licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the
article (e.g. in Word or Tex form) to their personal website or
institutional repository. Authors requiring further information
regarding Elsevier’s archiving and manuscript policies are
encouraged to visit:
Isolation and characterization of a neural progenitor cell line
from tilapia brain
Chiu-Ming Wen
a,⁎
, Yeong-Hsiang Cheng
b, Ya-Fen Huang
a, Chung-Shung Wang
aa
Department of Life Sciences, National University of Kaohsiung, Kaohsiung, Taiwan, ROC
b
Department of Animal Science, National I-Lan University, I-Lan, Taiwan, ROC
Received 19 September 2007; received in revised form 16 November 2007; accepted 19 November 2007 Available online 23 November 2007
Abstract
Astroglial cell lines have many applications for advancing neural developmental and functional studies. However, few astroglial cell lines have been reported from fish. In this study, we report the characterization of the immortal cell line TB2 isolated from adult tilapia brain tissue. The cell line was established at 25 °C in L15 medium supplemented with 15% fetal bovine serum. Most of the cells displayed a fibrous morphology and were immunoreactive for A2B5 antigen, glial fibrillary acidic protein (GFAP), keratin, vimentin, and the gap junction protein connexin 43 (Cx43). They weakly expressed glutamine synthetase (GS), S100 protein, and the neural stem cell markers Sox2 and brain lipid binding protein (BLBP). In contrast to astroglia in vivo, most TB2 cells also expressed galactocerebroside (GalC), substance P (SP), and tyrosine hydroxylase (TH). By immunoblot and RT-PCR, the cells also expressed myelin basic protein (MBP), proteolipid protein (PLP), and Cx35. On a poly-L-lysine-coated substrate in vitro, TB2 cells showed increases in neuronal dopamine decarboxylase (DDC) and microtubule-associated protein 2 (MAP2), indicating that they can initiate differentiation into neurons. Taken together, the results suggest that TB2 cells are astroglial progenitor cells (neural stem cells) and may develop into oligodendrocytes and neurons in a suitable environment. The present study advances our knowledge of fish astroglia. However, the factors that affect neural development in fish remain unknown, as do the characteristics of the intermediate differentiation stages between stem cells and mature nerve cells. The TB2 cell line will promote these investigations.
© 2007 Elsevier Inc. All rights reserved.
Keywords: A2B5; Connexin; GFAP; Glutamine synthetase; Oligodendrocyte; Tyrosine hydroxylase
1. Introduction
Recent studies of the mammalian central nervous system (CNS) have demonstrated that certain astroglial cells, such as radial glial cells in the ependyma and subependyma regions, can generate neurons as well as astrocytes and oligodendrocytes, and suggest that these cells may be neural stem cells (Doetsch, 2003; Kristen and McCarthy, 2006). In contrast to mammals, radial glial cells are the most abundant astroglial cells in the fish CNS, even in adulthood (Kálmán, 1998). Although it is known that radial glial cells generate neurons in the fish CNS throughout the lifespan of the fish (Adolf et al., 2006; Pellegrini
et al., 2007), it remains unclear whether fish astroglial cells besides radial glial cells can generate neurons. Furthermore, little is known about the specific molecular expression profiles of progenitor, intermediate, and mature neural cells in the fish CNS. Knowledge of the molecular markers of the cells benefits us to understand the fish CNS development.
Astroglial lineage cells express a unique intermediate filament molecular marker, glial fibrillary acidic protein (GFAP) (Dahl and Bignami, 1973). In mammals, glutamine synthetase (GS) and S100 protein have been used as mature astrocyte-specific markers, although the proteins are also present in some progenitor cells (Akimoto et al., 1993; Bass et al., 1998; Deloulme et al., 2004) and oligodendrocytes (Cammer, 1990; Richter-Landsberg and Heinrich, 1995). Immature oligodendrocytes contain A2B5, O1, or O4 antigens on their surface, whereas mature oligodendrocytes express galactocerebroside (GalC), myelin basic protein (MBP), and
Comparative Biochemistry and Physiology, Part A 149 (2008) 167–180
www.elsevier.com/locate/cbpa
⁎ Corresponding author. Department of Life Sciences, National University of Kaohsiung, No. 700, Kaohsiung University Rd., Nan-Tzu Dist., 811, Kaohsiung, Taiwan, ROC. Tel.: +886 7 5919231.
E-mail address:wenchiumin@nuk.edu.tw(C.-M. Wen).
1095-6433/$ - see front matter © 2007 Elsevier Inc. All rights reserved. doi:10.1016/j.cbpa.2007.11.005
proteolipid protein (PLP) (Baumann and Pham-Dinh, 2001). Neuronal precursors have 5A5 and HuC/D, and neurons exhibit microtubule-associated protein 2 (MAP2), acetylated tubulin, and neurofilament proteins (Eddé et al., 1991; Li et al., 2004). Gap junction proteins also can be used to classify nerve cells. Connexin 43 (Cx43) is found in astrocytes, radial glia cells, and ependymal cells, whereas Cx36 (Cx35 in fish) is found in neurons and Cx32 is present in oligodendrocytes (Belliveau and Naus, 1994; Bittman and LoTurco, 1999; Rash et al., 2001; Wallraff et al., 2004). A2B5+oligodendrocyte-type 2 astrocyte (O-2A)–like cells from trout (Jeserich and Stratmann, 1992; Sivron et al., 1992) and GFAP+ multipolar cells from goldfish (Bastmeyer et al., 1989) also demonstrate immature oligoden-drocyte markers, such as O1, O4, and 6D2 antigens and myelin glycoprotein IP2 in vitro. Radial glial cells in the fish CNS, as in the mammalian CNS, are defined by their long radial processes containing vimentin and GFAP. In mammals, several specific markers have been used to clarify subtypes of radial glia and intermediate cell types, such as A2B5+ glial–restricted precursors and 5A5+ neural-restricted precursors (Li et al., 2004); however, little is known about the subtype of fish radial glia. In vivo, fish radial glia (including Müller cells in the retina) can be defined by expression of aromatase B, astrocyte-specific glutamate transporter (GLAST), brain lipid binding protein (BLBP), Pax6 transcription factor, and Sox2 (Kinoshita et al., 2005; Adolf et al., 2006; Raymond et al., 2006; Pellegrini et al., 2007), but we know nothing regarding the in vitro situation of these cells.
Neural cell lines, particularly immortal cell lines, are beneficial because they largely produce pure neuron or glial cell populations and thus allow the study of biochemical and physiological functions. Until now these cell lines have been limited to mammals, and studies on fish neural development have mainly depended on immunohistochemistry. However, the cell-specific markers that have been useful in identifying mammalian neural cells are also valuable for recognizing fish neural cells. Here we report the molecular characteristics of a glial cell line isolated from adult tilapia brain using immuno-cytochemistry, immunoblotting, and reverse transcription-PCR (RT-PCR). Many of the fibrous cells had uni- or bipolar radial processes that labeled with anti-GFAP and anti-vimentin antibodies and were similar to radial glial cells. A subpopulation of the cells additionally labeled with anti-A2B5 and thus may be recognized as O-2A progenitor cells. The present study illustrates that an astroglial cell line from adult fish brain has the potential to generate oligodendrocytes and neurons. 2. Materials and methods
2.1. Cell cultures
Tilapia (Oreochromis sp.) about 13 cm in length obtained from a local breeding farm were maintained in circulating fresh water tanks at ambient temperature (25–30 °C). Fish were anaesthetized with 3-aminobenzoic acid ethyl ester (MS-222, 1:1,000 dilution; Sigma, St. Louis, MO, USA) and decapitated aseptically, then the entire brain was removed, minced, and
washed several times in an antibiotic solution (Ca2+- and Mg2+ -free Dulbecco's phosphate-buffered saline (PBSA) containing streptomycin (500μg/mL) and penicillin (500 IU/mL)). A cell suspension was produced by grinding the tissue fragments with two sterilized ground-slides. The suspension was centrifuged at 300 g for 10 min at 4 °C and the cell pellet was resuspended in 10 mL L15 growth medium (Gibco, Grand Island, NY, USA) containing antibiotics (penicillin (50 IU/mL) and streptomycin (50μg/mL)) and 15% fetal bovine serum (FBS, Hyclone, Logan, UT, USA). The cells (5 mL each) were seeded into 25-cm2tissue culture flasks (Nunc, Roskilde, Denmark), and incubated at 25 °C. The medium was replaced weekly until passage.
