Retinal Vein Occlusion and the Risk of Stroke
Development: A Five-year Follow-up Study
JAU-DER HO, SHIOW-WEN LIOU, AND HERNG-CHING LIN ● PURPOSE: To investigate the risk of stroke
develop-ment following the occurrence of retinal vein occlusion (RVO).
● DESIGN: Retrospective nationwide population-based administrative database study.
● METHODS:Data were collected from Taiwan National Health Insurance Research Database, which comprises 1,073,891 random subjects from 23 million Taiwan residents. The study cohort comprised of all patients with a first-time diagnosis of either central or branch RVO from January 1999 to December 2001 (nⴝ 350). The comparison cohort comprised randomly selected patients (n ⴝ 2,100) matched with the study group for age, gender, and the date of ambulatory care visit. Each sampled patient was tracked for five years. Cox propor-tional hazard regressions were utilized to compute the five-year stroke-free survival rate after adjusting for possible confounding factors.
● RESULTS: Stroke developed in 35.1% and 19.9% of RVO patients and comparison group patients, respec-tively. After adjusting for demographic characteristics and comorbidities, RVO was not associated with an increased risk of stroke development (adjusted hazard ratio, 1.01; 95% confidence interval [CI], 0.65 to 1.57) among subjects of any age. However, RVO patients age 60 to 69 years had a 2.34-fold (95% CI, 1.05 to 5.24) higher risk of suffering a stroke.
● CONCLUSIONS: There was no overall association of RVO with stroke except in the 60-to 69-year subgroup. The possible causes include: an actually increased risk of stroke development in the 60- to 69-year group, chance finding, the presence of selection biases, small numbers of stroke patients in the <50 and 50 to 59 age groups, or a lack of power in the >70-year group. (Am J Ophthal-mol 2009;147:283–290. © 2009 by Elsevier Inc. All rights reserved.)
R
ETINAL VEIN OCCLUSIVE (RVO) DISORDERS, WHICH include central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO), collectively constitute one of the major causes of severe vision impair-ment and blindness.1,2The systemic risk factors associated with RVO include old age,3hypertension,4diabetes mel-litus,4 and cardiovascular diseases.4 Many of these are associated with the development of stroke, which is the most common cause of serious disability in adults.5,6 Therefore, it would be of clinical relevance to investigate whether RVO is a predictor for the future development of stroke.To the best of our knowledge, there have been two published single hospital-based studies that compared the rate of stroke development after RVO occurrence with a control cohort. Mansour and associates7investigated the risk of mortality and morbidity in 78 patients with CRVO with a mean follow-up of 7.2 years. Thirteen subjects died at an average of 7.0 years after CRVO. None of the 11 subjects with known causes of death died of stroke. One surviving subject developed stroke three years after CRVO. They concluded that patients with CRVO did not carry a higher risk of mortality or morbidity than matched controls derived from national surveys. In a study by Tsaloumas and associates,8which involved 549 RVO cases with a mean follow-up period of 9.08 years, there was no statistical difference between the RVO group and the background population in the rates of stroke development, although there was a trend towards a greater number of deaths from stroke in the RVO group (19% vs 13.5%).
Using a population-based dataset from Taiwan, this study investigated the relationship between RVO occur-rence and the risk of future stroke development.
METHODS
● DATABASE: This study used a database released by the Taiwan National Health Research Institute (NHRI) in 2008. This database is composed of 1,073,891 random subjects, about 5% of the entire enrollees in the National Health Insurance (NHI) program. It was created by the NHRI using a systematic sampling method to randomly sample a representative database from the entire database. There were no statistically significant differences in age, gender, or costs between the sample group and all enroll-ees. This database consists of medical claims for
ambula-Accepted for publication Aug 5, 2008.
From the Department of Ophthalmology (J.-D.H.), Taipei Medical University Hospital; Department of Ophthalmology (J.-D.H., S.-W.L.) and School of Health Care Administration (H.-C.L.), Taipei Medical University; Department of Ophthalmology (S.-W.L.), Taipei City Hos-pital; and the Department of Ophthalmology (S.-W.L.), National Taiwan University Hospital, Taipei, Taiwan.
