Glutamine reduces the expression of LFA-1 and Mac-1 on leukocytes in mice with arsenic exposure
Ya-Ling Luoa, Hui-Ting Yanga, Chun-Sen Hsub, Wan Chun Chiua, Sung-Ling Yeha Institute of Nutrition and Health Sciences, Taipei Medical Universitya, Department of Obstetrics and Gynecology, Taipei Medical University-Municipal Wan-Fang Hospitalb, and Taipei, Taiwan
Running title: glutamine reduces integrin expression in arsenic exposure
Correspondence to: Sung-Ling Yeh, PhD
School of Nutrition and Health Sciences Taipei Medical University
250 Wu-Hsing Street, Taipei, Taiwan 110, ROC Tel: 8862-27361661 ext. 6551-115
Abstract
This study was designed to investigate the effect of GLN supplementation on leukocyte integrin expression and in vitro splenocyte cytokine production in mice with arsenic exposure. Mice were assigned to the control and experimental groups. Control group drank deionized water, whereas experimental group drank water containing 50ppm sodium arsenite instead. Each control and experimental group was divided into 2 subgroups respectively. One subgroup fed a common
semipurified diet, the other one was supplemented with glutamine (GLN), replacing 25% of total amino acid nitrogen. After feeding for 5 weeks, all mice were
sacrificed for the analytical measurements. The results showed that plasma GLN levels in arsenic group were significantly lower than those in control groups, GLN supplementation reversed the depletion of plasma GLN levels. Under the condition of arsenic exposure, β2 intergins including LFA-1 and Mac-1 expression on leukocyte were significantly reduced in GLN supplemented group than those without GLN, and had no difference from the control groups. There were no differences in interleukin -4, interleukin-6, interferon-γ and tumor necrosis factor-α production between the 2 arsenic exposure groups, when splenocytes were stimulated with mitogen. These results suggest that arsenic exposure resulted in depletion of plasma GLN and GLN supplementation normalized the plasma GLN levels and reduced the intergins LAF-1, Mac-1 expression on leukocyte. However, GLN administration seemed to have no effect on in vitro cytokine secretion in mice with arsenic exposure.
1. Introduction
Arsenic is a ubiquitous element widely distributed in the environment. The main source of arsenic exposure for the general population is through ingestion of high arsenic drinking water (1). Chronic arsenic exposure is associated with an increased risk of vascular diseases including ischemic heart disease, cerebrovascular disease, and carotid atherosclerosis (2,3). The pathogenic mechanisms of arsenic atherogenicity are not completely understood. Previous reports have shown that arsenic results in the generation of reactive oxygen species both in vivo and in vitro (3-5). Oxidative stress may have impact on the atherogenic process by modulating intracellular signaling pathways in vascular tissues to affect inflammatory cell adhesion, migration and proliferation (6). Blood leukocytes, mediators of host defense and inflammation, localize in the earliest lesions of atherosclerosis (7). The initial sign of inflammation is the capture of leukocyte from the blood stream and their subsequent rolling along the endothelium of postcapillary venules (8). Patches of arterial endothelial cell express selective adhesion molecules on their surface that binds to various classes of leukocytes (7). Leukocyte function associated antigen (LFA)-1 and macrophage antigen (Mac)-1 are members of β2 intergins (CD18) that are predominantly involved in leukocyte trafficking and extravasation (9). LFA-1 (CD11a/CD18) is exclusively expressed on leukocytes and interacts with its ligands intercellular adhesion molecule (ICAM) to promote a variety of homotypic and hererotypic cell adhesion required for normal and pathologic functions of the immune system (9). Mac-1 (CD11b/CD18) is abundant in neutrophil and contributes to neutrophil migration into the sites of inflammation (9-11). Activation of β2 intergins is required for a stronger attachment to the endothelium and subsequent
transmigration (9-11). Excessive expression of LAF-1 and Mac-1 may enhance the inflammatory response and have deleterious effects including tissue destruction,
ischemia-reperfusion injury and autoimmune disease (12). Rahimi et al (13)
suggested that CD11a, CD11b, CD18 may be used as indicators for the progression of coronary artery disease.