The primary cultures were passaged 6 weeks after the culture was initiated. The cells were detached using 0.25% trypsin-EDTA solution (Sigma) and the subculture split 1:2 for four passages; at the fifth passage, cells were split 1:4. The cells were designated TB2.
2.2. Antibodies
The primary antibodies and their concentrations are listed in Table 1. Specificities of the antibodies have been described previously (Dahl et al., 1985; Mastronardi et al., 1993; Vecino et al., 1997; Mack et al., 1998; Wang, 2001; Sueiro et al., 2003; Raymond et al., 2006). Anti-keratin was used to identify epithelial cells and antibodies against GFAP, Cx43, GS, and
Table 1
Primary antibodies used, target, source, and dilution for immunocytochemistry Antigen Target Species and clone Company Dilution A2B5 Oligodendrocyte
progenitor cells
Mouse 105 Sigma 1:100
Human BLBP Radial glia Rabbit polyclonal Abcam 1:1000 Human DDC Dopamine neurons Mouse
DDC-109
Sigma 1:200
Human Cx36 Neuronal gap junctions
Rabbit polyclonal Zymed 1:200
Human Cx43 Astroglial gap junctions
Rabbit polyclonal Sigma 1:200
GABA GABA neurons Mouse GB-69 Sigma 1:200 Bovine GalC Young and adult
oligodendrocytes Rabbit polyclonal
Chemicon 1:100
Porcine GFAP Astroglia Mouse GA5 NeoMarker 1:200 Sheep GS Astrocytes Mouse GS-6 Chemicon 1:200 Human HuC/D Neuronal precursor cells Mouse 16A11 Molecular probe 1:100 Human Keratin
Epithelial cells Mouse C-11 NeoMarker 1:200
Bovine MAP2 (2a + 2b)
Neurons Mouse AP20 NeoMarker 1:200
Human MBP Mature oligodendrocytes
Rabbit polyclonal NeoMarker 1:200
Bovine NF-200
Neurons Rabbit polyclonal Sigma 1:1000
Porcine NF-H Neurons Mouse NF-01 Acris 1:200 Human Sox2 Neural stem cells Rabbit polyclonal Abcam 1:1000 Bovine S100 Astrocytes Rabbit
polyclonal
NeoMarker 1:200
Human TH Dopamine neurons Mouse TH-2 Sigma 1:200 Porcine
Vimentin
Neural progenitor cells
S100 to specify astroglial cells. Anti-A2B5 labels oligoden-drocyte progenitor cells, including O-2A progenitors, and anti-GalC and anti-MBP antibodies identify more mature oligoden-drocytes. Anti-vimentin, anti-BLBP, anti-HuC/D, and anti-Sox2 antibodies were used to recognize radial glial cells. Anti-MAP2, anti-neurofilament heavy protein (anti-NF-H and anti-NF200), and anti-Cx36 antibodies were used to identify neurons. Specific neuronal subtypes were identified with anti-TH and anti-dopamine decarboxylase (DDC) for dopaminergic neurons and anti-GABA for GABAergic neurons. Secondary antibodies used in this study were FITC-conjugated goat antibodies against rabbit IgG, mouse IgG, and mouse IgM and TRITC-conjugated goat anti-mouse IgM for immunofluorescence staining. For immunoperoxidase staining, the secondary antibodies were HRP-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG. All the secondary antibodies, TrueBlue™ and diaminobenzidine (DAB) peroxidase substrate solutions were purchased from KPL (Gaithersburg, MD, USA).
2.3. Immunocytochemistry
A volume containing 2–3×104TB2 cells was plated on 12-mm diameter uncoated or poly-L-lysine-coated (0.01% w/v;
Sigma) cover slips (Glaswarenfabrik, Sondheim, Germany) in 4-well plates (Nunc) and cultured at 25 °C for two days. Staining for surface antigens, such as A2B5 and GalC, was carried out in live cultures or after cell fixation in PBSA-buffered formalin (10%, v/v) for 10 min. For other antigens, cells were fixed 10 min in cold methanol for immunofluores-cence or fixed 20 min in methanol containing 0.3% H2O2for
immunoperoxidase cytochemistry. Fixed cells were rinsed thoroughly in PBST (0.05% (v/v) Tween 20 in PBSA), blocked for 15 min with blocking buffer (10% BSA in PBSA), and then incubated with primary antibodies diluted in blocking buffer for 1 h at room temperature or at 4 °C overnight. After rinsing three times with PBST, the cells were incubated with a species-specific secondary antibody diluted in blocking buffer for 30 min. Negative controls (omission of the primary antibody or use of epithelial cells from fish fin in place of TB2 cells) were included in each experiment.
In double-labeling experiments, cells were fixed and labeled with mouse anti-GFAP and HRP-conjugated anti-mouse IgG, followed by incubation in DAB solution to develop the color reaction. After thorough rinsing, the cells were incubated with 0.3% H2O2solution to inhibit the peroxidase, then sequentially
incubated with rabbit anti-S100 and HRP-conjugated anti-rabbit IgG and then visualised with TrueBlue™. In some cases, the cells were counterstain with orcein (KPL). Double immunofluores-cence using secondary antibodies conjugated to FITC and TRITC failed due to emission bleed-through between channels with our epifluorescence microscopes (Olympus IX51 and CKX41). 2.4. Immunoblotting
Brain tissues from tilapia and mouse, were used as positive controls and tilapia spleen tissue as a negative control. The tissues were washed with PBSA and homogenized separately
with a glass homogenizer in extraction buffer containing NaCl (0.15 M), Tris–HCl (0.01 M; pH 7.4), EDTA (2 mM), SDS (1%), and protease inhibitor cocktail (Set I; Merck Ltd., Taipei, Taiwan). TB2 cells grown in 75-cm2flasks (Nunc) for 60–80 passages were rinsed three times with PBSA and collected by scraping into the extraction buffer. The suspensions were centrifuged at 40,000 g for 30 min at 4 °C and the supernatants were collected. Protein concentration was measured with a protein assay kit (Bio-Rad, Hercules, CA, USA).
The extracts were combined with an equal volume of 2× electrophoresis sample buffer containing mercaptoethanol. Proteins (10 μg/lane) were separated by electrophoresis on a 10% SDS-polyacrylamide slab gel and then electrophoretically transferred to a polyvinylidene fluoride transfer membrane (Pall, Taipei, Taiwan). Blots were blocked in the blocking buffer for 1 h and then incubated with the Cx43, Cx36, anti-GFAP, anti-GS, anti-NF200, anti-NF-H, anti-MBP, anti-S100, or anti-TH for an additional 1 h at room temperature. Blots were thoroughly washed with PBST and incubated for 1 h at room temperature with HRP-conjugated anti-mouse IgG (1:20,000) or anti-rabbit IgG (1:50,000). After thorough rinsing, the blots were visualized by chemiluminescence (SuperSignal® West Pico; Pierce Biotechnology, Inc., Rockford, IL, USA) or TMB™ peroxidase substrate (KPL).