Inquiries to Herng-Ching Lin, School of Health Care Administration, Taipei Medical University, 250 Wu-Hsing Street, Taipei 110, Taiwan; e-mail:henry11111@tmu.edu.tw
tory care, inpatient care, dental services, and prescription drugs as well as the registration files of the insured from January 1996 to December 2006.
● STUDY SAMPLE: Our study design featured a study cohort and a comparison cohort. The study cohort com-prised all patients who visited ambulatory care physicians from January 1999 to December 2001 for a diagnosis of RVO (ICD-9-CM codes 362.35 or 362.36). In order to assure that selected cases were new episodes to avoid the potential confounding factors of chronicity, we excluded those who had been treated for RVO in ambulatory care during the previous three-year period (n⫽ 51), and also patients previously diagnosed with stroke (ICD-9-CM codes 430-438) (n⫽ 21). Ultimately, we were left with a sample of 350 eligible patients.
We randomly selected 2,100 patients (six for every RVO patient) from the database matched with the study group in terms of gender, age (⬍ 50, 50 to 59, 60 to 69, and ⱖ70 years) and the date of ambulatory care visit as our comparison cohort. Again, patients with a prior diagnosis of RVO or stroke were not included in the comparison cohort.
Each patient was tracked from his or her index ambu-latory care visits for five years to distinguish all patients who had developed any type of stroke (ICD-9-CM codes 430-438). In addition, to calculate the stroke-free sur-vival time after the index ambulatory care visit for a five-year period, the data were also linked to death certificate data in Taiwan, with cases censored if indi-viduals died from nonstroke causes during that time (340 patients died from nonstroke causes: 67 from the study cohort and 273 from the comparison cohort).
Regression modeling also adjusted for patient’s age, gender, and the geographic location of the community in which the patient resided (Northern, Central, Eastern, and Southern Taiwan) as well as comorbidities. Selected co-morbid medical disorders included hypertension, diabetes, hyperlipidemia, and renal disease, because these conditions may exacerbate the risk of stroke.5,6,9 –11
● STATISTICAL ANALYSIS: The SAS statistical package SAS System for Windows, version 8.2 (SAS Institute Inc, Cary, North Carolina, USA) was used to perform the analyses in this study. Pearson 2tests were performed to examine differences between the two cohorts in terms of TABLE 1. Demographic Characteristics and Comorbid Medical Disorders for Retinal Vein Occlusion Patients and Comparison
Group Patients in Taiwan (n⫽ 2,450)
Variable
Retinal Vein Occlusion Group Comparison Group
P value
Total No. Column % Total No. Column %
Gender Male 187 53.4 1122 53.4 1.000 Female 163 46.6 978 46.6 Age (yrs) ⬍50 56 16.0 336 16.0 1.000 50 to 59 72 20.6 432 20.6 60 to 69 113 32.3 678 32.3 ⱖ70 109 31.1 654 31.1 Hypertension Yes 299 85.4 133 6.3 ⬍.001 No 51 14.6 1967 93.7 Diabetes Yes 152 43.4 65 3.1 ⬍.001 No 198 56.6 2035 96.6 Hyperlipidemia Yes 186 53.1 27 1.3 ⬍.001 No 164 46.9 2073 98.7 Renal disease Yes 88 25.1 10 0.5 ⬍.001 No 262 74.9 2090 99.5 Geographic region Northern 154 44.0 905 43.1 .732 Central 81 23.1 541 25.8 Southern 106 30.3 596 28.4 Eastern 9 2.6 58 2.7 Yrs⫽ years.
demographic characteristics, selected comorbid medical disorders, and the risk of developing stroke. The five-year stroke-free survival rate was then estimated using the Kaplan-Meier method, using the log-rank test to examine the differences in the risk of developing stroke between the two cohorts in different age groups. Thereafter, separate Cox proportional hazard regressions in different age groups were performed to compute the five-year stroke-free sur-vival rate after adjusting for possible confounding factors. A significance level of 0.05 for the regression coefficients was selected to determine the significance of predictors in the models.