Glutamine (GLN) is the most abundant free amino acid in plasma and tissue pool. It is a critical substrate for enterocytes and rapid proliferating immune cells (14,16). A variety of studies have demonstrated that GLN has immuno-enhancing properties (16-19). Previous reports have revealed that a relatively GLN-deficient state is created by the catabolic process, and GLN supplementation can correct this nutritional deficiency and hence improve outcome (20,21). GLN is required during catabolic processes to manifest optimal tissue response to catabolism, inflammation and infection, and is considered an essential amino acid during certain inflammatory conditions (22-24). Study by Fukatsu et al. (25) showed that compared with conventional total parenteral nutrition, GLN-supplemented parenteral nutrition reduced ICAM-1 expression in intestinal homogenates. Also, Arndt et al. (26) demonstrated that GLN administration reduced leukocyte adhesion and transmigration in indomethacin-induced intestinal inflammation in the rat. As we know, there is no study investigating the effect of GLN on the inflammatory response under the
condition of arsenic exposure. Therefore, the aim of this research was to study the effect of GLN supplemention on leukocyte integrin expression and in vitro spenocyte cytokine production in mice with arsenic exposure.
MATERIALS AND METHODS Animals
Male BALB/c mice weighing 10-15 g (4 weeks of age) were used in this study. All rats were housed in temperature- and humidity-controlled rooms and were allowed
free access to standard rat chow for 1 wk prior to the experiment. The care of the animals followed the guidelines for the care and use of laboratory animals established by the Animal Care Committee of Taipei Medical University, and protocols were approved by that committee.
Study protocol. All mice were assigned to the control and experimental groups.
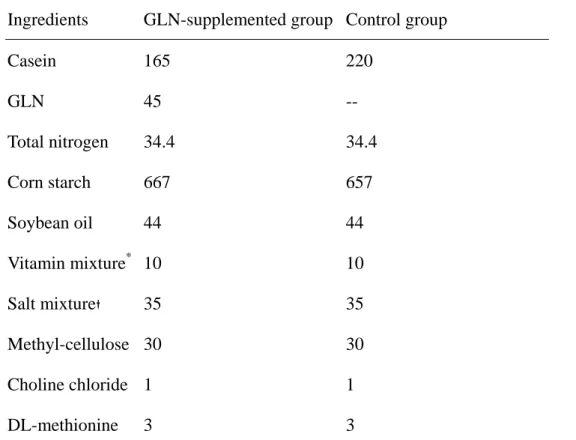
Mice in control group drank deionized water, whereas experimental group drank water containing 50ppm sodium arsenite (NaAsO2) instead. Each control and experimental group was divided into 2 subgroups. One subgroup fed a common semipurified diet, the other one was supplemented with GLN, replacing 25% of total amino acid nitrogen (Table 1). There were 4 groups in this study: CC group, no arsenic and GLN were administered; CG group, no arsenic but supplemented with GLN; AC, with arsenic but no GLN supplemented; AG group, with arsenic and GLN supplementation. Food and water intake were recorded everyday during the
experimental period. After 5 weeks, all mice were anesthetized and sacrificed by heart puncture.
Plasma GLN level analysis. Blood samples were collected in tubes containing
heparin and immediately centrifuged. Plasma amino acid was analyzed by standard ninhydrin technology (Beckman Instrument, model 6300, Palo Alto, CA), after deprotienization of the plasma with 5% salicylic acid (27).
Flow cytometric analysis. To determine the integrin expression on leukocyte, 100 μl whole blood containing fluorescein-conjugated rat anti-mouse CD11a, CD11b (Serotec, Oxford, UK) and phycoerythrin-conjugated rat anti-mouse CD18 (Serotec) were used to identify LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18), respectively. After staining for 15 min, 1 mL red blood cell (RBC) lysing buffer (Serotec) was added to lyse the RBCs and to fix the stained leukocytes. Fluorescence data were collected on 1 x 105 viable cells and analyzed by flow cytometry (Coulter, Miami, FL,
USA).
