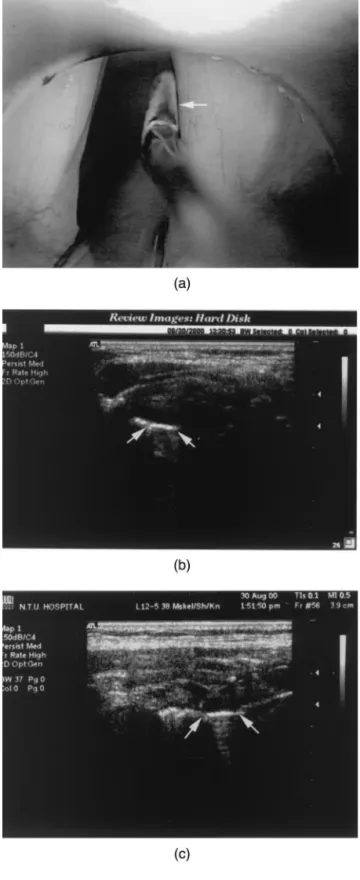
microsurgery was placed at the lumen side of the normal VF as US landmark (Fig. 3). The microsurgical instru-ment was moved back and forth to show the true VF in the B-scan images.
Ten men, aged 34⫾ 8 years (range 24–50 years) without any history of voice disorder were involved in the mucosal HDV study. During US scanning, they were in sitting position and were asked to utter a sustained vowel “aa” that corresponded to the individual’s most comfortable pitch. The fundamental frequency of the voice was recorded on tape and measured on site with a digital oscilloscope (TDS-224, Tektronix, Wilsonville, OR) equipped with a fast Fourier transform (FFT) mod-ule (TDS2MM). The fundamental frequency measured for the utterances “aa” ranged from 98⫾ 12 Hz. For the HDV measurements at a comfortable intensity, the sub-jects were asked to give a sustained vowel with the mean sound pressure level (SPL) kept at about 55– 65 dB that was measured at about 20 cm away from the mouth. The US B-scan was carried out first to locate the true VF, and then the machine was shifted to color mode for the tissue motion measurements. At least 20 frames were recorded in the sustained vowel uttering (Fig. 4), and at least 5 measurements were taken for analysis in each subject. Because the direction of HDV is parallel to the US scanning line in the coronal section, one can obtain the HDV directly from the color of the pixels at the area of true VF cover.
The RGB (red, green and blue) codes given by the velocity color-bar of CDI were first analyzed. The red code value (R) was found ranging from 57 to 239 with a second order relation to the velocity (Fig. 5). After de-coding the red color (R) of the CDI pixels in the vibrating portion of VF, the movement velocities (V) were calcu-lated by fitting R-value on the regression line:
V⫽ 0.0017R2⫹ 0.2082 R ⫺ 19.263.
(1) The HDV value can be determined from the maximal velocity in the vibrating portion of VF that was averaged over the 20 frames recorded.
Fig. 3. (a) The identification of the structure of the vocal fold cover was performed in the B-scan by using the metallic instrument contacting the right vocal fold free margin as a contrast (arrow). (b) In the horizontal section, the cover of the vocal fold was a hypoechoic band. The structure was verified by the metallic comet-tail shadow (arrow). (c) In the coronal section, the cover was identified as a hypoechoic area and it
RESULTS
Under general anesthesia in laryngomicrosurgery, the VFs were in the lateral position. By attaching a metal instrument to the VF free margin, the cover of VF was identified as a hypoechoic band medical to the vocal ligament and vocal muscle in the B-scan image. This structure was easily verified by the US comet-tail shadow that is commonly seen in US images for metallic objects (Fig. 3b and c).
In the real-time B-scan of VF, the adduction and abduction motion of VF reflected on the movement of arytenoid cartilages and vocal ligaments. Again, the cover of VF was not clearly visualized in the US images. During the utterance of the sustained vowel, a slow wave-like movement was observed in the cover area. By
applying Doppler spectral analysis to the motion in this area, the pitch of the spectral acoustic signals was per-ceptually indistinguishable from the uttering vowel.