2.5. RT-PCR
Total RNA from tilapia brain tissue and TB2 cells at passage 60–80 were isolated with TRIZOL (Invitrogen, Taipei, Taiwan) and used in RT-PCR studies. RNA concentration and purity were determined by measuring the absorbance at 260 nm. RNA (2μg/ sample) was used to generate cDNA using the MMLV-RT kit (Promega, Taipei, Taiwan). PCR reactions were performed in Taq DNA Pol 2.0× Master Mix (Ampliqon, Trugene Diagnos-tics, Inc., Taipei, Taiwan) containing 1μM each of forward and reverse primers. The PCR thermocycle consisted of an initial denaturation at 95 °C for 1 min, followed by 35 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, with a final elongation at 72 °C for 5 min. The forward and reverse primer sequences and the length of amplified products are as follows: Cx35 (a/tgtg/ tgtggtgatcttccg, tgtcttctccgtgggtct, 619 bp); Cx43 (ggctgctcatccc-caactg, gactgctcattctgctgctgg, 325 bp); GFAP (tggtatcgctcgaagtt-tgc, aggtcctggtactcctgca, 290 bp); GS (aagggctccaacagcgacat, cagccagcaccgttccagtt, 537 bp); PLP (caaatgtttccggtcagg, ccgaa-ggtctgcttgaca, 345 bp). The amplicons that had the predicted size were extracted and purified using a Gene-Spin 1-4-3 DNA Ex-traction kit (Protech Technology Enterprise Co., Taipei, Taiwan), and sequenced using an ABI 3730 sequencer (Genomics Biosci & Tech Co., Taipei, Taiwan). The sequences were subjected to BLASTN search.
3. Results
3.1. Establishment of the TB2 cell line
Cell lines and immortal cell lines especially, as they have a homogeneous cell population, are easy to maintain, and produce
large numbers of cells, are useful for neural developmental and functional studies. To develop a fish astroglial cell line, we cultured tilapia brain that was dissociated by mechanical means. One day after plating, cells and cell clumps were observed adhering to the culture flasks. The cultures usually grew to confluence over the next two weeks. Cell clumps with radial
fibers were commonly observed in the primary and subsequent cultures (Fig. 1). The cells expressed GFAP (Fig. 1A) and vimentin (Fig. 3) and had the appearance of radial glial cells. In the primary cultures, macrophages and neurons as well as dendritic melanocytes (Fig. 2A) and multipolar cells (Fig. 2B) appeared frequently in small numbers. A confluent monolayer
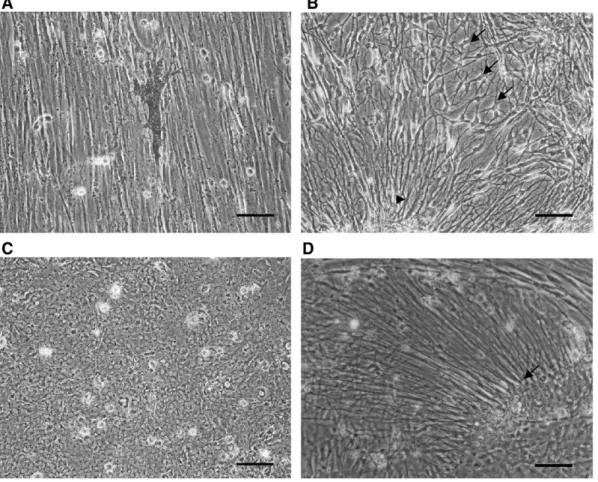
Fig. 1. Morphological and immunocytochemical characterization of TB2 cells at the fourth passage. (A) TB2 cells were labeled with anti-porcine GFAP and TRITC-conjugated anti-mouse IgG. (B) Phase contrast micrograph. Arrows indicate the cells have a long fiber. Scale bars = 100μm.
Fig. 2. Phase contrast micrographs of TB2 cells at the fourth passage. (A) A dendritic melanocyte growing on the fibrous monolayer. (B) Multipolar cells (arrows) and radial cells (arrow head). (C) Epithelial monolayer. (D) Numerous long fibers (arrow) extended from a cell clump (unipolar cells). Scale bar = 100μm.
usually consisted of slender fibroblast-like cells, which grew in parallel, but islands of epitheloid cells were also occasionally noted (Fig. 2C). Unipolar radial glial cells or tanycyte-like cells appeared in large number, usually in high-density cultures (Fig. 2D). The fibrous cells were not fibroblasts, as they labeled with anti-keratin (data not shown). Because they contain GFAP (Fig. 1A), vimentin (Fig. 3), and A2B5 (Fig. 4), we identified them as O-2A progenitor-like cells. Stellate astrocytes that commonly appear in in vitro mammalian CNS cultures were not observed in this study, as also reported in previous studies (Bastmeyer et al., 1989; Tocher and Wilson, 1990; Sivron et al., 1992; Fröjdö et al., 2002). After serial passage, the melanocytes, macrophages, and neurons disappeared gradually, leaving nearly all fibrous cells. The cell line was designated TB2 and was subcultured more than 100 times over five years.
3.2. TB2 cell identification
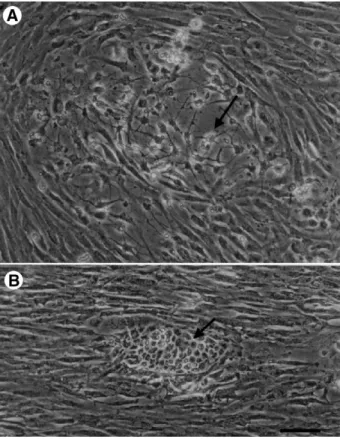
Specific molecular markers have been useful to monitor molecular events in glial and neuronal development and to clarify cell identity. We observed an increase in multipolar cells (Fig. 5A) following longer culture times. The multipolar cells adhered to the flask loosely, and so washed out easily in routine subculture. However, they formed islets when the medium was replaced with fresh growth medium (Fig. 5B). The multipolar
cells had higher GS and S100 (Fig. 6A, B) but lower GFAP (Fig. 6A) and A2B5 (Fig. 4) expression than the fibroblast-like cells in the same field. The multipolar cells may be derived from the O-2A progenitor-like cells, as suggested by their abundant growth under serum-free conditions (data not shown) and long-term culture. The higher GS and S100 expression of these cells initially suggested that they may be astrocytes, but a subpopulation of oligodendrocytes is also known to express high levels of GS and S100 (Fressinaud et al., 1991; Tansey et al., 1991; Richter-Landsberg and Heinrich, 1995; Hachem et al., 2005). Furthermore mammalian O-2A cells in serum-free medium mature to oligodendrocytes (Raff et al., 1983). Taken together with the fact that the multipolar cells have lower GFAP and A2B5 than O-2A progenitor cells and that these cells express MBP (Fig. 6C), we concluded that the multipolar cells are oligodendrocytes.
Although TB2 cells at early passages contained predominantly O-2A progenitor-like cells, these may transform after serial subculture. Thus, we tested some of the markers that have been useful for classifying mammalian neural cells on TB2 cells. Similar to the early subcultures, TB2 cells at 60–80 passages showed a fibrous morphology and parallel cell arrangement at high density, whereas radial glia-like cells were observed frequently at low cell density. All of these cells labeled with
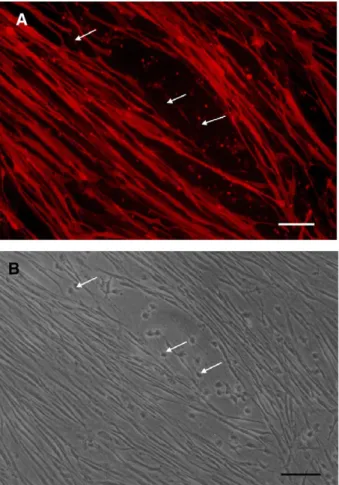
Fig. 3. TB2 cells contain vimentin. (A) Immunofluorescence microscopy of TB2 cells at the fifth passage labeled with vimentin and FITC-conjugated anti-mouse IgG. (B) Phase contrast micrograph. Scale bars = 100μm.
Fig. 4. Most TB2 cells contain A2B5. (A) Immunofluorescence microscopy of TB2 cells at the fifth passage labeled with anti-A2B5 and TRITC-conjugated anti-mouse IgM antibodies. (B) Phase contrast micrograph. Arrows indicate the multipolar A2B5−cells. Scale bars = 100μm.