In vitro cultures of splenocytes. Splenocytes were obtained by mechanical disruption of the spleen with a spatula on a stainless steel mesh. Cell suspensions were passed through a sterile nylon mesh to remove debris. RBCs were lysed using sterile distilled water for 15 s, and immediately neutralized to isotonic cell
suspensions. After washing with PBS 3 times (300 xg for 5 min), splenocytes were resuspended in RPMI-1640 with antibiotics and fetal calf serum. The number of isolated splenocytes was determined by a hemacytometer count using the trypan blue dye exclusion method.
Cytokine assay. Phytohemagglutinin (PHA, 200 ng/mL; Sigma) and
lipopolysaccharide (LPS, 1ug/mL; Sigma) were used to stimulate cytokine production by isolated splenocytes lymphocytes in culture. Triplicate wells of 96-well
flat-bottomed microtiter plates (Falcon, Becton Dickinson, Oxford, CA, USA) were seeded with splenocytes (2.5 x 106 cells/mL in RPMI-1640) and mitogen. The control well contained cells plus an equal volume of medium. After incubating PHA or LPS for 24 h at 37 oC in a CO2 incubator, supernatants were centrifuged and stored at –70 oC until being analyzed for cytokine. Concentrations of interleukin (IL)-2, IL-4, interferon (IFN)-γ in PHA-stimulated, IL-6 and tumor necrosis factor-α in LPS-stimulated splenocyte supernatants were determined by commercially available enzyme-linked immunosorbent assay (ELISA) kits (Amersham Pharmacia Biotech, Buckinghamshire, UK).
Statistical analysis. Data are expressed as the mean ± SD. Differences among
groups were analyzed by two-way ANOVA with Fisher’s test. A p value of < 0.05 was considered statistically significant.
RESULTS
Body weight and plasma GLN levels. There were no differences in initial body
weights among the groups. No differences in food and water intake were observed across the groups during the experimental period (data not shown). After feeding for 5 weeks, the body weights of mice in arsenic group without GLN (AC) were
significantly lower than the control group (CC), whereas there were no differences in body weights between arsenic group with GLN supplementation (AG) and the control group (Fig. 1). Plasma GLN levels in AC group were significantly lower than those in control groups (CC & CG), however, under the condition of arsenic exposure, GLN supplementation reversed the depletion of plasma GLN levels and had no difference from the control groups (Fig. 2).
Leukocyte integrin expression. LFA-1 and Mac-1 expression on leukocyte were
significantly lower in AG group than those in AC group, and had no difference from the control groups (Fig. 3A &3B)
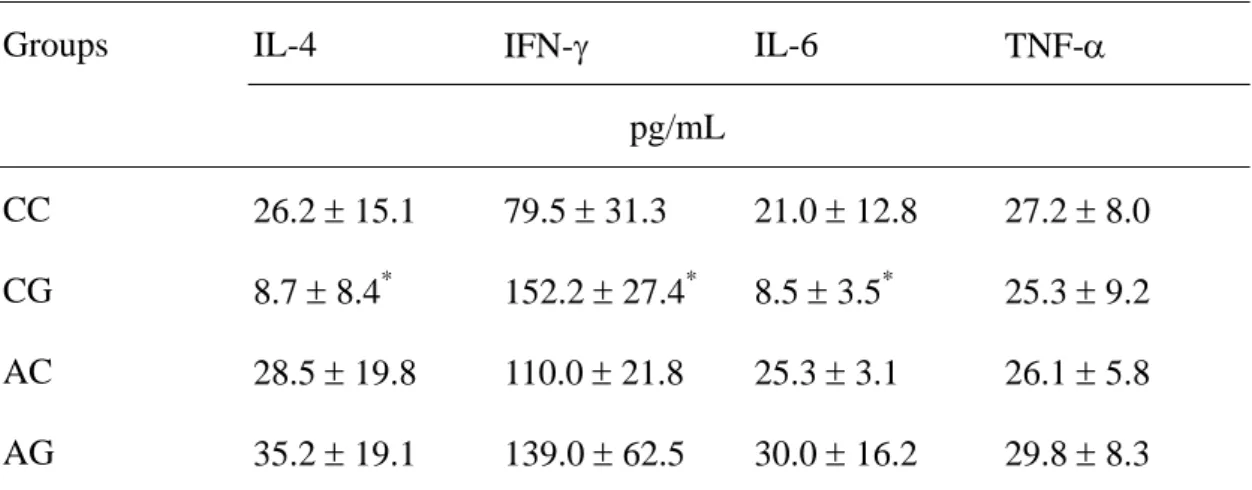
In vitro cytokine secretion. IL-2 levels were not detectable. IL-4 and IL-6
production by splenocytes were significantly lower, whereas IFN-γ levels were higher in CG group than the CC group. There were no differences in IL-4, IL-6, IFN-γ and TNF-α production in arsenic exposure groups despite GLN was supplemented or not (Table 2).