RESULTS
TABLE 1 SHOWS THE DISTRIBUTIONS OF DEMOGRAPHIC
characteristics and comorbid medical disorders for these two cohorts. After matching for age, gender, and the date of the ambulatory care visit, patients with RVO were more likely to have comorbidities such as hypertension (P ⬍ .001), diabetes (P⬍ .001), hyperlipidemia (P ⬍ .001), and renal disease (P ⬍ .001) compared to patients in the comparison group. There was no significant difference in the distribution of geographic regions between the two cohorts.
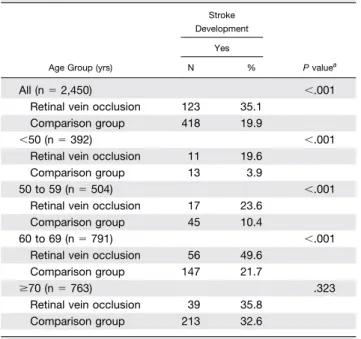
Table 2presents the distribution of stroke development during the five-year follow-up period between these two cohorts. Of the total sample of 2,450 patients, 541 patients
(22.1%) developed a stroke during the follow-up period, 123 (35.1% of the patients with RVO) from the study cohort and 418 (19.9% of the patients in the comparison group) from the comparison cohort. After categorization into the different age groups, we found that patients with RVO in the age group 60 to 69 years had the highest percentage (49.6%) of developing stroke during the fol-low-up period.
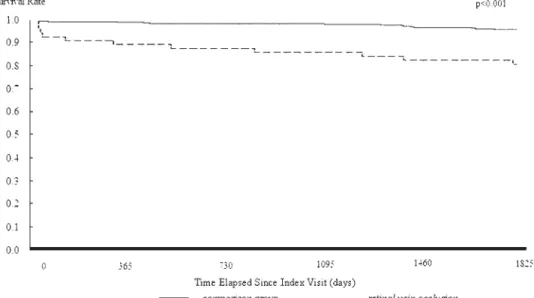
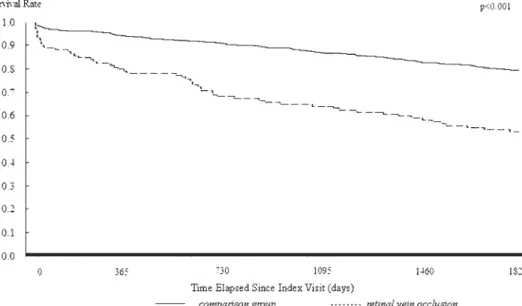
Table 2also shows the results of the log-rank tests. The log rank tests consistently indicated that patients with RVO had significantly lower five-year stroke-free survival rates than patients in the comparison group for the total sample and in the different age groups (all P ⬍ .001) except in the age group of ⱖ70 years (P ⫽ .323). The results of Kaplan-Meier survival analysis are presented in
Figures 1through 4.
Table 3provides details of the adjusted hazard ratios for stroke during the follow-up period by cohort for the total sample and in the different age groups. After adjusting for patient age, gender, and comorbid medical disorders, RVO was no longer a significant predictor for the development of stroke during the five-year follow-up period in the total sample or in the age groups of ⬍50 and 50 to 59 years. However, the regression model showed that RVO re-mained a statistically significant predictor for the develop-ment of stroke after adjusting for possible confounding factors among the age group of 60 to 69 years; the adjusted hazard of developing stroke during the five-year follow-up period was 2.34 (95% confidence interval [CI], 1.05 to 5.24; P⬍ .05) times greater for patients with RVO than for patients in the comparison group.
DISCUSSION
IN THIS STUDY, IN WHICH DATA ON 350 RVO PATIENTS
were analyzed, we found that RVO patients younger than 70 years had a significantly higher risk for stroke develop-ment in the five-year follow-up period. Our findings also confirmed that old age, hypertension, diabetes, hyperlipid-emia, and renal diseases are systemic risk factors for RVO. After adjusting for age, gender, and comorbid medical disorders (hypertension, diabetes, hyperlipidemia, and re-nal diseases), RVO was not associated with an increased risk of stroke development during the follow-up period among subjects at any age. However, RVO patients in the age group of 60 to 69 years were found to have a 2.34-fold (95% CI, 1.05 to 5.24) higher risk of suffering a stroke during the follow-up period than the matched comparison cohort.
