Research Notes
353 Received for publication April 17, 1997.
Accepted for publication October 20, 1998.
1Present address: Chang Jung University, No. 396, Sec. 1, Chang
Jung Road, Tatan Tsun, Kway Jen, Tainan, Taiwan, R.O.C.
2To whom correspondence should be addressed: bjs12@cornell.edu 3GIBCO Laboratories, Grand Island, NY 13072. A modified D-MEM
devoid of arginine was used in these studies.
4HyClone Laboratories, Inc., Logan, UT 84321.
Abbreviation Key:D-MEM = Dulbecco’s modified Eagle medium; FBS = fetal bovine serum; LPS = lipopolysaccharide.
The Recycling of L-Citrulline to L-Arginine in a
Chicken Macrophage Cell Line
C.-L. SU1 and R. E. AUSTIC2
Department of Animal Science, Cornell University, Ithaca, New York 14853
ABSTRACT L-Arginine is the only biological sub-strate of nitric oxide synthase in a reaction yielding NO and citrulline as co-products. The resynthesis of L-arginine from L-citrulline has been observed in murine macrophages. However, it is not known whether avian macrophages have a similar capacity for the synthesis of arginine. The present studies were carried out to determine whether L-citrulline can support NO
(meas-ured as nitrite) production in the HD11 cell, a chicken macrophage cell line. When added to media lacking L-arginine, L-citrulline supported a low level of nitrite accumulation: about 4 to 11% of the amount of nitrite formed from an equivalent concentration of L-arginine. Aspartic acid was not limiting for NO production from citrulline.
(Key words: nitric oxide, nitrite, HD11 cell, citrulline, aspartic acid)
1999 Poultry Science 78:353–355
INTRODUCTION
Nitric oxide is a cytotoxic agent of macrophages (Stuehr and Marletta, 1987; Hibbs et al., 1988; Marletta et
al., 1988) synthesized from L-arginine (Hibbs et al.,
1987b), the only known physiological substrate. Cul-tured mouse (Baydoun and Mann, 1994) and rat (Wu and Brosnan, 1992) macrophages have been shown to synthesize L-[guanidino-14C]arginine from L-[ureido-14C]citrulline, indicating the presence of argininosuc-cinate synthase and argininosuccinase activities in these cells. The addition of L-citrulline to arginine-devoid culture media has resulted in NO generation in murine macrophages, indicating further that significant recy-cling of citrulline to arginine can occur (Baydoun and Mann, 1994; Nussler et al., 1994; Norris et al., 1995).
It is not known whether chicken macrophages can convert citrulline to arginine. Experiments were carried out, therefore, to determine whether NO is produced when L-citrulline is substituted for L-arginine in culture media for chicken macrophages.
MATERIALS AND METHODS
Cells of the chicken macrophage cell line HD11 (Beug
et al., 1979) were maintained in culture and plated at a
density of 106 cells in 24-well plates as previously described (Su and Austic, 1998). Adhered cells were cultured for 24 h in (Dulbecco’s modified Eagle medium (D-MEM)3+ 10% fetal bovine serum (FBS)4containing 1 mg/mL of Escherichia coli lipopolysaccharide (LPS) and different concentrations of arginine and citrulline. Arginine concentrations ranged from 0 to 3 mM and L-citrulline concentrations ranged from 0 to 10 mM in the first experiment. Both amino acids were included in the medium at 0.1 or 2.0 mM concentrations in the second experiment, which also included aspartic acid as a variable. Three culture wells per treatment were used in the first experiment and two or three wells per treatment were used in the second experiment. A final experiment, using three culture wells per treatment in each of two complete blocks of time, was carried out to determine the effect of 0.4 mM L-citrulline on nitrite production when the aspartic acid concentration of the medium was varied in 0.2 mM increments from 0 to 1.2 mM.
Nitrite accumulation in culture media was used as the indication of NO synthesis (Hibbs et al., 1987a). The nitrite assay was based on the procedure of Ding et al. (1988) with minor modifications.
Differences among treatment means were determined by Duncan’s multiple range test (Duncan, 1955).