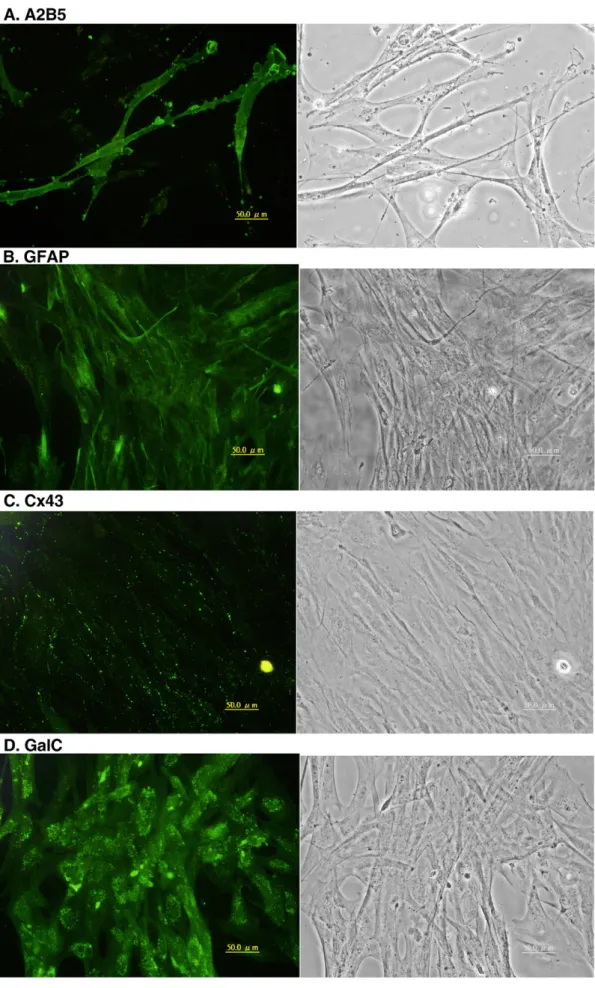
anti-vimentin and anti-keratin antibodies, as did cells at early passages, yet no more than 20% of the cells labeled with anti-A2B5 (Fig. 7A) and about 70% reacted with anti-GFAP (Fig. 7B). The A2B5 labeling was usually spread homogeneously on the cell surface, whereas GFAP labeling appeared filamentous throughout the cytoplasm and often appeared in only one or some of the cell processes. Most of the A2B5+ cells exhibited a bipolar morphology similar to mammalian O-2A cells. By contrast, the GFAP+ cells displayed a heterogeneous phenotype, including polygonal, multipolar, and spindle-shaped cells. Staining for Cx43, a gap junction protein commonly found in radial glial cells, ependymal cells, and astrocytes in the CNS, appeared as punctate labeling between the cells (Fig. 7C). Most of the cells still stained weakly for GS and S100 protein (data not shown). GalC-positive cells were observed more frequently after serial passages (Fig. 7D), but MBP-labeled cells, even in serum-free cultures, were still sparse (data not shown). Because subpopulation of radial glial cells, tanycytes, and oligodendrocyte progenitor cells in mam-malian CNS have been suggested to be stem cells that can generate neurons (Kondo and Raff, 2000; Xu et al., 2005; Itoh et al., 2006), we performed immunocytochemistry using antibodies against the neuronal stem cell markers Sox2, BLBP, and HuC/D. Sparse labeling was observed for BLBP and HuC/D (data not shown); despite of the labeling for Sox2 (Fig. 7E) was denser.
Although most cells reacted intensely with anti-TH (Fig. 7F) and anti-substance P (SP) (Fig. 7G) antibodies, they labeled sparsely with anti-GABA (Fig. 7H), anti-DDC, anti-MAP2, and
anti-NF-Fig. 5. Phase contrast micrographs of TB2 cells at the ninth passage grown in low-serum and normal medium. (A) Increases in the number of multipolar cells (arrow) among the parallel growing cells were usually observed in serum-reduced (3% FBS) medium. (B) A cell islet (arrow) was observed. Before replace medium, groups of multipolar cells were marked. Next day after the low serum medium was replaced with growth medium (15% FBS), aggregations of the marked multipolar cells were noted. Scale bar = 100μm.
Fig. 6. Multipolar TB2 cells contain more S100, GS, and MBP and less GFAP than fibrous TB2 cells. (A) Double-labeling immunoperoxidase of TB2 cells at the ninth passage first labeled with mouse anti-porcine GFAP and HRP-conjugated goat anti-mouse IgG and colorized by DAB, and then labeled with rabbit anti-bovine S100 and HRP-conjugated goat anti-rabbit IgG and visualized with TrueBlue™. (B) Immunoperoxidase staining using monoclonal anti-sheep GS. (C) Immunoperoxidase staining using rabbit anti-human MBP. Both of the cells in B and C are visualized with TrueBlue™ and counterstained with orcein to label the nuclei. Scale bar = 100μm.
Fig. 7. TB2 cells at passage 65 were labeled with a panel of antibodies. (A) A2B5, (B) GFAP, (C) Cx43, (D) GalC, (E) Sox2, (F) TH, (G) SP, and (H) GABA. The left column is the florescence images and the right column is the contrast phase contrast micrographs. Scale bar = 50μm.
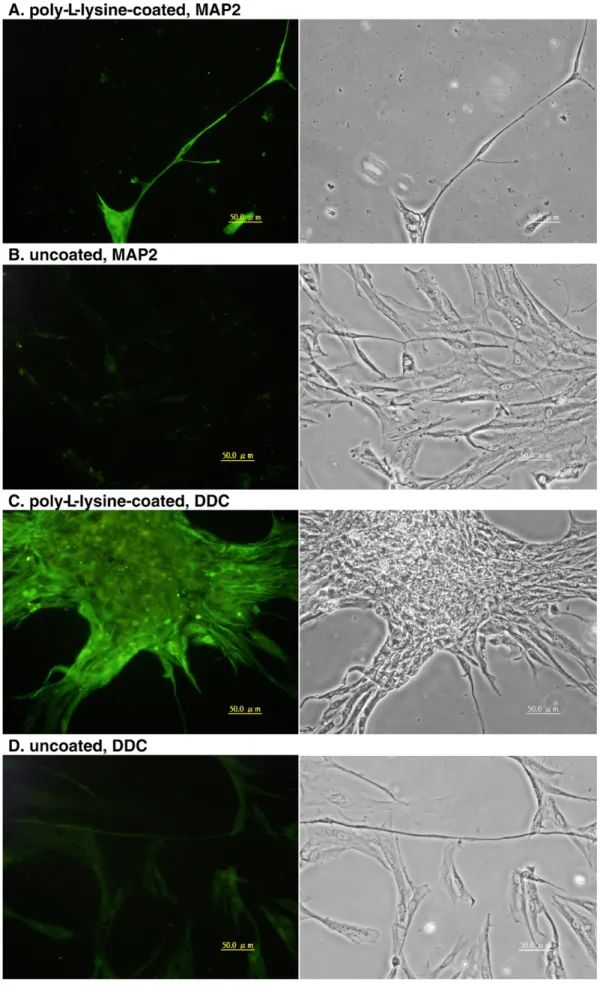
Fig. 8. TB2 cells at passage 65 grown on poly-L-lysine-coated (A and C) and uncoated (B and D) cover slips were labeled with anti-MAP2 (A and B) or anti-DDC (C and D) antibodies. The left is fluorescence images and the right is the corresponding phase contrast micrographs. Scale bars = 50μm.
H antibodies (data not shown). The polyclonal anti-NF200 labeled the control epidermal cells due to non-specific cross-reactivity with non-NF proteins (data not shown). Although few typical neurons were observed in TB2 cultures, many SP-positive cells were found. One reason for this is that anti-SP polyclonal antibody cross-reacts with other neurokinins (Auclair et al., 2004); another reason is that fish astroglial cells have abundant SP, like mammalian reactive astrocytes (Michel et al., 1986; Lin, 1995).
Substrates such as poly-L-lysine promote neural cell
maturation (Yavin and Yavin, 1980). TB2 cells plated on poly-L-lysine-coated cover slips adhered faster than on an uncoated surface. However, cells usually formed clumps by the next day and usually dislodged from the cover slips in five days. The cells on poly-L-lysine-coated cover slips showed more intense MAP2 and DDC labeling (Fig. 8) than those grown on uncoated cover slips. This result confirms that poly-L-lysine can promote the expression of neuron-specific proteins.
