行政院國家科學委員會專題研究計畫成果報告
以基因微陣列分析肝細胞再生時,特種基因及基因族群之基因表現在程
度、型態、時程的變遷,並鑑別其扮演之角色
ANALYZING THE REGULATING GENES OF LIVER
REGENERATION IN CHANGING DEGREE, PATTERN, TIMING
AND VERIFYING THE ROLES OF SPECIFIC AND CLUSTER GENES
BY cDNA MICROARRY
計畫編號:NSC 92-2314-B-002-276
執行期限:92 年 8 月 1 日至 94 年 7 月 31 日
主持人:賴鴻緒 台大醫學院 外科
中文摘要 有關肝細胞在肝損傷後可以再生,雖 已是公認的事實,許多研究也證實多種營 養素、賀爾蒙、生長因子、藥劑等,可直 接或間接影響肝細胞再生,但肝細胞再生 基因控制之詳細機轉,則仍不明瞭。 任何細胞之分裂與再生,必然與細胞 核內 proto-oncogene 之表現有相當大的關 聯,肝細胞再生也不例外。在多項以mRNA 定量的研究報告中指出,肝臟經部分切除 後,剩餘肝臟內某些基因,如c-fos、c-myc、 p53、p21、gas-6 及 ras 家族基因等的表現 的確有增加現象。Arora 等人發現抗 c-myc 物質可經由p-450 3A 活性之調控可抑制肝 細胞再生;Ozeki 及 Tsukamoto 發現 retinoic acid 可抑制 c-fos 及 c-jun 之表現,促成肝 細胞凋亡而使再生程度減少;而其他基因 如p21 及 gas-6 等之表現也曾被提出與肝細 胞再生有關,但大量搜尋相關基因變化之 研究,則尚未有報告。吾人初步以 384 點 肝臟相關之cDNA 基因微陣列(microarray) 研究,發現肝細胞再生過程中,有59 種基 因表現明顯增加,19 種明顯降低,但因其 變化程度、型態、時程與基因種別、基因 族群均十分複雜,無法清楚分析,其扮演 之角色也尚難了解。更大量的 cDNA 基因 微陣列搜尋,以進一步分析及了解基因管 控之機轉仍屬必要。 本計劃以重約200 克之 Wistar 雄性大 鼠做實驗,測定肝細胞再生過程中,超過 6000 種確定基因種別之基因表現,並分析 其變遷程度、型態、時程及各基因族群之 相關性,以了解其在肝細胞再生中確實扮 演之角色。所有大鼠均接受約百分之七十 之肝臟部分切除手術,各於術前及術後2、 4、6、12、24、72 小時及 5、7、10 天後犧 牲取樣,測定:(1)剩餘肝臟之重量比值; (2)剩餘肝臟之有絲分裂指標;(3)以基因微 陣列尼龍膜(6144 identified cDNA clones, Wittech Co, Taipei, Taiwan)、肝細胞 mRNA 標號、hybridization 及影像分析等方法,測 定6144 種基因表現之變遷程度、型態及時 程;(4)將基因表現變化大者,依特性分為 免疫、賀爾蒙、生長因素、酵素、及血管 新生因子等基因族群,並比較其程度、型 態及時程之差異性。結果發現:(1)剩餘肝 臟重量比值,於切肝術後 72 小時即恢復 90%以上;(2)有絲分裂於術後 48 小時大量 出現,術後72 小時逐漸減少;(3)所有基因表現變遷之型態及時程共分為72 種,包括 第2、、6、12、24、72 小時及、7 天單一 尖峰型、雙尖峰、遞增型、遞減型、突出 型、凹陷型和混和型等,每種型態包括 40 至 218 種基因;(4)包括免疫、賀爾蒙、生 長因子、酵素及血管新生因子等基因族 群,均有明顯的變遷;(5) fas-associating protein with death domain, carnitine palmitoyltransferase 1, fas death domain-associating protein, 及 steroid O-acyltransferase 1 等早期變化基因可能與 肝再生之啟動有關;(6) transforming growth factor beta 2 及 beta receptor 等中期變化基 因可能與肝再生之分化有關;(7) TGF-β regulated gene 3 及 small inducible cytokine A2 等晚期變化基因可能與肝再生之終止 有關。 關鍵字:肝細胞再生、部分肝臟切除術、 proto-oncogene、基因微陣列、基因表現型 態、基因族群 ABSTRACT
Although there are much controversy on the initiation, regulation, metabolic changes, and termination of liver regeneration after partial hepatectomy that well initiate proliferation of the remaining hepatocytes, several factors, such as hormones, growth factors, nutritional components, and pharmacological agents, have been demonstrated to directly or indirectly affect liver regeneration. However, the regenerative mechanism and genetic control of liver after major tissue loss is still not clear.