SU AND AUSTIC 354
FIGURE 1. Nitrite accumulation in culture media containing various concentrations of L-arginine (A) or L-citrulline (B). Cells were activated with lipopolysaccharide (LPS) (1 mg/mL) in the presence of various concentrations of L-arginine (0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 1.0, and 3.0 mM) or L-citrulline (0.0, 0.2, 0.4, 0.6, 1.0, 3.0, 6.0, and 10.0 mM). Nitrite accumulation in culture media was determined after 24 h of culture. Results are means of three observations. Pooled SEM = 2.06 for arginine levels and pooled SEM = 0.98 for citrulline levels.
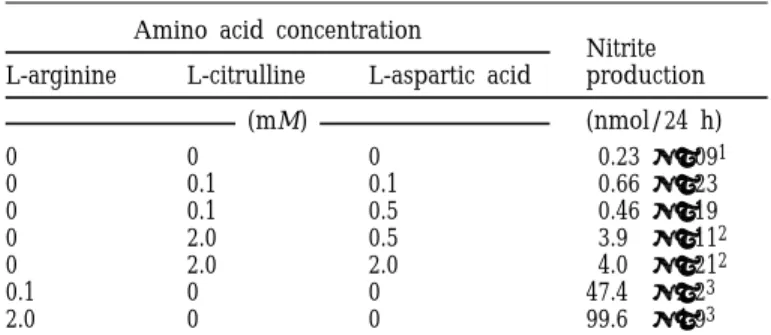
TABLE 1. Comparison of arginine and citrulline as precursors of NO in HD11 cells
1Mean±SD of two observations for all treatments lacking citrulline
and three observations for those involving citrulline.
2Significantly different from the group not supplemented with
arginine, citrulline, and aspartic acid (P < 0.05).
3Significantly different from citrulline-supplemented groups (P <
0.05).
Amino acid concentration
Nitrite production L-arginine L-citrulline L-aspartic acid
(mM) (nmol/24 h) 0 0 0 0.23 ±0.091 0 0.1 0.1 0.66 ±0.23 0 0.1 0.5 0.46 ±0.19 0 2.0 0.5 3.9 ±0.112 0 2.0 2.0 4.0 ±0.212 0.1 0 0 47.4 ±2.23 2.0 0 0 99.6 ±4.93
RESULTS
The amount of nitrite that accumulated in the culture medium increased significantly (P < 0.05) at each concentration of arginine up to 0.4 mM, at which concentration 135 nmol of nitrite accumulated in the medium during 24 h of cell culture (Figure 1A). The accumulation of nitrite was similar at 0.5 and 1 mM arginine but declined slightly (P < 0.05) at 3 mM arginine. Only 5.8 nmol of nitrite accumulated when 0.4 mM L-citrulline was included in the medium (Figure
1B). Nitrite accumulation increased to 15 nmol as the L-citrulline concentration of the medium was raised to 3 and 6 mM and declined slightly, but significantly (P < 0.05), when the concentration of citrulline was raised from 6 to 10 mM. The yield of nitrite from 1 and 3 mM citrulline was approximately 8 and 10% of the yield of nitrite from 1 and 3 mM L-arginine, respectively. The maximum accumulation of nitrite from citrulline (i.e., at 3 and 6 mM) was 11% of the maximum from arginine (i.e., at 0.4, 0.5, and 1.0 mM). In the second experiment (Table 1), the maximum yield of nitrite from 2 mM citrulline was 3.7% of the yield obtained from 2 mM L-arginine. The yield did not differ (P > 0.05) when media contained 0.5 mM or 2 mM L-aspartic acid. In the last experiment (data not shown) the accumulations of nitrite in the medium did not differ (P > 0.05) among treatments, ranging from 0 to 1.2 mM of aspartic acid. The mean accumulation of nitrite from 0.4 mM citrulline at all concentrations of aspartic acid was 4.3 nmol/24 h.