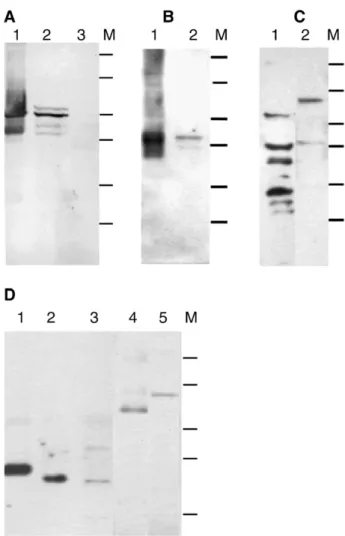
We next performed immunoblotting with antibodies against GFAP and other neuronal proteins to confirm the immunocy-tochemistry results. The immunoblotting, as Fig. 9 shows, revealed a major (about 50 kDa) and three minor (about 52, 43, and 41 kDa) GFAP polypeptides in the TB2 cell homogenate; this is in contrast to the two GFAP polypeptides (about 50 and 42 kDa) we observed in the fish brain homogenate (Fig. 9A). Others have similarly found GFAP polypeptides of about 50– 52 kDa in fish (Dahl et al., 1985; Blaugrund et al., 1991; Nona et al., 1989; Fröjdö et al., 2002). This report is the first demonstration of 41 and 43 kDa GFAP polypeptides in fish. The significance of various GFAP isoforms is still unknown; however, they may be the results of differential splicing and/or post-translation modification (Blaugrund et al., 1991) in either a single cell type or disparate astroglial cell types (Alunni et al., 2005). We suggest that the heterogeneous GFAP expression observed by immunoblotting is a reflection of the hetero-geneous astroglial cell population in the TB2 culture.
A single 42-kDa polypeptide in tilapia brain and TB2 cell homogenate was recognized by the GS antibody (Fig. 9B). The protein mass is similar to that reported previously for zebrafish and stingray (Smith et al., 1987; Peterson et al., 2001). Two blurry MBP bands (of about 72 and 45 kDa) and four S100 polypeptides (of approximately 60, 40, 30, and 24 kDa) were observed in the TB2 homogenate (Fig. 9C). TH and Cx35, with protein masses of about 70 kDa and 35 kDa, respectively, were also present in the TB2 cell homogenate (Fig. 9D).
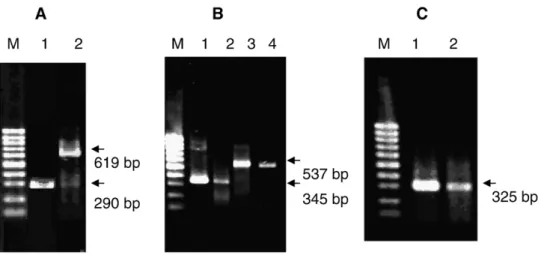
3.3. TB2 cells express glia- and neuron-specific genes To confirm that the data obtained from the above immu-nocytochemistry and immunoblotting were not the results of nonspecific cross reactions, and to verify that TB2 cells express glia- and neuron-specific genes, we performed RT-PCR on total RNA from TB2 cells. Electrophoretic analyses of the RT-PCR products are shown inFig. 10. Obvious bands at about 290 bp, 325 bp, 619 bp, 345 bp, and 545 bp for GFAP, Cx 43, Cx35, PLP, and GS, respectively, were observed. Sequencing of the GFAP amplicon demonstrated that the sequence (GenBank
Accession no. EU142910) were 100% identical to the Nile tilapia GFAP mRNA sequence (Accession no. AB109167) and the GS amplicon (Accession no. EU142911) had 99% identity with the GS mRNA sequence (Accession no. AF503208) at nucleotides 303–848. The sequences of the PLP (Accession no. EU142912), Cx43 (Accession no. EU142908), and Cx35 (Accession no. EU142909) amplicons had 100%, 100%, and 95% identity, respectively, to the Mozambique tilapia PLP mRNA sequences at nucleotides 515–170 (Accession no. AY522623), Cx43 mRNA sequences at nucleotides 118–442 (Accession no. AY455925), and Cx35 mRNA sequences at nucleotides 3–621 (Accession no. AY455924). Thus, TB2 cells express astroglial-specific genes (GFAP, Cx43, and GS), an
Fig. 9. Immunoblot analysis of TB2 cells at passages 65-75. (A) Reactivity with anti-porcine GFAP. Lane 1, tilapia brain homogenate; lane 2, TB2 cell homogenate; lane 3, tilapia spleen homogenate. (B) A single (42 kDa) band reacted with anti-GS in tilapia brain (lane 1) and TB2 cell (lane 2) homogenates. (C) Two anti-MBP (lane 1) and seven anti-S100 (lane 2) antibody-reactive polypeptides were detected in the TB2 cell homogenate. (D) A single polypeptide labeled with anti-Cx36 was observed in the homogenates of mouse brain (lane 1) and tilapia brain (lane 2). However, the antibody labeled an additional polypeptide in the TB2 cell homogenate (lane 3). The protein mass is 36 kDa in mouse and 35 kDa in tilapia. Anti-TH is shown labeling 60-kDa and 70-kDa polypeptides in the homogenates of mouse brain (lane 4) and TB2 cells (lane 5), respectively. M: molecular weight markers include 120-, 76-, 50-, 39-, 26-, and 19-kD. The 19-kD marker in D was cut off.
oligodendrocyte-specific gene (PLP), and a neuron-specific (Cx35) gene.
4. Discussion
Cell culture is useful for studies of molecular events during differentiation. Most studies with cultured neural cells have been carried out on cells isolated from the brains of embryonic or newborn rodents and relatively few studies have reported neural cells isolated from fish brains. Bastmeyer et al. (1989) first isolated and cultivated astroglial cells and oligodendrocytes from goldfish optic nerves and reported that a subpopulation of the cells has GFAP and O1 antigens. In addition, A2B5+O-2A progenitor-like cells have been reported in cultures isolated from goldfish and trout brain (Jeserich and Stratmann, 1992; Sivron et al., 1992). Polygonal type-1 astrocyte-like cells also have been obtained from the brain tissues of rainbow trout (Tocher and Wilson, 1990; Fröjdö et al., 2002). Studies on fish glial cells in vitro, although scarce, suggest that the characteristics of fish glial cells differ from those of glial cells from adult mammals. Most astroglial cells in the fish CNS (including adult) are radial glia and tanycytes (Wicht et al., 1994; Pepperell and Levine, 1996; Kálmán, 1998; Chiba, 2000; Kálmán and Gould, 2001; Arochena et al., 2004; Lazzari and Franceschini, 2004). Therefore, one would expect that astroglia cell cultures derived from fish brain tissue would contain a substantial number of either radial glial cells or tanycytes, but that has not been the case.
In this study, fibroblast-like cells were the most common cells in the primary culture. The fibroblast-like cells may be radial glial cells or tanycytes, as they had GFAP+and vimentin+uni- or bipolar radial process. Islands of epitheloid polygonal cells also were observed in the primary cultures and in several subsequent sub-cultures. The epitheloid cells may be ependymal cells, although cilia were not demonstrated. Neurons and oligodendrocytes ad-hered poorly to the tissue culture flasks and did not proliferate, as also previously reported (Fröjdö et al., 2002). After serial sub-culture, however, only fibroblast-like cells remained in the culture.
4.1. TB2 cells derived from radial glial cells
The TB2 cell line established in this study expresses GFAP, vimentin, Cx43, GS, and S100 protein, although the two latter proteins are expressed sparsely, suggesting that the cells are astroglial cells. In fish, tanycytes as well as radial glial cells, possess radial processes, share the characteristics of astroglia including GFAP, S100, and vimentin (Chiba, 2000; Arochena et al., 2004; Lazzari and Franceschini, 2004). Therefore, one can easily define tanycytes in vivo by their location in the CNS, but cannot distinguish in vitro unciliated tanycytes from radial glia. As a matter of fact, tanycytes in the fish CNS are a subtype of radial glial cells (radial ependymoglia) (Lazzari and Franceschini, 2004). A subpopulation of tanycyte has cilia on the apex (Shioda et al., 1977), and therefore can be labeled with anti-acetylated tubulin antibody. TB2 cells did not label with anti-acetylated tubulin (data not shown) and did not contain cilia; therefore, taken together with the fact that TB2 cells share many charac-teristics with radial glial cells and radial glial cells are the most dominant astroglial cell in the fish CNS, we suggest that TB2 cells are derived from radial glia.