The regenerating liver is a system in which the relationships between proto-oncogene expression and cell replication should be examined during a
physiologic growth response. Proto-oncogene expression after partial hepatectomy should be specific, sequential, and highly regulated. As measured by levels of mRNAs, the changes have been detected in the expression of c-fos, c-myc, p53, p21,
gas-6 and the ras gene family (c-Ha-ras, c-Ki-ras, and N-ras). In contrast, expression of c-src and c-abl does not change after partial hepatectomy while c-mos transcripts cannot be detected in normal or regenerating liver. Arora et al reported that c-Myc antisense limits rat liver regeneration by regulating cytochrome p-450 3A activity. Ozeki and Tsukamoto found that retinoic acid can repress c-fos and c-jun expression and induce apoptosis in regenerating liver. Our previous study monitored the variation of regulating genes by 384 liver-related gene cDNA microarray nylon membrane, and found that there are 59 proto-oncogenes expression increased markedly and 19 decreased significantly during liver regeneration. However, the changing degree, patterns, timing and gene grouping were very sophisticated and not clear. Mass survey and more detailed analysis by more cDNA microarry method should be very important.
Male Wistar rats around 200g will be used as subject. Partial hepatectomy around 70% were performed. They were sacrificed before and 2, 4, 6, 12, 24, 72 hours and 5, 7, 10 days after hepatectomy. We have measured: (1)weight of remnant liver; (2)mitotic index; (3)genomic survey of the gene expression by microarray of 6144 identified cDNA clones on nylon membrane (Wittech Co., Taipei, Taiwan), labeling of liver mRNA hybridization and image analysis; and (4)Grouping of genes expression into immune, nutrition, hormone, growth factor, enzyme, oncologic and embryonic subgroups, and compare the expression degree, changing pattern and specific timing.
The results were: (1) the remnant liver weight increased to 90% in 72h after partial hepatectomy; (2) the mitosis of hepatocytes increased marked at 48h then decreased at 72 after partial hepatectomy; (3) analyzing the gene expression of microarray chips, the variation could be classified into 72 different patterns in cluding the patterns with a single peak at 2, 4, 6, 12, 24, 72h and 5, 7d after
partial hepatectomy; (4) gene clusters of immune, hormone, growth factor, enzyme and angiogenesis have changed markedly; (5) early stage changed genes including fas-associating protein with death domain, carnitine palmitoyltransferase 1, fas death domain-associating protein, and steroid O-acyltransferase 1 could be related to the initiation of liver regeneration; (6) intermediate stage changed genes including transforming growth factor beta 2 and beta receptor could be related to the differentiation of liver regeneration; (7) late stage changed genes including TGF-β regulated gene 3 and small inducible cytokine A2 could be related to the termination of liver regeneration.
Key words: liver regeneration, partial
hepatectomy, proto-oncogene, microarray, genetic changing pattern, gene cluster
INTRODUCTION
Hepatocytes have a quiescent and highly differentiated phenotype. They rarely divide in adult humans or animals while remaining in the G0-phase of the cell cycle. However,
their capacity to replicate is not lost and is readily activated after liver resection or after injury induced by chemicals or drugs. Therefore, hepatocytes constitute a conditional renewal cell system that may proliferate in vivo under well-defined conditions.1-3 It seems that liver ”knows” when to start and when to stop growing, and thereby accurately regulates its mass.4,5 Partial hepatectomy triggers hepatocyte proliferation whereas excessive liver mass is regulated by apoptosis. The process of initiation and the control of the final size of the regenerated liver have been the subject of research for many years.6-9 Genetic regulation should have played an important role during the liver regeneration, however, the knowledge on the genetic mechanism is still limited.