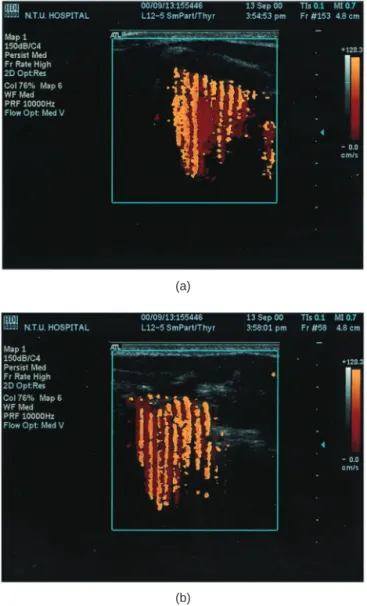
As soon as the US system shifted to the color mode, there were very strong color signals in the vibratory area of VF during phonation (Fig. 4a and b). The pseudocolor signals appeared not only in the cover area of VF, but also in the adjacent region where the vibration waves spread out. When the voice ceased, either in Valsalva status or in quiet respiration position, the color signals disappeared immediately. The Doppler signals appar-ently came from the fluctuations of VF cover toward or away from the US scan head. After decoding the color signals, the HDV was about 68⫾ 10 cm/s in the 10 men phonating at their most comfortable pitch and intensity.
DISCUSSION
Phonation is one of the major functions in the larynx. This process involves the vibration of VF that modulates the glottal airflow into voice (Hirano 1975). The VF is a multilayered structure anatomically, and can be divided into a cover and a body functionally. The cover is much more pliable than the body and is the main vibratory portion of the VF (Saito et al. 1983). For an elastic medium, the travelling were velocity (TWV) is correlated directly to its stiffness or elastic modulus and is inversely proportional to the mass density (Berke 1992).
TWV⫽
冑
⌬Y (2)where⌬Y is the change in elastic modulus and is the mass density. Therefore, the stiffness of the cover is the key factor that affects the formation of the vibratory mucosal
(a)
(b)
Fig. 4. Color Doppler images of vocal fold vibration in (a) horizontal plane and in (b) coronal plane. The vibratory motion of the mucosa-air column interface generates the color artifact
patterns underneath.
Fig. 5. Calibration curve for the vocal fold displacement ve-locity (V) vs. the red code (R) of the color-map in CDI. The horizontal displacement velocity (HDV) can be measured,
plane, and the vertical mucosal wave travels from the infraglottic to the supraglottic margin of the VF structure and spreads to the adjacent tissues. Based on Hirano’s body-cover theory (Hirano 1974), the stiffness of the VF was contributed by both the extrinsic longitudinal ten-sion on the cover and the internal stiffness of the under-lying body. In vivo canine experimental evidence showed that the mucosal muscular tension, VF stiffness and VWV were well correlated (Titze et al. 1993), and the VWV ranged from 0.9 to 1.6 m/s and was linearly related to HDV (Sloan et al. 1993).
Nasri et al. (1994) developed a videostroboscopic sys-tem to visualize the upper edge of the VF in the canine larynx without touching the VF. The levels of phonation were controlled by stimulating the dog’s superior and re-current laryngeal nerves. To calculate the HDV, the VF surface displacement, given by the two video images taken at the most open and the most closed positions, was divided by the time interval. Assuming that the dog’s VF mass density was 1 g/cm3, the HDV calculated was about 0.32 to 1.0 m/s and the VWV ranged from 0.76 to 2.2 m/s. The HDV was shown to correlate linearly with mucosal travel-ing wave velocity. Therefore, the HDV may be used to reflect the voice disorder associated with the change of mucosal stiffness or mass density.