Discussion
The metabolism of arsenic has an important role in its toxicity. In the trivalent state, inorganic and organic arsenic may react with critical thiols in protein and inhibit their activity (28). In this study, we found that body weight in mice exposure with arsenic was significantly lower than the control groups, although the food and water intake
was comparable among groups. This result indicated that the dosage of arsenic administered in this study allowed survival but resulted in metabolic stress in mice. Study showed that arsenite causes the oxidative damage of the protein of pyruvate dehydrogenase, and thus inhibit the enzyme activity (29). Since pyruvate
dehydrogenase catalyzes a reaction that controls the pathway of glycolysis to the citric acid cycle, the inactivation of the enzyme may interfere energy metabolism and consequently resulted in weight loss.
LFA-1 and Mac-1 are thought to play central roles in mediating the firm
adhesion of leukocytes to endothelial cells, a critical step to the subsequent leukocyte transmigration. Excessive expression of LAF-1 and Mac-1 may induce
inflammatory response and tissue injury (11-13). In this study we found that arsenic group with GLN (AG) had lower LAF-1 and Mac-1 expression than group without GLN (AC). This finding indicated that GLN supplementation reduce leukocyte integrin expression and may consequently attenuate the inflammatory response induced by arsenic. Compared with the control groups, we did not observe a higher integrin expression in arsenic groups. Arsenic exposure is expected to result in higher adhesion molecule expression because previous reports have shown that arsenic induced the generation of reactive oxygen species, and up-regulated the inflammatory mediators (3, 4, 30). We speculate that the lower body weight in the arsenic exposure groups may lead to an inferior nutrition status, and thus interfere the synthesis and/or the expression of the adhesion protein.
Cytokines are peptides produced by cells of the immune system that act as mediators of the immune response. We did not determine plasma cytokines in this study, because previous study reported that plasma IL-1, TNF, and IFN-γ levels are rarely detected in the plasma of injured patients (31). Previous reports by our
laboratory also showed that IL-1β, IL-2, and IFN-γ were undetectable in a septic animal model (32, 33). Therefore, we analyzed the production of cytokines
including IL-2, IL-4, IL-6, IFN-γ and TNF-α by splenocytes after mitogen stimulation to investigate the effect of GLN supplementation on the systemic immune response under arsenic exposure. IL-2 and IFN-γ are produced by Th1 lymphocytes. Th1 cytokines enhance cell-mediated immunity. IL-4 is a Th2 cytokine that enhance humoral immunity. The effects of Th1 or Th2 lymphocytes are counter-regulatory (34). IL-6, and TNF-α are pro-inflammatory cytokines. The results showed that GLN supplementation reduced IL-6 production, enhanced IFN-γ and suppressed IL-4 secretion in non-arsenic exposure groups. The finding is partly consistent with the report by Rohde et al (35), their study also showed that IFN-γ were enhanced by GLN supplementation. Since there were no differences in the cytokines between arsenic exposure groups despite GLN was supplementation or not, cytokine modulation may not responsible for reducing leukocyte integrin expression in GLN supplemented group.