It is reported that proto-oncogene expression after partial hepatectomy is
specific, sequential, and highly regulated.10,11 Changes have been detected in the expression of c-fos, c-myc, p53 and the ras gene family (c-Ha-ras, c-Ki-ras, and N-ras).12-14 In contrast, expression of c-src and c-abl does not change after partial hepatectomy while c-mos transcripts cannot be detected in normal or regenerating liver.15,16 Recently, p21 cyclin-dependent kinase (CDK) inhibitor, Fas, interleukin (IL)-18, and several caspases which inceased apoptosis, and Bcl-2, heat shock proteins, glutathione-S-transferase genes which down regulated cell proliferation were noted to be involved in liver regeneration.17 D6.1A gene was proved relate to stimulation of cell proliferation and differentiation.18 Fox MIB transcription factor was proved contribute to the decline in liver regeneration in the aging process.19 Insulin like growth factor binding protein 1 (IGFBP-1), HURP mRNA were also noted involve in the process of liver regeneration.20,21 The mass survey about the variation of all the regulating proto-oncogenes expression according time sequence, which is not reported yet, should be very important for investigating the genetic mechanism of liver regeneration.
This study was conducted to find out the variation patterns of more than 6,000 regulating genes expressions by cDNA microarray during liver regeneration after partial hepatectomy in rats.
MATERIALS AND METHODS Experimental Protocol
Sixty male Wistar rats (purchased from Charles River, Osaka, Japan) weighing approximately 200g were used as subjects. All of them received partial hepatectomy and they were sacrificed before and 2, 4, 6, 8, 12, 24, 48, 72 hours and 5, 7 days after hepatectomy. Six were sacrificed each time and the remnant livers were removed immediately for further tests.
All rats are anesthetized by intraperitoneal pentobarbital (10mg/kg) injection. A midline laparotomy was performed. Partial hepatectomy was then carried out by means of aseptic extirpation of the median and left lateral lobes (around 70%) according to the procedure of Higgins and Anderson.9
Measurements
(1)Evaluation of the remnant liver
Observation of the liver surface and color. Then weighing the liver immediately after sacrifice, and the ratio of remnant liver weight/body weight will be calculated.
(2)Mitotic index of remnant liver
The small pieces of liver tissue for hisopathological examination at certain postoperative period will be fixed in 10% neutral formalin, embedded in paraffin, sectioned and stained with hematoxylin-eosin for microscpic observation. The mitotic index will be determined by counting the number of parenchymal cells undergoing mitosis in 50 randomly-selected fields under magnification ×400.
(3)Genomic survey of remnant liver by cDNA microarray
a. Non-isotopic labeling of liver mRNA Total RNA was extracted from remnant livers of sacrificed rats in each postoperative time sequence. The tissue was homogenized in 3 ml of solution A containing 4M guanidine thiocyanate, 0.5% sarcosyl, 25 mM sodium citrate, and 0.1M betamercaptoethanol at pH 7.0, followed by phenol extraction, isopropanol precipitation, and ethanol precipitation. Quality of RNA was examined by agarose gel electrophoresis. Messenger RNA was purified using Qiagen Oligotex extraction Kit.
b. Hybridization and image analysis of
microarray
The membrane containing 384 spots cDNA was pre-hybridized in 5 ml 1×hybridization buffer containing 5×SSC, 0.1% SDS, 1% BM blocking buffer (Boehringer Mannheim), and 10 ug/ml denatured salmon sperm DNA, at 60ºC for 1 h. The probe was mixed with 2 ul of 10 ug/ul poly d(A) 10 and 2 ul of 10 ug/ul human Cot-1 DNA (Gibco BRL) and 40 ul of 2×hybridization buffer to a final volume of 80 ul, followed by denaturation of the probe mixture at 95ºC for 5 min and then cooling on ice. The membrane was then washed with TBS buffer three times for 5 min each. The membrane was then treated with 5 ml X-gal substrate containing 1.2 mM X-gal, 1 mM MgCl2, 3 mM K3Fe(CN)6, 3 mM K4Fe(CN)6
in TBS buffer for 45 min at 37ºC with gentle shaking, followed by mini-Q water wash and air dry. Color image was generated using UMAX PowerLook 3000 flatbed scanner at a resolution 3048 dpi and processed by ScanAlyze.