Mansour and associates’ study7reported no higher risk of stroke development and mortality following CRVO occur-rence. Their study adjusted for age, gender, hypertension, and diabetes when making the comparison between the CRVO patients and the control cohort. This study did not perform a sub-analysis for different age groups. Tsaloumas TABLE 2. Distribution of Stroke Development During the
Five-Year Follow-up Period Between Retinal Vein Occlusion Patients and Comparison Group Patients
in Taiwan
Age Group (yrs)
Stroke Development P valuea Yes N % All (n⫽ 2,450) ⬍.001
Retinal vein occlusion 123 35.1 Comparison group 418 19.9
⬍50 (n ⫽ 392) ⬍.001
Retinal vein occlusion 11 19.6 Comparison group 13 3.9
50 to 59 (n⫽ 504) ⬍.001
Retinal vein occlusion 17 23.6 Comparison group 45 10.4
60 to 69 (n⫽ 791) ⬍.001
Retinal vein occlusion 56 49.6 Comparison group 147 21.7
ⱖ70 (n ⫽ 763) .323
Retinal vein occlusion 39 35.8 Comparison group 213 32.6 Yrs⫽ years.
a
and associates8found no statistical difference between the RVO group and the background population in the rates of stroke development, although there was a trend towards a greater number of deaths from stroke in the RVO group. The results of those two studies were similar to ours in terms of the total sample. However, subanalysis of our sample found that RVO patients in the age group of 60 to 69 years had a 2.34-fold (95% CI, 1.05 to 5.24) higher risk of suffering a stroke. When comparing the results of previous studies with those of ours, several differences in the methodology must be noted. First, those two previous studies relied upon data from a single hospital, which might not have been sufficiently representative to draw unequivocal conclusions. Second, there was a difference in the selection of the comparison group. In our study, we
matched patients’ age, gender, and date of ambulatory visit in selecting the comparison group. In Mansour and asso-ciates’ study, they matched age, gender, and race. In Tsaloumas and associates’ study, the comparison group was the general population in West Midland of the United Kingdom at 23 to 92 years of age (the age range of RVO patients in their study). They did not match other param-eters. Third, there was a difference in whether adjustment for confounding factors (eg, comorbid medical conditions) and a sub-analysis for different age groups were performed. Ueda and associates12examined 31 RVO patients with-out neurologic signs or symptoms using magnetic reso-nance imaging (MRI). They found that asymptomatic cerebral infarction was more common in these RVO patients than in the control group. The authors suggested
FIGURE 1. Stroke-free survival rates for retinal vein occlusion (RVO) and comparison group patients age <50 years in Taiwan.
that patients with retinal vascular obstruction (including RVO) should be ranked as a moderate risk group for stroke. Furthermore, patients with retinal vascular obstruction and hypertension were in a high-risk group for stroke. In a population-based study utilizing pooled data from the Atherosclerosis Risk in Communities Study and the Car-diovascular Health Study, it was found that RVO was associated with carotid artery plaque, which was a poten-tial source of ischemic stroke.13On the other hand, recent data from another population-based study did not confirm such an association.14
There are several other studies that focused on the issue of whether RVO is associated with an increased cerebro-vascular or overall mortality. Rubinstein and Jones15 fol-lowed the long-term prognosis in 143 RVO patients and
found that the number of deaths from vascular disease— cerebral or cardiac—was about double the national figure. In a study of 191 CRVO patients, Elman and associ-ates16found that the mortality was not higher in CRVO patients.