Study by Hong et al (36) revealed that GLN supplemented nutrition protects the liver during hepatic injury by preserving glutathione stores. An in vitro study by Babu et al (37) also found that GLN reversed beneficial effect in preventing liver damage possibly mediated via GSH synthesis. GSH is a major antioxidant and a vital component of host defense. GLN was found to be rate limiting for GSH synthesis and availability of GLN is critical in the generation of GSH stores (38). In this study we found that group with arsenic exposure had significantly lower plasma GLN levels than control groups, this finding was compatible with the previous reports that plasma GLN was reduced during a catabolic conditions such as inflammatory, infection, and injury (18,22-24). Previous report showed that trivalent organic
arsenicals inhibit glutathione reductase activity. Inhibition of this enzyme may result in depletion of reduced form of glutathione (GSH), and decreased ability of cells to protect against oxidants (39). In this study we found that GLN supplementation reversed the depletion of plasma GLN induced by arsenic exposure. Whether supplementation of GLN restored the GSH levels, improved the antioxidant status of the animals and thus decreased LFA-1 and Mac-1 expression requires further
investigation.
In summary, this study showed for the first time that arsenic exposure resulted in
depletion of plasma GLN and GLN supplementation normalized the plasma GLN levels and reduced the intergins LAF-1, Mac-1 expression on leukocyte. However, the effects of GLN on in vitro cytokine secretion were not obvious in mice with arsenic exposure.
Acknowledgements
This study was supported by research grant NSC 92-2321-B-038-008 from the National Science Council, ROC.
Reference
1. World Health Organization, Environmental Health Criteria 18: Arsenic. Geneva, Switzerland: World Health Organization ; 1981
2. Engel RR and Smith AH (1994) Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Arch Environ Health 49(5): 418-427
3. Thomas DJ, Styblo M, Lin S., 2001. The cellular metabolism and systemic toxicity of arsenic. Toxicology & Applied Pharmacology 176, 127-144 4. Chen A, Cao EH, Zhang TC, Qin JF (2002) Arsenite-induced reactive oxygen
species and the repression of alpha-tocopherol in the MGC-803 cells. European Journal of Phamacology 448, 11-18.
5. Flora SJ., (1999) Arsenic-induced oxidative stress and its reversibility following combind administration of N-acetylcysteine and meso 2,3-dimercaptosuccinic acid in rats. Clinical Experimental Pharmacol Physiology 26, 865-869
6. Patel RP, Moellering D, Murohy-Ullrich J, Jo H, Becman JS, Darley-Usmar VM., (2000) Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radical Biology & Medicine 28, 1780-1794
7. Libby, P., Ridker, P. M., Maseri, A., 2002. Inflammation and atherosclerosis. Circulation 105, 1135-1143.
8. Ley, K., Tedder, T. F., 1995. Leukocyte interactions with vascular endothelium: new insight into selectin-mediated attachment and rolling. Journal of Immunology 155, 525-528.
9. Henderson, R. B., Lim, L. H. K., Tessier, P. A., Gavins, F. N. E., Mathies, M., Perretti, M., Hogg, N., 2001. The use of lymphocyte function-associated antigen (LFA)-1-deficient mice to determine the role of LFA-1, Mac-1 and α4 integrin in the inflammatory response of neutrophils. Journal of Experimental Medicine 194, 219-226.
10. Gao, J. X., Issekutz, A. C., 1996. Mac-1 (CD11b/CD18) is the predominant β2integrin mediating human neutrophil migration through synovial and dermal fibroblast barriers. Immunology 88, 463-470.