Data analysis
The images captured by a scanner could be digitized by a commercial analysis software such as GenePix 3.0 (Axon instruments) or the program written in-house. These softwares are free and available from Stanford university and Massachusetts Institute of Technology once got the license permission for non-profit use.
RESULTS
The rats were living well during experimental stage. After sacrifice, the remnant liver weight increased to 90% in 72h after partial hepatectomy. The mitosis index showed that the mitosis of hepatocytes in the remnant liver increased to 104±12 at 48h, then decreased to 24±6 at 72h after partial hepatectomy.
The mRNA extracted from the remnant liver was collected at the indicated intervals
(before and 2, 4, 6, 12, 24, 48, 72 hours and 5, 7 days after partial hepatectomy) and analyzed by Northern blot hybridization as shown in Fig 1. The mRNA examined by agarose gel electropgoresis were well qualified in the 28s/18s ratio of 2, and the 260nm/280nm OD ratio of 2 by spectrophotometry.
The colorimetric image of cDNA microarray hybridization with 6,144 putative genes on the nylon membrane of the remnant liver before and 6, 24, 72 hours after partial hepatectomy were shown in Fig 2. The scattering of the scanned spots were not eventually even and the deep-colored spots were not much in the cDNA microarray chip from the normal liver tissue before hepatectomy. The spots increased in number and in the density on the chips at 6, 24, 72, hours after partial hepatectomy. The variation was uneven and not in a regular pattern.
When the microarray chip was analyzed by a flatted scanner and the GenePix in each time sequence, the variations of all the 6,144 proto-oncogenes expression could be classified into 72 different patterns (Fig.3). The various patterns of the genes expressions include the pattern containing a single peak which occurred at 2h (c29, 86 genes), 4h (c7, 80 genes), 6h (c0, 178 genes), 12h (c24, 117 genes), 48h (c64, 71 genes), 72h (c40, 73 genes), 5d (c69, 141 genes), and 7d (c44, 93 genes). In addition, typical double-peaks patterns occurred at different time sequence such as 12h, 5d (c16, 58 genes) and 6h, 5d (c68, 70 genes). Moreover, patterns showing increasing trend since 4h (c61, 65 genes) or patterns illustrating a decreasing trend since 4h (c21, 67 genes) were also noticed in some proto-oncogenes expressions. Beyond that, the protruding types of patterns from 12h to 24h (c25, 48 genes) and from 6h to 72h (c33, 56 genes), or the excavated types of patterns from 4h to 5d (c38, 98 genes) and 6h to 5d (c47, 101 genes; c55, 65 genes) were found. Mixed types of time-dependent curves were detected as other unclassified variation
patterns of genes expressions. The name, NCBI number and features of the chosen representative genes which were contained in the 72 patterns of genes expressions were listed in Table 1. Each category of gene expression pattern contained 40 to 218 identified proto-oncogenes.
The genes with a single peak at 2h (e.g. fas-associating protein with death domain, NCBI# NM_152937), 4h (e.g. carnitine palmitoyltransferase 1, NCBI# NM_031559), 6h (e.g. fas death domain-associating protein, NCBI# NM_007829), 12h (e.g. steroid O-acyltransferase 1, NCBI# NM_009230), and the genes with an enhancing trend (e.g. protein kinase, DNA activated catalytic polypeptide, NCBI# NM_011159), a protruding increasing curve (e.g. killer cell lectin-like receptor, NCBI# NM_012745), occurred before 24h after partial hepatectomy might be considered as the genes closely related G0 to G1 phase. The genes with a
diminished trend (e.g. fibroblast growth factor 1, NCBI# NM_010197) or an
excavated curve (e.g. glutathione-S-transferase alpha, NCBI#
NM_017013) which occurred before 24h are also considered to be the down-regulation genes in this phase. These genes changed markedly before 24h might be considered as the initiation related genes of liver regeneration. Further verification by real time polymerase chain reaction (RT-PCR) or Western blot of these genes should be needed. As for the further process of cell cycles into S, G2 and M periods, the trigged hepatocytes
might just go on in a nature course. However, further study is needed to evaluate whether other genes involved in the whole cell cycles or not.