For VF during phonation, the vibrating frequency typically ranges from about 70 to 500 Hz in men and 150 to 1000 Hz in women (Hirano 1981); the frame rate of the commercially available US systems is still not able to catch up with the VF movement in real time. However, there have been a few reports that used US imaging to demonstrate the morphology, movement and vibration of the VF. In the study by Raghavendra et al. (1987), the vocal muscles were hypoechoic structures and the vocal ligaments were echogenic bands that appeared as free margins of the VF. In their study, the adduction and abduction movements of the bilateral vocal ligaments and the arytenoid cartilages were identified during nor-mal breathing using US. However, the true VF could be visualized in only two thirds of the women and in less than half of the men whose mean age was about 25. The cover of VF was not visible in the B-mode imaging,
image. It is, thus, difficult to study the VF vibration using conventional US imaging. Moreover, for the vibration frequency as high as several hundred Hz, to quantify the VF uttering vibration with US is generally not acceptable in the previous reports. Hertz et al. (1970) traced the continuous movement of vocal ligament during VF vi-bration with an M-mode-like design, and the echo trace was similar to photoglottography. Schindler et al. (1990) used a Doppler US flowmeter to detect the VF vibration velocity in terms of frequency shift; the Doppler fre-quency was found to vary from a few hundred Hz to 8000 Hz in the range of audible frequency. In our study, the frame rate of B-mode scan was about 15–24 Hz, depending on the 2-D scanning line density. With appro-priate focusing, the real-time US display of VF vibration showed a slow wave-like motion at the cover area. This motion observed in B-mode was actually the “strobe motion” of the VF movement during phonation.
In the present study, CDI was used to visualize the structural characteristics of the larynx, and it provided an excellent tool to visualize the tissue movements over a large area (Ferrara and DeAngelis 1997; Hoskins and McDicken 1997). Mostly this technique was used to study the blood flows of vessels over a certain region. However, McDicken et al. (1992) applied CDI to inves-tigate the myocardial contraction velocity. Using a sim-ilar idea, elastographic imaging is being developed and aimed to differentiate the various biomechanical at-tributes of tissues (Ophir et al. 2000).
Baken and Orlinkoff (2000) conducted an extensive review of the clinical measurements of phonation func-tion associated with the voice disorder. In the literature, Ooi et al. (1995) applied the CDI qualitatively to identify vocal cord palsy based on the asymmetry of image pat-terns. Friedman (1997) applied B-mode US to the diag-nosis of vocal cord paralysis in infants and children. In this study, we used CDI to estimate the VF stiffness via the measurement of HDV of the VF during phonation. The vibratory motion of the mucosa-air column interface generated a strong colored US artefact at the cover area of the VF during utterance. The color signals were ag-gregated in the cover and the adjacent area, where the
vibratory wave spread. The color signals appeared only in the phonation period or lightly during breathing. After the phonation ceased, the color signals disappeared. By applying the Doppler spectral analysis to the US echo in the cover area, the pitch of the spectral audio output was perceptually indistinguishable from the uttering vowel. Therefore, we had enough evidence to confirm that the color artefact resulted from the vibration of the VF.
Because the mucosal cover moved mediolaterally in the direction toward and away from the scan head in the VF coronal view, the HDV of the cover was retrieved from the corresponding CDI based on the velocity color map. The HDV followed approximately a sine curve, in which the peak velocity appeared at the neutral position and zero velocity appeared at its maximal excursion. It should be noted that the magnitude of velocity displayed in CDI is an averaged speed of the moving objects. The HDV was defined as the highest velocity obtained in the area of mucosal cover that averaged for the 20 frames of images taken continuously.
Our results of HDV obtained in the 10 normal men uttering a sustained vowel in their most comfortable pitch and intensity fall in the same range as the data reported in the literature (Titze et al. 1993; Nasri et al. 1994). We also observed that the magnitude of HDV increased, not only with the pitch of the phonation, but also with the intensity of the voice (i.e., the sound pressure level). How this rela-tionship deviates from the sinusoidal wave linear theory requires further study. The present method is intended to give a quantitative estimation of the mechanical properties of the VF and facilitates the potential for further research in laryngeal function.
To our best knowledge, this is the first time that the HDV along the VF is reported in human subjects with normal active phonation using our US system. Using CDI in the measurement of HDV of the VF cover has the advantages over laryngostroboscopy that the VF vibra-tion can be monitored continuously during phonavibra-tion, with no irritating instruments in the mouth and with no interference to the articulatory movements of the tongue. It can provide a simple, absolutely noninvasive and pain-less procedure in routine laryngeal functional examina-tion. Moreover, the structural bounding of the VF to its surrounding tissues, in other words, the global nature of VF stress wave propagation, can also be observed.
SUMMARY
In the literature, the characteristics of the mucosal wave have been shown to correlate directly with the me-chanical properties of the VF. The present study indicates that the measurement of HDV using the CDI is feasible and may become a promising technique for assessing the active phonation function of the larynx in humans.
Acknowledgment—This research was supported by Grant NSC
89-2314-B-002-441.