4.2. TB2 cells generate O-2A progenitor-like cells
In vivo, fish radial glial cells (including Müller cells in the retina) can be defined by expression of GFAP, vimentin, aro-matase B, and BLBP (Kinoshita et al., 2005; Raymond et al., 2006), but we know nothing regarding the in vitro expression profile of these cells. TB2 cells also express Sox2, BLBP, and HuC/D; however, expression of these three proteins was less than that of GFAP and vimentin. Although the expression of stem cell and neuron progenitor markers such as BLBP, Sox2, and HuC/D were not very obvious, TB2 cells may be neural progenitor cells. A2B5+ O-2A progenitor-like cells have been reported in primary cultures obtained from trout (Jeserich and Stratmann, 1992; Sivron et al., 1992) and goldfish (Bastmeyer et al., 1989). The O-2A progenitor-like cells contain the
oligodendrocyte-Fig. 10. Glia- and neuron-specific mRNAs detected by RT-PCR. RT was performed with 2μg total RNA and 50 ng of the resulting cDNAwas amplified by PCR. PCR products were separated by agarose gel electrophoresis and stained with ethidium bromide. (A) Amplicons of GFAP (lane 2) and Cx35 (lane 3) are shown. (B) The PLP amplicon (lanes 2 and 3) and the GS amplicon (lanes 4 and 5) from RT-PCR using total RNA from tilapia brain (lanes 2 and 4) or from TB2 cells (lanes 3 and 5). (C) Amplicon of Cx43 from TB2 cells (lane 2) and tilapia brain (lane 3). Lane 1: DNA size markers: 100-bp DNA ladder.
specific markers GalC, IP2, O1, O4, and 6D2 antigens, but were not observed to generate mature ramified oligodendrocytes in vitro as mammalian O-2A cells do (Jeserich and Stratmann, 1992; Sivron et al., 1992). However, the O-2A progenitor-like cells were observed to lose A2B5 expression and increase expression of 6D2 during maturation (Jeserich et al., 1993). In this study, the O-2A progenitor-like cells gave rise to multipolar presumptive oligodendrocytes that expressed GS, S100, and MBP and low levels of A2B5 and GFAP and increased in number under serum-reduced conditions. Our results, similar to the previous studies, suggest that O-2A progenitor-like cells from tilapia spontaneously generate immature oligodendro-cytes, especially in serum-reduced or serum-free medium. Although the TB2 cells revealed expression of mature oligodendrocyte markers such as GalC, MBP, and PLP, mature ramified oligodendrocytes were rarely observed in the culture. Jeserich and Stratmann (1992)suggested that further maturation of immature oligodendrocytes requires more stimulation factors. GalC was reported to rapidly disappear from the surface of cultured trout oligodendrocytes (Jeserich and Rauen, 1990). In this study, however, we did not note any loss of GalC. No stellate astrocytes that commonly appear and mature from O-2A progenitor cells in mammals were observed in previous or the present study (Jeserich and Stratmann, 1992; Sivron et al., 1992). In fact, fish brain has few if any stellate astrocytes (Kálmán, 1998; Kálmán and Gould, 2001; Lazzari and Franceschini, 2004). 4.3. TB2 cells may generate neurons
Fish radial glia and mammalian radial glia are well known to be able to generate neurons. As the TB2 cell line was demonstrated to contain radial glial cells, we were interested in testing whether the cells could generate neurons. In this study, several neuron-specific molecules, Cx35, DDC, GABA, MAP2, SP, and TH, were found in TB2 cultures, suggesting that the cells may spontaneously generate neurons during culture. We noted that the TB2 cells may be promoted or inhibited to differentiate by environment factors such as poly-L-lysine and serum. Recently, neural stem cells have been isolated and cultivated from the adult fish CNS that can form neurospheres and generate astroglia and neurons in vitro (Hinsch and Zupanc, 2006).
The substrate on which cells adhere affects cell morphology, maturation, and survival. Some astroglia from the fish CNS have a fibroblast morphology on poly-D-lysine -coated surfaces
but show a protoplasmic phenotype on laminin-coated or in typical culture flasks (Fröjdö et al., 2002). Our results and previous studies (Fröjdö et al., 2002; Hinsch and Zupanc, 2006) also indicate that fish neural cells mature better on a poly-L
-lysine- or poly-D-lysine-coated surface than on laminin-coated
or uncoated cell culture surfaces, but cell proliferation and survival rates respond in the opposite direction.
4.4. TB2 cells show characteristics intermediate between glia and neurons
The TB2 cells that express oligodendrocyte- or neuron-specific proteins are not believed to be neurons or oligodendrocytes that
originated from the primary fish brain culture because the TB2 cells were subcultivated for many generations and hence low proliferating neurons and oligodendrocytes would have been completely lost during the routine subculturing. Because several types of astroglial progenitor cells were observed in the cultures, it is possible that the different types of astroglial progenitor cells can generate neurons and oligodendrocytes. The different astroglial progenitor cells may have originated from the primary cultured fish brain tissue; however, they would need to have similar growth rates so as not to be lost during serial subculturing. Another possibility is that the astroglial progenitor cells arose from one type of radial glial cell but for lack of the proper differentiation factors, the intermediate cells co-exhibit neuron-, oligodendro-cyte- and astroglia-specific proteins.
There are two TH genes in teleosts, TH1 is found in brain and kidney while TH2 is only found in brain (Candy and Collet, 2005). By RT-PCR, expression of TH1 mRNA in TB2 cells was just revealed (unpublished result) but the expression of TH2 mRNA has not study yet. The TH+cells may generate L-dopa, which is converted to dopamine and melanin by DDC and tyrosinase, respectively. Based on the granular autofluorescence we have observed around the nuclei (data not shown), we suggest that TB2 cells have dopamine, despite the fact that DDC expression is low in routine cultivation. The significance of TB2 cells exhibiting TH is unclear. However, TH immunoreaction also was observed in the ependymal cells of dogfish, sturgeon, gar, and goldfish spinal cord (Sueiro et al., 2003; Wai et al., 2007). Coexpression of GFAP and TH has revealed in Müller cells from chicken embryos (Kubrusly et al., 2005), in mouse neural progenitor cells (Von Visger et al., 1994), and in human glioblastoma cells (Bonfigli et al., 2006). Additionally, a subpopulation of type-2 astrocytes expresses the neuron-specific antigens Tau, MAP2, and TH (Schinstine and Iacovitti, 1996). Beside, TH was observed frequently in the glial cells in vitro from other fish brain (Wang, 2001; unpublished results). These results suggest that TH is produced before the cells differentia-tion into neurons and thus can be observed in the non-neuronal cells. Mreover, intermediate cells between oligodendrocyte and astrocyte that coexpress GalC and GFAP were observed from cultured rat A2B5+O-2A cells (Raff et al., 1984).
TB2 cells show characteristics that have not been observed in fish astroglial cells in vivo. However, we suggest that TB2 is a normal cell line that maintains gap junctions and contact inhibition. As described above, cells co-expressing neuron- and glia-specific molecules are not uncommon. This phenomenon has been reported in glial tumors (Tlhyama et al., 1993), gene transfer-immortalized neural cell lines (Tlhyama et al., 1993; Marone et al., 1995; Bongarzone et al., 1998), and normal primary cultures (Müller et al., 1997; Rosser et al., 1997). Subtypes of astrocytes in mice have been implicated as stem cells and form hybrid asterons which coexpress astrocytic and neuronal markers (Laywell et al., 2000, Laywell et al., 2005). If TB2 cells are neural progenitor (stem) cells, it is not surprising we observed glial and neuronal markers in the cultures.