Utilizing the pooled data from two population-based cohorts, Cugati and associates17 found that RVO was not associated with cerebrovascular-related mortality among participants of all age groups, although it was found in men that the presence of RVO was associated with a nonsig-nificant 2.3-fold higher risk of cerebrovascular mortality for all ages. It should be noted that only 96 RVO patients were collected in that study, and seven of them died of cerebrovascular diseases. A truly statistically significant trend might be masked when the case number is not large
FIGURE 3. Stroke-free survival rates for RVO and comparison group patients age 60 to 69 years in Taiwan.
enough. Therefore, the possibility that RVO patients had a significantly higher risk of cerebrovascular mortality cannot be excluded based only on the results of that study. In another population-based study (Beijing Eye Study18), which analyzed 61 RVO patients at baseline from 4,335 subjects, RVO was significantly associated with an in-creased overall mortality rate in subjects⬍70 years or ⬍65 years old. In contrast, another study examining 329 BRVO patients from two secondary referral centers in Denmark found no significant difference in mortality between BRVO patients and the background population in all age groups.18It must be noted that stroke mortality (or overall mortality) is not equal to stroke development when inter-preting the results of these studies.
The workforce of ophthalmologists is adequate on the relatively small island of Taiwan, with an ophthalmologist-to-population ratio of 1:14 375 (cf 1:23 523 in the United Kingdom19). The barrier to medical access is negligible because the NHI System in Taiwan allows patients to visit any ophthalmology clinic or department of ophthalmology of any hospital freely without referral by a general practi-tioner, and patients pay only about US $5 to 15 at each visit (the per capita gross national product for Taiwan was US $14,721 in 200020). In view of the severity of the RVO disorder and the above-mentioned factors, we believe most patients with RVO would seek medical help soon after disease onset, and the number of untreated RVO cases would be small. This small number of untreated RVO cases TABLE 3. Adjusted Hazard Ratioaand 95% Confidence Interval for the Development of Stroke During the Five-Year Follow-up
Period for Retinal Vein Occlusion Patients and Comparison Group Patients in Taiwan
Variable Stroke Occurrence All (n⫽ 2,450) Age Group⬍50 (n⫽ 392) Age Group 50 to 59 (n⫽ 504) Age Group 60 to 69 (n⫽ 791) HR, 95% CI HR, 95% CI HR, 95% CI HR, 95% CI Cohort
Retinal vein occlusion 1.01 (0.65 to 1.57) 3.23 (0.73 to 14.35) 0.61 (0.17 to 2.19) 2.34b(1.05 to 5.24)
Comparison group (reference group) 1.00 1.00 1.00 1.00
Gender
Male 1.06 (0.87 to 1.30) 1.12 (0.45 to 2.71) 1.16 (0.66 to 2.05) 1.09 (0.78 to 1.51)
Female (reference group) 1.00 1.00 1.00 1.00
Geographic region
Northern (reference group) 1.00 1.00 1.00 1.00
Central 1.35 (1.07 to 1.72) 1.10 (0.30 to 4.01) 2.77c(1.34 to 5.72) 1.29 (0.87 to 1.92) Southern 0.99 (0.77 to 1.27) 1.95 (0.70 to 5.45) 2.13b(1.06 to 4.31) 0.79 (0.52 to 1.20) Eastern 1.53 (0.86 to 2.73) 4.88 (0.62 to 38.45) 2.42 (0.47 to 12.50) 1.96 (0.76 to 5.05) Hypertension Yes 2.05c(1.45 to 2.90) 0.48 (0.10 to 2.35) 2.66 (0.95 to 7.46) 1.88b(1.08 to 3.28) No (reference group) 1.00 1.00 1.00 1.00 Diabetes Yes 1.20 (0.82 to 1.75) 0.88 (0.15 to 5.10) 0.69 (0.25 to 1.96) 1.00 (0.55 to 1.82) No (reference group) 1.00 1.00 1.00 1.00 Hyperlipidemia Yes 1.40 (0.91 to 2.15) 2.69 (0.45 to 16.09) 2.22 (0.74 to 6.68) 1.31 (0.66 to 2.61) No (reference group) 1.00 1.00 1.00 1.00 Renal disease Yes 1.17 (0.70 to 1.93) 1.04 (0.16 to 7.02) 1.97 (0.60 to 6.42) 0.87 (0.39 to 1.93) No (reference group) 1.00 1.00 1.00 1.00 Age (yrs) ⬍50 (reference group) 1.00 50 to 59 1.96c(1.19 to 3.22) 60 to 69 4.80d(3.07 to 7.53) ⱖ70 7.09d(4.54 to 11.1)
CI⫽ confidence interval; HR ⫽ hazard ratio; yrs ⫽ years.