DISCUSSION
The results of the present studies suggest that L-citrulline is converted to a limited extent to L-arginine and can serve as a precursor of NO in HD11 cells. Nitrite formation in the murine monocyte/macrophage cell line J774 is about 8 nmol after 24 h of culture in the presence of 0.4 mM L-citrulline (Baydoun and Mann, 1994), which is about twice the amount of nitrite formed in HD11 cells. More nitrite (∼ 14 nmol/12 h) is formed by murine macrophage cell line, RAW 264.7 cultured in media containing 0.5 mM L-citrulline (Nussler et al., 1994), but little, if any, nitrite is formed by murine peritoneal macrophages cultured in media containing 0.1 mM L-citrulline (Wu and Brosnan, 1992). Although the yield of nitrite from L-citrulline is only slightly lower, the efficiency of the apparent recycling of L-citrulline to L-arginine in HD11 cells may be much lower than in J774 cells because the former appear to
RESEARCH NOTE 355 produce less nitrite from citrulline, but have a greater
capacity for nitrite synthesis. Only 70 nmol of nitrite was formed in J774 cells, for example, when the concentra-tion of L-arginine was 0.4 mM, but 135 nmol of nitrite was formed in HD11 cells. The efficiency of apparent recycling in HD11 cells appears to be lower than that of RAW 264.7 cells because the latter have high yield of nitrite from citrulline, but nitrite accumulation in media containing 0.4 mM L-arginine is only slightly higher (∼150 nmol/24 h) than that of HD11 cells (Nussler et al., 1994).
Aspartic acid, the source of nitrogen for amination of the ureido group of the citrulline molecule in vivo, does not appear to limit the conversion of citrulline to arginine by HD11 cells cultured in D-MEM.
Citrulline is a product of NO synthase activity. The ability of chicken macrophages to recycle citrulline to arginine may be important in the cellular dynamics of arginine metabolism in macrophages, especially when dietary arginine concentration is low or the availability of arginine to the macrophage is otherwise limited.
ACKNOWLEDGMENTS
This research was supported, in part, by a gift from Degussa Corp., Kennesaw, GA 30144. The authors wish to thank Karen Golemboski and Rodney R. Dietert, Department of Microbiology and Immunology, College of Veterinary Medicine, Cornell University for provid-ing the initial stock of HD11 cells for these experiments and for their technical advice.
REFERENCES
Baydoun, A. R., and G. E. Mann, 1994. Selective targeting of nitric oxide synthase inhibitors to system y+ in activated macrophages. Biochem. Biophys. Res. Commun. 200: 726–731.
Beug, H., A. Von Kirchbach, G. Doderlein, J.-F. Conscience, and T. Graf, 1979. Chicken hematopoietic cells
trans-formed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentia-tion. Cell 18:375–390.
Ding, A. H., C. F. Nathan, and D. J. Stuehr, 1988. Release of reactive nitrogen intermediates and reactive oxygen inter-mediates from mouse peritoneal macrophages. J. Im-munol. 141:2407–2412.
Duncan, D. B., 1955. Multiple range and multiple F tests. Biometrics 11:1–42.
Hibbs, J. B., Jr., R. R. Taintor, and Z. Vavrin, 1987a. Macrophage cytotoxicity: Role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235: 473–476.
Hibbs, J. B., Jr., R. R. Taintor, Z. Vavrin, and E. M. Rachlin, 1988. Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem. Biophys. Res. Commun. 157: 87–94.
Hibbs, J. B., Jr., Z. Vavrin, and R. R. Taintor, 1987b. L-Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J. Immunol. 138:550–565.
Marletta, M. A., P. S. Yoon, R. Iyengar, C. D. Leaf, and J. S. Wishnok, 1988. Macrophage oxidation of L-arginine to nitrite and nitrate: Nitric oxide is an intermediate. Biochemistry 27:8706–8711.
Norris, K. A., J. E. Schrimpf, J. L. Flynn, and S. M. Morris, Jr., 1995. Enhancement of macrophage microbicidal activity: Supplemental arginine and citrulline augment nitric oxide production in murine peritoneal macrophages and pro-mote intracellular killing of Trypanosoma cruzi. Infect. Immun. 63:2793–2796.
Nussler, A. K., T. R. Billiar, Z.-Z. Liu, and S. M. Morris, Jr., 1994. Coinduction of nitric oxide synthase and ar-gininosuccinate synthetase in a murine macrophage cell line. J. Biol. Chem. 269:1257–1261.
Stuehr, D. J., and M. A. Marletta, 1987. Synthesis of nitrite and nitrate in murine macrophage cell lines. Cancer Res. 47: 5590–5594.
Su, C.-L., and R. E. Austic, 1998. The utilization of dipeptides containing L-arginine by chicken macrophages. Poultry Sci. 77:1852–1857.
Wu, G., and J. T. Brosnan, 1992. Macrophages can convert citrulline into arginine. Biochem. J. 281:45–48.