In conclusion, we have isolated and established an immortal cell line, TB2, from tilapia brain. The immunocytochemical analyses indicate that TB2 cells are astroglial progenitor cells.
We propose that fish astroglial progenitor cells in vitro may express both specific glial and neuronal molecules and that the cells may generate oligodendrocytes or neurons under the appropriate conditions. The factors that promote cell maturation are uncertain; however, we suggest that they may differ from those for mammalian neural cells. As a neural progenitor cell line, TB2 has much potential for advancing investigations of the factors that affect fish neural development. TB2 cells also have the potential to reveal specific molecular markers for fish neural progenitor cells as well as aid in functional studies.
References
Adolf, B., Chapouton, P., Lam, C.S., Topp, S., Tannhauser, B., Strahle, U., Gotz, M., Bally-Cuif, L., 2006. Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278–293.
Akimoto, J., Itoh, H., Miwa, T., Ikeda, K., 1993. Immunohistochemical study of glutamine synthetase expression in early glial development. Brain Res. Dev. Brain Res. 72, 9–14.
Alunni, A., Vaccari, S., Torcia, S., Meomartini, M.E., Nicotra, A., Alfei, L., 2005. Characterization of glial fibrillary acidic protein and astroglial architecture in the brain of a continuously growing fish, the rainbow trout. Eur. J. Histochem. 49, 157–166.
Arochena, M., Anadón, R., Díaz-Regueira, S.M., 2004. Development of vimentin and glial fibrillary acidic protein immunoreactivities in the brain of gray mullet (Chelon labrosus), an advanced teleost. J. Comp. Neurol. 469, 413–436. Auclair, F., Lund, J.P., Dubuc, R., 2004. Immunohistochemical distribution of
tachykinins in the CNS of the lamprey Petromyzon marinus. J. Comp. Neurol. 479, 328–346.
Bass, D., Dalencon, D., Fressinaud, C., Vitkovic, L., Sarlieve, L.L., 1998. Oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells express glutamine synthetase: developmental and cell type-specific regulation. Mol. Psychiatry 3, 356–361.
Bastmeyer, M., Beckmann, M., Nona, S.M., Cronly-Dillon, J.R., Stuermer, C.A., 1989. Identification of astrocyte- and oligodendrocyte-like cells of goldfish optic nerves in culture. Neurosci. Lett. 101, 127–132.
Baumann, N., Pham-Dinh, D., 2001. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 81, 871–927. Belliveau, D.J., Naus, C.C., 1994. Cortical type 2 astrocytes are not dye coupled
nor do they express the major gap junction genes found in the central nervous system. Glia 12, 24–34.
Bittman, K.S., LoTurco, J.J., 1999. Differential regulation of connexin 26 and 43 in murine neocortical precursors. Cereb. Cortex 9, 188–195.
Blaugrund, E., Cohen, I., Shani, Y., Schwartz, M., 1991. Glial fibrillary acidic protein in the fish optic nerve. Glia 4, 393–399.
Bonfigli, A., Zarivi, O., Colafarina, S., Cimini, A.M., Ragnelli, A.M., Aimola, P., Natali, P.G., Ceru, M.P., Amicarelli, F., Miranda, M., 2006. Human glioblastoma ADF cells express tyrosinase,L-tyrosine hydroxylase and melanosomes and are sensitive toL-tyrosine and phenylthiourea. J. Cell. Physiol. 207, 675–682.
Bongarzone, E.R., Fosterm, L., Byravan, S., Casaccia-Bonnefil, P., Schonmann, V., Campagnoni, A.T., 1998. Two neuronal cell lines expressing the myelin basic protein gene display differences in their in vitro survival and in their response to glia. J. Neurosci. Res. 54, 309–319.
Cammer, W., 1990. Glutamine synthetase in the central nervous system is not confined to astrocytes. J. Neuroimmunol. 26, 173–178.
Candy, J., Collet, C., 2005. Two tyrosine hydroxylase genes in teleosts. Biochim. Biophys. Acta 1727, 35–44.
Chiba, A., 2000. S-100 protein-immunoreactive structures in the brains of the elasmobranchs scyliorhinus torazame and mustelus manazo. Neurosci. Lett. 279, 65–68.
Dahl, D., Bignami, A., 1973. Immunochemical and immunofluorescence studies of the glial fibrillary acidic protein in vertebrates. Brain Res. 61, 279–293. Dahl, D., Crosby, C.J., Sethi, J.S., Bignami, A., 1985. Glial fibrillary acidic (GFA) protein in vertebrates: immunofluorescence and immunoblotting study with monoclonal and polyclonal antibodies. J. Comp. Neurol. 239, 75–88.
Deloulme, J.C., Raponi, E., Gentil, B.J., Bertacchi, N., Marks, A., Labourdette, G., Baudier, J., 2004. Nuclear expression of S100B in oligodendrocyte progenitor cells correlates with differentiation toward the oligodendroglial lineage and modulates oligodendrocytes maturation. Mol. Cell. Neurosci. 27, 453–465. Doetsch, F., 2003. The glial identity of neural stem cells. Nat. Neurosci. 6,
1127–1133.
Eddé, B., Rossier, J., Le Caer, J.P., Berwald-Netter, Y., Koulakoff, A., Gros, F.P.D., 1991. A combination of posttranslational modifications is respon-sible for the production of neuronal alpha-tubulin heterogeneity. J. Cell. Biochem. 46, 134–142.
Fressinaud, C., Weinrauder, H., Delaunoy, J.P., Tholey, G., Labourdette, G., Sarlieve, L.L., 1991. Glutamine synthetase expression in rat oligodendrocytes in culture: regulation by hormones and growth factors. J. Cell. Physiol. 149, 459–468. Fröjdö, E.M., Westerlund, J., Isomaa, B., 2002. Culturing and characterization
of astrocytes isolated from juvenile rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. A 133, 17–28.
Hachem, S., Aguirre, A., Vives, V., Marks, A., Gallo, V., Legraverend, C., 2005. Spatial and temporal expression of S100B in cells of oligodendrocyte lineage. Glia 51, 81–97.
Hinsch, K., Zupanc, G.K.H., 2006. Isolation, cultivation, and differentiation of neural stem cells from adult fish brain. J. Neurosci. Methods 158, 75–88. Itoh, T., Satou, T., Nishida, S., Hashimoto, S., Ito, H., 2006. Cultured rat
astrocytes give rise to neural stem cells. Neurochem. Res. 31, 1381–1387. Jeserich, G., Rauen, T., 1990. Cell cultures enriched in oligodendrocytes from the central nervous system of trout in terms of phenotypic expression exhibit parallels with cultured rat Schwann cells. Glia 3, 65–74.
Jeserich, G., Stratmann, A., 1992. In vitro differentiation of trout oligodendrocytes: evidence for an A2B5-positive origin. Brain Res. Dev. Brain Res. 67, 27–35. Jeserich, G., Strelau, J., Tönnies, R., 1993. An immunomagnetic cell separation technique for isolating oligodendroglial progenitor cells of trout CNS. NeuroProtocols 2, 219–224.
Kálmán, M., 1998. Astroglial architecture of the carp (Cyprinus carpio) brain as revealed by immunohistochemical staining against glial fibrillary acidic protein (GFAP). Anat. Embryol. 198, 409–433.
Kálmán, M., Gould, R.M., 2001. GFAP-immunopositive structures in spiny dogfish, Squalus acanthias, and little skate, Raia erinacea, brains: differences have evolutionary implications. Anat. Embryol. 204, 59–80.
Kinoshita, M., Fukaya, M., Tojima, T., Kojima, S., Ando, H., Watanabe, M., Uranom, A., Ito, E., 2005. Retinotectal transmission in the optic tectum of rainbow trout. J. Comp. Neurol. 484, 249–259.
Kondo, T., Raff, M., 2000. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 289, 1754–1757. Kristen, B.C., McCarthy, K.D., 2006. GFAP-positive progenitor cells produce
neurons and oligodendrocytes throughout the CNS. Mol. Cell. Neurosci. 31, 676–684.