aAdjusted for patients’ gender, geographic region, hypertension, diabetes, hyperlipidemia, and renal disease. bP⬍ .05.
cP⬍ .01. dP⬍ .001.
would be categorized as non-RVO and would have a small chance of being selected as a part of the comparison cohort. This would minimally reduce the power of analysis. However, this study limitation also existed in previous hospital-based studies7,8 in which the patients sought medical help after they noted the onset of RVO, and the comparison group included subjects whose retinas were not examined. Besides, the interval between the RVO occur-rence and the presentation of the patients for examination in our study would be similar to or shorter than that of previous hospital-based studies.7,8 In the NHI System of Taiwan, for the purpose of auditing, the physician must key the diagnosis into the computer system during each med-ical visit and the data are sent to the NHI Bureau on-line immediately. Therefore, there will be no discrepancy in the date of actual visit and the date recorded in the national health statistics data.
The pathogenesis of RVO remains unclear but may be multifactorial. The postulated causes include thrombosis in venules resulting from compression by an adjacent athero-sclerotic arteriole, local alteration to blood flow from unfavorable physiological conditions, or hematological abnormalities.21–25Retinal blood vessels share similar an-atomic, physiological, and embryological characteristics to cerebral vessels. The retina is an extension of the dien-cephalon, and possesses a blood-retinal barrier that is analogous to the blood-brain barrier.26Pathologic changes in the retinal vessels may reflect similar changes in cerebral vessels.27 In addition, retinal vessels are unique in that they can be noninvasively and directly examined in vivo. Examination of signs in the retina might be a safe and easy way to help evaluate the risk of stroke and related cerebrovascular diseases. Our results that RVO occurrence was associated with a 2.34-fold higher risk of stroke development in the 60- to 69-year age group supports this view.
The underlying pathophysiological mechanisms respon-sible for RVO development may be dissimilar in different age groups. For example, retinal vasculitis (inflammation of the vessel) is an important cause of RVO in young adults.28 On the other hand, RVO in the elderly may be more related to atherosclerotic changes of neighboring arte-rioles. It is possible that some of the underlying pathophys-iological mechanisms (eg, atherosclerosis of all vessels in the entire body) have a stronger association with stroke development than others (eg, retinal vasculitis). There-fore, it is possible (or reasonable) that there is an age difference regarding the association of RVO with stroke. It is suggested that further study be performed to clarify the mechanisms involved in the higher risk of develop-ing stroke in RVO patients of the 60- to 69-year age group.
The findings of this study need to be interpreted in the context of the following limitations. First, diagnoses of RVO, stroke, or any other comorbid medical conditions that were totally dependent upon ICD codes may be less
accurate than those obtained through a standardized pro-cedure. This is a major limitation of this study as compared to studies that used standardized grading of retinal photo-graphs. However, the NHI Bureau of Taiwan randomly samples a fixed percentage of claims from every hospital and randomly interviews patients and reviews charts each year to verify the diagnosis validity and the quality of care. Any hospital with outlier charges or outlier practice patterns or that is suspected of malpractice faces the risk of an audit and subsequent heavy penalties by the NHI Bureau when discrepancies, overcharging, or malpractice are discovered. Second, the study found no association of RVO with stroke in the total sample, except in the 60- to 69-year subgroup. This may be attributable to chance. On the other hand, another possibility is that there were only approximately 10 stroke cases in the ⬍50- and 50- to 59-year age groups, and only the 60- to 69-year age group may have had sufficient power to detect the association. Further studies should be performed to confirm or refute our findings. Third, data on some variables, such as smoking, dietary habits, and body mass index, which might contribute to stroke development, were not available in our database. In addition, data on blood pressure and blood glucose levels were not available. Simply including hyper-tension and diabetes in the model might not adequately adjust for the confounding effects of blood pressure and blood glucose levels. These factors may have compromised our findings. Fourth, we used the ICD-9-CM diagnosis code 362.35 (CRVO) as the definition for CRVO and the diagnosis code 362.36 (venous tributary [branch] occlu-sion) as the definition for BRVO, with these two codes comprising RVO as a whole. However, since this was a retrospective study and the diagnosis codes were deter-mined by a variety of doctors, we have reason to believe that some of the CRVO and BRVO cases may have been given one of the more nonspecific diagnosis codes, includ-ing 362.3 (RVO) or 362.30 (RVO, unspecified). Such factors were, however, unlikely to invalidate our findings since less specific diagnoses would be expected to be independently given to RVO patients who would develop stroke and those who would not develop stroke in the follow-up period. Fifth, most of the residents in Taiwan are of Chinese ethnicity. Generalizability of the results to other racial/ethnic group is unclear given that strokes in Chinese/Asians might not be similar to strokes in other ethnic groups. Finally, our data only allowed us to trace the medical history of the sampled patients back to the year 1996. We cannot be certain that patients in the comparison group had no RVO prior to three years before the study baseline date. This could compromise our findings.