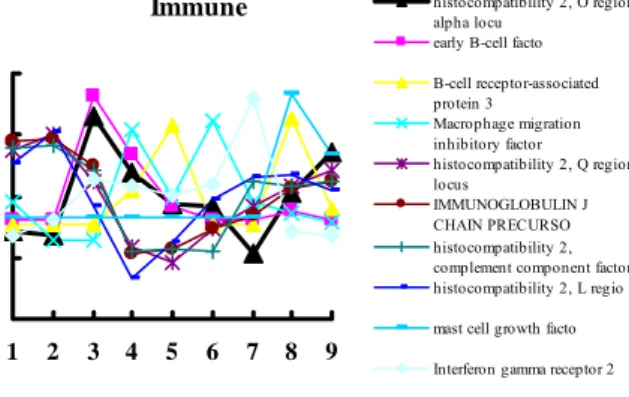
As for subgrouping the genes into clusters of immune, hormone, growth factor, enzyme and angiogenesis, many genes in these clusters were found to have markedly changed in different patterns. The name and changing pattern of these genes clusters were shown in Fig. 4-8.
DISCUSSION
The multistep process of liver regeneration constitutes at least 2 critical phases: the transition of the quiescent hepatocyte into the cell cycle (priming) and the progression beyond the restriction point in the G1 phase of the cycle.2 Four
transcription factors, NFKB, STAT3 (both are strongly induced by TNF), AP-1, and C/EBP β are activated after partial hepatectomy and they may play important roles in the initiation of liver regeneration.23-26 Harber et al. proved that 70 genes were induced with relation to liver regeneration during 9 days after partial hepatectomy.27 Since the initiation and termination of liver regeneration is a self-regulating growth process, the progression phase must be dependent on proto-oncogene regulatory mechanisms. Thereafter, mass survey and analysis of genes expression, although not reported yet, should be very important in the investigation of the genetic mechanism of liver regeneration.
Analysis of genes expression by cDNA microarray technology led to the identification of many regeneration-related genes expressions during liver regeneration stage after partial hepatectomy. We analyzed 6,144 genes according the time sequency after partial hepatectomy by PCR-amplified cDNA fragments and arraying machine, and found that a lot of genes changed there expressions in totally 72 patterns of changing curves. The extraction of mRNA in the regenerated liver was well qualified by both Northern blot hybridization and colorimetric image of microarray chips as shown in Fig.1 and Fig.2. The quantative analysis of time-dependent patterns of the genes expression by the computer showed the variation of patterns including a single peak at early, intermediate, late phase, or two separated peaks, a protruding curve, an excavated curve, ascending or descending trends with a different number of genes. It’s hard to conclude how many or which genes
are involved in the regenerating mechanism. However, many of these genes play very important roles at some specific timing during the liver regeneration. The effects may be direct or indirect, enhanced of diminished.
Quantitative gene expression profiles as shown in this study proved that a lot of proto-oncogenes in the remnant liver were found to have their expressions changed and were classified into 72 categories of patterns according to the time sequence during liver regeneration from 2 hours to 7 days after partial hepatectomy. It should be an important implication for the further investigation about the genetic mechanisms and, furthermore, the gene therapy for enhancing the liver regeneration.
REFERENCES
1. George K, Marie C. Liver regeneration. Science 1997;276:60-6.
2. Kountouras J, Boura P, Lygidakis NJ. Liver regeneration after hepatectomy. Hepato-Gastroenterology
2001;48:556-62.
3. Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today 1997;27:518-26.
4. Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, et al. Liver regeneration is an angiogenesis-associated phenomenon. Ann Surg 2002;236:703-11.
5. Gaglio PJ, Liu H, Dash S. Liver regeneration investigated in a non-human primate model (Macaca mulatta). J Hepatol 2002;37: 625-32.
6. Higgins GM, Anderson RM. Experimental pathology of the liver; I. Restoration of the liver by the white rat following partial surgical removal. Arch Pathol 1931;12:186-202.
7. Lai HS, Chen Y, Chen WJ. Carnitine contents in remnant liver, kidney and
skeletal muscle after partial hepatectomy in rats: randomized trial. World J Surg 1998;22:42-7.
8. Court FG, Wemyss-Holden SA, Dennison AR, Maddern GJ. The mystery of liver regeneration. Br J Surg 2002;89:1089-95.
9. Mitsue S, Hamanoue M, Tanabe G, Ogura Y, Yoshidome S, Aikou T, et al. Expression of HGF and TGF-beta 1 mRNA after partial hepatectomy in rats with liver cirrhosis. Surg Today 1995;25:237-43.
10. Fausto N, Shank PR. Oncogene expression in liver regeneration and hepatocarcinogenesis. Hepatology 1983;3:1016-23.
11. Fausto N, Mead JE, Braun L, Thompson NL, Panzica M, Goyette M, et al. Proto-oncogene expression and growth factors during liver regeneration. Symp Fundam Cancer Res 1986;39:69-86. 12. Thompson NL, Mead JE, Braun L,
Goyette M, Shank PR, Fausto N. Sequential protooncogene expression during rat liver regeneration. Cancer Res 1986;46:3111-7
13. Corral M, Paris B, Guguen-Guillouzo C, Corcos D, Kruh J, Defer N. Increased expression of the N-myc gene during normal and neoplastic rat liver growth. Exp Cell Res 1988;174:107-15.
14. Fausto N. Hepatic regeneration. In: Zakim D, Boyer TD eds. Hepatology. Pholadelphia, W.B. Saunders Co. 1990:Pp 49-65
15. Fausto N, Shank PR. Analysis of proto-oncogene expression during liver regeneration and hepatic carcinogenesis. In: Okuda K, Ishak KG, eds. Neoplasms of the liver. New York, Springer-Verlag, 1987:Pp57-61.
16. Goyette M, Petropoulos CJ, Shank PR, Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol 1984;4:1493-8.
17. Morita T, Togo S, Kubota T, Kamimukai N, Nishizuka I, Kobayashi T, et al. Mechanism of postoperative
liver failure after excessive hepatectomy investigated using a cDNA microarray. Hepat Biliar Pancr Surg 2002;9:352-9. 18. Tanaka F, Hori N, Sato K. Identification
of differentially expressed genes in rat hepatoma cell lines using subtraction and microarray. J Biochem 2002;131:39-44.
19. Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proceed Nat Aca Sci USA 2001;98:11468-73.
20. Leu JI, Crissey MA, Craig LE, Taub R. Impaired hepatocyte DNA synthetic response posthepatectomy in insulin-like growth factor binding protein 1-deficient mice with defects in C/EBP beta and
mitogen-activated protein kinase/extracellular signal-regulated
kinase regulation. Mol Cell Bio 2003;23:1251-59.
21. Tsou AP, Yang CW, Huang CY, Yu RC, Lee YC, Chang CW, et al. Identification of a novel cell cycle regulated gene, HURP, overexpressed in human hepatocellular carcinoma. Oncogene 2003;22:298-307.
22. Chen JJ, Peck K, Hong TM, Yang SC, Sher YP, Shih JY, et al. Global analysis of gene expression in invasion by a lung cancer model. Cancer Res 2001;61:5223-30.
23. FitzGerald M, Webber E, Donovan J, Fausto N. Rapid DNA binding by nuclear factor kB in hepatocytes at rhe
start of liver regeneration. Cell Growth Diff 1995;6:417-27.