REFERENCES
Baken RJ, Orlinkoff RF. Clinical measurement of speech and voice. 2nd ed. Thomson Learning. San Diego: Singular Publishing, 2000. Berke GS. Intraoperative measurement of the elastic modulus of the vocal fold. Part 1. Device development. Laryngoscope 1992;102: 770 –778.
Ferrara K, DeAngelis G. Color flow mapping. Ultrasound Med Biol 1997;23:321–345.
Friedman EM. Role of ultrasound in the assessment of vocal cord function in infants and children. Ann Otol Rhinol Laryngol 1997; 106:199 –209.
Hanson DG, Jiang J, D’Agostino M, Herzon G. Clinical measurement of mucosal wave velocity using simultaneous photoglottography and laryngostroboscopy. Ann Otol Rhinol Laryngol 1995;104:340 – 349.
Hertz CH, Lindstrom K, Sonesson B. Ultrasonic recording of the vibrating vocal folds. Acta Otolaryng 1970;69:223–230. Hirano M. Morphological structure of the vocal cord as a vibrator and
its variations. Folia Phoniatr 1974;26:89 –94.
Hirano M. Phonosurgery: Basic and clinical investigations. Otologia (Fukuoka) 1975;21(Suppl. 1):239 –262.
Hirano M. Clinical examination of voice. Wien-New York: Springer-Verlag, 1981.
Hoskins PR, McDicken WN. Colour ultrasound imaging of blood flow and tissue motion. Br J Radiol 1997;70:878 – 890.
Isshiki N. Phonosurgery. Theory and practice. Tokyo: Springer-Verlag, 1989.
McDicken WN, Sutherland GR, Moran CM, Gordon LN. Colour Doppler velocity imaging of the myocardium. Ultrasound Med Biol 1992;18:651– 654.
Miles KA. Ultrasound demonstration of vocal cord movements. Br J Radiol 1989;62:871– 872.
Miller RH, Nemechek AJ. Airway evaluation and imaging. In: Bailey BJ, Pillsbury HC, Driscoll BP, eds. Head and neck surgery— Otolaryngology. 2nd ed. Philadelphia PA: Lippincott-Raven, 1998: 597– 608.
Nasri S, Sercarz JA, Berke GS. Noninvasive measurement of traveling wave velocity in the canine larynx. Ann Otol Rhinol Laryngol 1994;103:758 –766.
Ooi LL, Chan HS, Soo KC. Color Doppler imaging for vocal cord palsy. Head Neck 1995;17:20 –23.
Ophir J, Garra B, Kallel F, et al. Elastographic imaging. Ultrasound Med Biol 2000;26:S23–S29.
Raghavendra BN, Horii SC, Reede DL, et al. Sonographic anatomy of the larynx, with particular reference to the vocal cords. J Ultrasound Med 1987;6:225–230.
Saito S, Fukuda H, Kitahara S, et al. Pellet tracking in the vocal fold while phonating— experimental study using canine larynges with muscle activity. In: Titze IR, Scherer RC, eds. Vocal fold physiol-ogy: Biomechanics, acoustics and phonatory control. Denver: Den-ver Center for the Performing Arts, 1983:169 –182.
Schindler O, Gonella ML, Pisani R. Doppler ultrasound examination of the vibration speed of vocal folds. Folia Phoniatr 1990;42:265–272. Sloan SH, Berke GS, Garratt BR, Kreiman J, Ye M. Determination of vocal fold mucosal wave velocity in an in vivo canine model. Laryngoscope 1993;103:947–953.
Tanaka S, Hirano M. Fiberscopic estimation of vocal fold stiffness in
vivo using the sucking method. Arch Otolaryngol Head Neck Surg
1990;116:721–724.
Titze IR. The physics of small-amplitude oscillation of the vocal folds. J Acoust Soc Am 1988;83:1536 –1552.
Titze IR, Jiang JJ, Hsiao TY. Measurement of mucosal wave propa-gation and vertical phase difference in vocal fold vibration. Ann Otol Rhinol Laryngol 1993;102:58 – 63.
Tran QT, Berke GS, Gerratt BR, Kreiman J. Measurement of Young’s modulus in the in vivo human vocal folds. Ann Otol Rhinol Lar-yngol 1993;102:584 –591.