In summary, this study demonstrates that although RVO was not associated with an increased risk of subsequent stroke development among subjects of any age, RVO is a significant predictor for the development of stroke after adjusting for possible confounding factors
among the age group of 60 to 69 years. The possible causes include: an actually increased risk of stroke development in the 60- to 69-year group, chance find-ing, the presence of selection biases, small numbers of stroke patients in the ⬍50- and 50- to 59-year age
groups, or a lack of power in the ⱖ70-year group. Further studies are expected to see if our data can be replicated and to help clarify the underlying pathophys-iological mechanisms of RVO and their associations with stroke development.
THE AUTHORS INDICATE NO FINANCIAL SUPPORT OR FINANCIAL CONFLICT OF INTEREST. INVOLVED IN DESIGN AND conduct of study (J.-D.H., H.-C.L.); collection (S.-W.L., H.-C.L.), management (J.-D.H., S.-W.L., H.-C.L.), and analysis and interpretation of the data (J.-D.H., S.-W.L., H.-C.L.); and preparation (J.-D.H., H.-C.L.), review (J.-D.H., S.-W.L., H.-C.L.), and approval of the manuscript (J.-D.H., S.-W.L., H.-C.L.). The Institutional Review Board of Taipei Medical University Hospital waived the need for approval for this study because the Taiwan National Health Insurance Research Database (NHIRD) contains only de-identified secondary data and is published by the National Health Research Institute of the government for research purposes. This study adhered to the Declaration of Helsinki and all laws in Taiwan.
REFERENCES
1. The Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol 1997;115:486 – 491.
2. Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Arch Ophthalmol 1986;104:34 – 41.
3. Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Arch Ophthalmol 1996;114:1243–1247. 4. Eye Disease Case-Control Study Group. Risk factors for
branch retinal vein occlusion. Am J Ophthalmol 1993;116: 286 –296.
5. Elkind MS, Sacco RL. Stroke risk factors and stroke preven-tion. Semin Neurol 1998;18:429 – 440.
6. Goldstein LB, Adams R, Becker K, et al. Primary prevention of ischemic stroke: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Circulation 2001;103:163–182.
7. Mansour AM, Walsh JB, Henkind P. Mortality and morbid-ity in patients with central retinal vein occlusion. Ophthal-mologica 1992;204:199 –203.
8. Tsaloumas MD, Kirwan J, Vinall H, et al. Nine year follow-up study of morbidity and mortality in retinal vein occlusion. Eye 2000;14:821– 827.
9. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the cardiovascular system. Circulation 2007;116: 85–97.
10. Baker ML, Hand PJ, Wang JJ, Wong TY. Retinal signs and stroke: revisiting the link between the eye and brain. Stroke 2008;39:1371–1379.
11. Wong TY, Mitchell P. The eye in hypertension. Lancet 2007;369:425– 435.
12. Ueda Y, Kanazawa S, Ohira A, et al. Retinal vascular obstruction and asymptomatic cerebral infarction. Jpn J Ophthalmol 2002;46:209 –214.