11. Plow, E. F., Zhang, L., 1997. A Mac-1 attack: integrin functions directly challenged in knockout mice. Journal of Clinical Investigation 99, 1145-1146. 12. Albelda, S. M., Smith, C. W., Ward, P. A., 1994. Adhesion molecules and
inflammatory injury. Federal Am Soc Exp Biological Journal 8, 504-512.
of Mac-1 and LFA-1 on leukocytes for prediction of late restenosis and their possible correlation with advanced coronary artery disease. Cytometry 53B, 63-69.
14. Smith RJ & Willmore DW (1990) Glutamine nutrition and requirements. Journal
of Parenteral and Enteral Nutrition 14, 94S-99S.
15. Souba WW (1991) Glutamine: a key substrate for the splanchnic bed. Annual
Review of Nutrition 11, 285-308.
16. Calder PC (1994) Glutamine and the immune system. Clinical Nutrition 13, 2-8 17. Heberer M, Babst R, Juretic A, et al.(1996) Role of glutamine in the immune
response in critical illness. Nutrition 12 (Suppl), S71-S72.
18. Gismondo MR, Drago L, Fassina MC, Vaghi I, Abbiati R & Grossi E (1998) Immunostimulating effect of oral glutamine. Digestive Diseases and Science 43, 1752-1754.
19. Wilmore DW & Shabert JK (1998) Role of glutamine in immunologic responses.
Nutrition 14, 618-626
20. Hammerquist F, Wernerman J, Al R, Vonder D & Vinners E (1989) Addition of glutamine to total parenteral nutrition after elective abdominal surgery spares glutamine in muscle, counteracts the fall in muscle protein synthesis and improves nitrogen balance. Annals of Surgery 209, 455-461.
21. Gianotti L, Alexander JW, Gennari R, et al. Oral glutamine decreases bacterial translocation and improves survival in experimental gut-origin sepsis. J Parenter Enter Nutr 1995;19:69
acid? Nutrition Reviews 48, 297-309.
23. Willmore DW. The effects of glutamine supplementation in patients following elective surgery and accidental injury. J Nutr 2001;131:2543S
24. Parry-Billings M, Evans J, Calder PC, et al. Does glutamine contribute to immunosuppression after major burns? Lancet 1990;336:523
25. Fukatsu K, Lundberg AH, Kudsk KA, Hanna MK and Zarzaur BL. Modulation of organ ICAM-1 expression during IV-TPN with glutamine. Shock 15:24-28, 2001 26. Arndt H. Kullmann F. Reuss F. Scholmerich J. Palitzsch KD. Glutamine
attenuates leukocyte-endothelial cell adhesion in indomethacin-induced intestinal inflammation in the rat. J Parenter Enter Nutr 23:12-18, 1999
27. Smith RJ & Panico K (1985) Automated analysis of o-phthalaldehyde derivatives of amino acids in physiological fluids of reverse phase high performance liquid chromatography. Journal of Liquid Chromatography 8, 1783-1795.
28. Hughes MF (2002) Arsenic toxicity and potential mechanisms of action. Toxicology Letters 133, 1-16.
29. Samikkannu T, Chen CH, Yih LH, Wang AS, Lin SY, Chen TC, Jan KY (2003) Reactive oxygen species are involved in arsenic trioxide inhibition of pyruvate dehydrogenase activity. Chemical Research Toxicology 16, 409-414.
30. Bunderson M, Coffin JD and Beall HD (2002) Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: possible role in atherosclerosis. Toxicology & Applied Pharmacology 184: 11-18 31. Cruickshank AM, Fraser WD, Burns HJG, Van Dame J & Shenkin A (1990)
varying intensities. Clin Sci 79, 161-165.
32. Yeh CL, Yeh SL, Lin MT & Chen WJ (2002) Effects of arginine-enriched total parenteral nutrition on inflammatory-related mediator and T cell population in septic rats. Nutrition 18, 631-635.
33. Yeh SL, Yeh CL, Lin MT, Lo PN, Chen WJ (2001) Effects of
glutamine-supplemented total parenteral nutrition on cytokine production and T cell population in septic rats. J Parenter Enter Nutr 25, 269-274.