Kubrusly, R.C.C., da Cunha, M.C.C., Reis, R.AdM., Soares, H., Ventura, A.L.M., Kurtenbach, E., de Mello, M.C.F., de Mello, F.G., 2005. Expression of functional receptors and transmitter enzymes in cultured Müller cells. Brain Res. 1038, 141–149.
Laywell, E.D., Rakic, P., Kukekov, V.G., Holland, E.C., Steindler, D.A., 2000. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. U. S. A. 97, 13883–13888.
Laywell, E.D., Kearns, S.M., Zheng, T., Chen, K.A., Deng, J., Chen, H.X., Roper, S.N., Steindler, D.A., 2005. Neuron-to-astrocyte transition: pheno-typic fluidity and the formation of hybrid asterons in differentiating neurospheres. J. Comp. Neurol. 493, 321–333.
Lazzari, M., Franceschini, V., 2004. Glial fibrillary acidic protein and vimentin immunoreactivity of astroglial cells in the central nervous system of the African lungfish, Protopterus annectens (Dipnoi: Lepidosirenidae). J. Morphol. 262, 741–749.
Li, H., Babiarz, J., Woodbury, J., Kane-Goldsmith, N., Grumet, M., 2004. Spatiotemporal heterogeneity of CNS radial glial cells and their transition to restricted precursors. Dev. Biol. 271, 225–238.
Lin, R.C., 1995. Reactive astrocytes express substance-P immunoreactivity in the adult forebrain after injury. NeuroReport 29, 310–312.
Mack, A.F., Germer, A., Janke, C., Reichenbach, A., 1998. Müller (glial) cells in the teleost retina: consequences of continuous growth. Glia 22, 306–313.
Marone, M., Quiñones-Jenab, V., Meiners, S., Nowakowski, R.S., Ho, S.Y., Geller, H.M., 1995. An immortalized mouse neuroepithelial cell line with neuronal and glial phenotypes. Dev. Neurosci. 17, 311–323.
Mastronardi, F.G., Boulias, C., Roots, B.I., Moscarello, M.A., 1993. Character-ization of basic proteins from goldfish myelin. J. Neurochem. 60, 153–160. Michel, J.P., Sakamoto, N., Bouvier, R., Tommasi, M., Pearson, J., 1986. Substance P-immunoreactive astrocytes related to deep white matter and striatal blood vessels in human brain. Brain Res. 377, 383–387.
Müller, R., Heinrich, M., Heck, S., Blohm, D., Richter-Landsberg, C., 1997. Expression of microtubule-associated proteins MAP2 and tau in cultured rat brain oligodendrocytes. Cell Tissue Res. 288, 239–249.
Nona, S., Swhab, S.A.S., Stafford, C.A., Cronly-Dillon, J.R., 1989. Glial fibrillary acidic protein (GFAP) from the goldfish: its localization in the visual pathways. Glia 2, 189–200.
Pellegrini, E., Mouriec, K., Anglade, I., Menuet, A., Page, Y.L., Gueguen, M.M., Marmignon, M.H., Brion, F., Pakdel, F., Kah, O., 2007. Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 501, 150–167.
Pepperell, C., Levine, R.L., 1996. Nonspecific esterase activity in astrocytes of the goldfish brain. J. Histochem. Cytochem. 44, 1195–1203.
Peterson, R.E., Fadool, J.M., Mcclintock, J., Linser, P.J., 2001. Müller cell differentiation in the zebrafish neural retina: evidence of distinct early and late stages in cell maturation. J. Comp. Neurol. 429, 530–540.
Raff, M.C., Miller, R.H., Noble, M., 1983. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 303, 390–396.
Raff, M.C., Williams, B.P., Miller, R.H., 1984. The in vitro differentiation of a bipotential glial progenitor cell. EMBO J. 3, 1857–1864.
Rash, J.E., Yasumura, T., Davidson, K.G., Furman, C.S., Dudek, F.E., Nagy, J.I., 2001. Identification of cells expressing Cx43, Cx30, Cx26, Cx32 and Cx36 in gap junctions of rat brain and spinal cord. Cell Commun. Adhes. 8, 315–320. Raymond, P., Barthel, L.K., Bernardos, R.L., Perkowski, J.J., 2006. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 6, 36.
Richter-Landsberg, C., Heinrich, M., 1995. S-100 immunoreactivity in rat brain glial cultures is associated with both astrocytes and oligodendrocytes. J. Neurosci. Res. 42, 657–665.
Rosser, A.E., Tyers, P., ter Borg, M., Dunnett, S.B., Svendsen, C.N., 1997. Co-expression of MAP-2 and GFAP in cells developing from rat EGF responsive precursor cells. Brain Res. Dev. Brain Res. 98, 291–295. Schinstine, M., Iacovitti, L., 1996. Expression of neuronal antigens by astrocytes
derived from EGF-generated neuroprogenitor cells. Exp. Neurol. 141, 67–78. Shioda, S., Honma, Y., Yoshie, S., Hosoya, Y., 1977. Scanning electron microscopy of the third ventricular wall in the lamprey, Lampetra japonica. Arch. Histol. Jpn. 40, 41–49.
Sivron, T., Jeserich, G., Nona, S., Schwartz, M., 1992. Characteristics of fish glial cells in culture: possible implications as their lineage. Glia 6, 52–66. Smith, D.D., Ritter, N.M., Campbell, J.W., 1987. Glutamine synthetase isozymes
in elasmobranch brain and liver tissues. J. Biol. Chem. 262, 198–202. Sueiro, C., Carrera, I., Rodriguez-Moldes, I., Molist, P., Anadon, R., 2003.
Development of catecholaminergic systems in the spinal cord of the dogfish Scyliorhinus canicula (Elasmobranchs). Dev. Brain Res. 142, 141–150. Tansey, F.A., Farooq, M., Cammer, W., 1991. Glutamine synthetase in
oligodendrocytes and astrocytes: new biochemical and immunocytochemical evidence. J. Neurochem. 56, 266–272.
Tlhyama, T., Lee, V.M., Trojanowski, J.Q., 1993. Co-expression of low molecular weight neurofilament protein and glial fibrillary acidic protein in established human glioma cell lines. Am. J. Pathol. 142, 883–892. Tocher, D.R., Wilson, R., 1990. Primary culture of astrocytic glial cells from
rainbow trout, Salmo gairdneri L., brain. J. Neurosci. Methods 33, 93–100. Vecino, E., Velasco, A., Caminos, E., Aijon, J., 1997. Distribution of S-100 immunorecativity in the retina and optic nerve head of the teleost Tinca tinca L. Microsc. Res. Tech. 36, 17–25.
Von Visger, J.R., Yeon, D.S., Oh, T.H., Markelonis, G.J., 2007. Differentiation and maturation of astrocytes derived from neuroepithelial progenitor cells in culture. Exp. Neurol. 70, 1079–1090.
Wai, M.S.M., Lorke, D.E., Zhang, A., Kung, H.-F., Yew, D.T., 1997. Study of the spinal cords of the sturgeon Acipenser schrenckii, gar Lepisosteus oculatus, and goldfish Carassius auratus by morphological, immunohisto-chemical, and biochemical approaches. Microsc. Res. Tech. 70, 1079–1090. Wallraff, A., Odermatt, B., Willecke, K., Steinhäuser, C., 2004. Distinct types of astroglial cells in the hippocampus differ in gap junction coupling. Glia 48, 36–43.
Wang, I.D., 2001. Establishment of the cell line derived from tilapia (Oreochromis mossambica × Oreochromis nilotica) brain and characteristics of its differentiation. Master's Thesis. National Taipei University of Education, Taipei, Taiwan. 69 p.
Wicht, H., Derouiche, A., Korf, H.W., 1994. An immunocytochemical investiga-tion of glial morphology in the Pacific hagfish: radial and astrocyte-like glia have the same phylogenetic age. J. Neurocytol. 23, 565–576.
Xu, Y., Tamamaki, N., Noda, T., Kimura, K., Itokazu, Y., Matsumoto, N., Dezawa, M., Ide, C., 2005. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 192, 251–264.
Yavin, Z., Yavin, E., 1980. Survival and maturation of cerebral neurons on