13. Wong TY, Larsen EK, Klein R, et al. Cardiovascular risk factors for retinal vein occlusion and arteriolar emboli: the Atherosclerosis Risk in Communities and Cardiovascular Health studies. Ophthalmology 2005;112:540 –547. 14. Cheung N, Klein R, Wang JJ, et al. Traditional and novel
cardiovascular risk factors for retinal vein occlusion: the multi-ethnic study of atherosclerosis. Invest Ophthalmol Vis Sci. Forthcoming.
15. Rubinstein K, Jones EB. Retinal vein occlusion: long-term prospects: 10 years’ follow-up of 143 patients. Br J Ophthal-mol 1976;60:148 –150.
16. Elman MJ, Bhatt AK, Quinlan PM, Enger C. The risk for systemic vascular diseases and mortality in patients with central retinal vein occlusion. Ophthalmology 1990;97: 1543–1548.
17. Cugati S, Wang JJ, Knudtson MD, et al. Retinal vein occlusion and vascular mortality: pooled data analysis of 2 population-based cohorts. Ophthalmology 2007;114:520 – 524.
18. Xu L, Liu WW, Wang YX, Yang H, Jonas JB. Retinal vein occlusions and mortality: the Beijing Eye Study. Am J Ophthalmol 2007;144:972–973.
19. Black P. Medical workforce in the hospital eye service. Available at: http://www.rcophth.ac.uk/docs/profstands/oph-thalmic-services/WorkforcePlanningOct2005.pdf. Accessed: July 25, 2008.
20. Data from Directorate-General of Budget, Accounting and Statistics, Executive Yuan, Republic of China (Taiwan). Available at: http://www.stat.gov.tw/public/Attachment/ 71231865371.XLS.Accessed: July 25, 2008.
21. Christoffersen N, Gade E, Knudsen L, Juel K, Larsen M. Mortality in patients with branch retinal vein occlusion. Ophthalmology 2007;114:1186 –1189.
22. Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res 2005;24: 493–519.
23. Greiner K, Hafner G, Dick B, Peetz D, Prellwitz W, Pfeiffer N. Retinal vascular occlusion and deficiencies in the protein c pathway. Am J Ophthalmol 1999;128:69 –74.
24. Gottlieb JL, Blice JP, Mestichelli B, Konkle BA, Benson WE. Activated protein C resistance, factor V Leiden, and central retinal vein occlusion in young adults. Arch Ophthalmol 1998;116:577–579.
25. Marcucci R, Bertini L, Giusti B, et al. Thrombophilic risk factors in patients with central retinal vein occlusion. Thromb Haemost 2001;86:772–776.
26. Tso MO, Jampol LM. Pathophysiology of hypertensive reti-nopathy. Ophthalmology 1982;89:1132–1145.
27. Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvascula-tures. J Anat 2005;206:319 –348.
28. Fong AC, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol 1993;37:393– 417.
Biosketch
Jau-Der Ho, MD, PhD, completed medical school from the National Taiwan University (NTU), and ophthalmology training at NTU Hospital. Dr Ho obtained his PhD degree from Chang-Gung University, Taiwan. He is a subspecialist in vitreoretinal diseases, cataract and vitreoretinal surgery. Dr Ho’s research interests include retinal cell biology and ocular pharmacology. He is currently an Associate Professor and Chair of the Department of Ophthalmology, Taipei Medical University Hospital of Taipei, Taiwan.
Biosketch
Shiow-Wen Liou, MD, PhD, completed medical school from the National Taiwan University (NTU), and did ophthalmology training at NTU Hospital, Taipei, Taiwan. Dr Liou received her PhD degree at Dokkyo Medical University in Japan and MHS degree from Johns Hopkins University, Baltimore, Maryland. Dr Liou is a subspecialist in cataract, strabismus, and oculoplastic surgery. Currently, Dr Liou serves as the superintendent of Zhongxing branch, Taipei City Hospital, and Professor of Ophthalmology in Taipei Medical University.