34. DiPiro JT (1997) Cytokine networks with infection: mycobacterial infectons, leishmaniasis, human immunodeficiency virus infection, and sepsis.
Pharmacotherapy 17, 205-223.
35. Rohde T, Maclean DA, Pedersen BK. (1996) Glutamine, lymphocyte proliferation and cytokine production. Scand J Immunology 44, 648-650
36. Hong RW, Rounds JD, Helton WS, Robinson MK, Wilmore DW. Glutamine preserves liver glutathione after lethal hepatic injury. Ann Surg 1992;215:114-119 37. Babu R, Eaton S, Drake DP, Spitz L, Pierro A. Glutamine and glutathione
counteract the inhibitory effects of mediators of sepsis in neonatal hepatocytes. J Pediatr Surg 2001;36:282-286
38. Welbourne TC. Ammonia production and GLN incorporation into GSH in the functioning rat kidney. Can J Biochem 1979;57:233-237
39. Styblo M, Serves SV, Cullen WR and Thomas DJ (1997) Comparative inhibition of yeast glutathione reductase by arsenicals and arsenothiols. Chem Res Toxicol 10: 27-33
Figure legends
Fig.1. Body weight of the groups. CC: control group without GLN and As; CG: control group with GLN but without As; AC: As group without GLN; AG: As group with GLN. *: significant difference from CC group.
Fig. 2. Plasma glutamine (GLN) concentrations of the groups. *: significant difference from the other groups.
Fig. 3. A) Leukocyte function associated antigen (LFA)-1 and B) Mac-1 expression (%) on whole blood as determined by cytometry. *: significant difference from the AC group. +: significant difference from the other groups.
Table 1 Composition of the semipurified diet (g/kg)
Ingredients GLN-supplemented group Control group
Casein 165 220 GLN 45 -- Total nitrogen 34.4 34.4 Corn starch 667 657 Soybean oil 44 44 Vitamin mixture* 10 10 Salt mixture 35 35 Methyl-cellulose 30 30 Choline chloride 1 1 DL-methionine 3 3 *
The vitamin mixture contained the following (mg/g): thiamin hydrochloride 0.6, riboflavin 0.6, pyridoxine hydrochloride 0.7, nicotinic acid 3, calcium pantothenate 1.6, D-biotin 0.02,
cyanocobalamin 0.001, retinyl palmitate 1.6, DL-α-tocopherol acetate 20, cholecalciferol 0.25, and menaquinone 0.005.
The salt mixture contained the following (mg/g): calcium phosphate diabasic 500, sodium chloride 74, potassium sulphate 52, potassium citrate monohydrate 220, magnesium oxide 24, manganese carbonate 3.5, ferric citrate 6, zinc carbonate 1.6, cupric carbonate 0.3, potassium iodate 0.01, sodium selenite 0.01, and chromium potassium sulphate 0.55.
Table 2. Concentrations of interleukin (IL)-4, interferon (IFN)-γ, IL-6 and tumor necrosis factor-α released by splenocytes after mitogen stimulation for 24 h.
Groups IL-4 IFN-γ IL-6 TNF-α
pg/mL
CC 26.2 ± 15.1 79.5 ± 31.3 21.0 ± 12.8 27.2 ± 8.0 CG 8.7 ± 8.4* 152.2 ± 27.4* 8.5 ± 3.5* 25.3 ± 9.2 AC 28.5 ± 19.8 110.0 ± 21.8 25.3 ± 3.1 26.1 ± 5.8 AG 35.2 ± 19.1 139.0 ± 62.5 30.0 ± 16.2 29.8 ± 8.3 Data are expressed as the mean ± standard deviation
24 26 28 30 32 34 CC CG AC AG groups weight (g)
0 100 200 300 400 500 600 CC CG AC AG groups GLN (nmol/mL) *
A) 0 5 10 15 20 CC CG AC AG groups LFA-1 (%) * B) 0 10 20 30 40 50 CC CG AC AG groups Mac-1 (%) †