24. Cressman DE, Diamond RH, Taub R. Rapid activation of the Stat3 transcription complex in liver
regeneration. Hepatology 1995;21:1443-1449.
25. Greenbaum LE, Li W, Cressman DE, Peng Y, Ciliberto G, Poli V, et al. CCAAT enhancer-binding protein beta is required for normal hepatocyte proliferation in mice after partial
hepatectomy. J Clin Invest 1998;102:996-1007.
26. Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signaling through the gp130/Jak/STAT pathway. Biochem J 1998;334:297-314.
27. Haber BA, Mohn KL, Diamond RH, Taub R. Induction patterns of 70 genes during nine days after hepatectomy define the temporal course of liver regeneration. J Clin Invest 1993;91:1319-2
Table 1. Representative genes contained in the 72 patterns of genes expressions*
Pattern Name NCBI number Features
Source Gene CDS
0 fas death domain-associated protein NM_007829 Mus musculus (house mouse) 1..2360 25..2244 1 myelocytomatosis oncogene BC006728 Mus musculus (house mouse) 1..1799 101..1420 2 MEK binding partner 1 (Mp1) mRNA, complete cd AF082526 Mus musculus (house mouse) 1..1315 147..521 3 myeloid cell leukemia sequence 1 NM_008562 Mus musculus (house mouse) 1..3464 76..1071 4 Bcl2-like 1 NM_009743 Mus musculus (house mouse) 1..1466 103..804 5 epidermal growth factor receptor NM_031507 Rattus norvegicus (Norway 1..4161 154..3783 6 IL2-inducible T-cell kinase NM_010583 Mus musculus 1..4294 93..1970 7 carnitine palmitoyltransferase 1 NM_031559 Rattus norvegicus (Norway 1..4377 103..2424 8 RAS p21 protein activator 1 NM_145452 Mus musculus 1..2626 1..2442 9 oncoprotein induced transcript NM_146050 Mus musculus (house mouse) 1..1299 257..928 10 small inducible cytokine A1 AF065928 Mus musculus (house mouse) 1..>276 48..>276 11 clusterin BC061534 Rattus norvegicus (Norway 1..1678 77..1420 12 apoptosis inhibitor BC062055 Rattus norvegicus (Norway 1..2907 643..2412 13 insulin-like growth factor 1 NM_184052 Mus musculus (house mouse) 1..2170 1344..184 14 pyruvate dehydrogenase BC002188 Mus musculus (house mouse) 1..1311 1..964 15 interleukin 1 receptor accessory protein NM_134103 Mus musculus (house mouse) 1..1916 133..1215 16 v-src suppressed transcript 5 XM_356456 Mus musculus (house mouse) 1..552 1..552 17 cadherin NM_199470 Mus musculus (house mouse) 1..2783 216..2561 18 glutamic acid decarboxylase NM_008077 Mus musculus (house mouse) 1..3198 185..1966 19 cytochrome P450, 2f NM_019303 Rattus norvegicus (Norway 1..1768 13..1488 20 fibroblast growth factor receptor 1 BC033447 Mus musculus (house mouse) 1..2877 29..2224 21 fibroblast growth factor 1 NM_010197 Mus musculus (house mouse) 1..3404 189..656 22 fos-like antigen NM_008037 Mus musculus (house mouse) 1..5825 171..1151 23 cell death-inducing DNA fragmentation factor, NM_009894 Mus musculus (house mouse) 1..1167 121..780 24 sterol O-acyltransferase 1 NM_009230 Mus musculus (house mouse) 1..3697 810..2432 25 killer cell lectin-like receptor, subfamily D, member NM_012745 Rattus norvegicus (Norway 1..1118 263..802 26 histocompatibility 2, Q region locus 1 NM_010390 Mus musculus (house mouse) 1..1092 1..1092 27 sterol carrier protein 2, liver BC018384 Mus musculus (house mouse) 1..2626 56..1699 28 integrin alpha 2 BC065139 Mus musculus (house mouse) 1..4235 109..3645 29 fas-associating protein with death domain NM_152937 Rattus norvegicus (Norway 1..1556 64..690 30 Fyn proto-oncogene NM_012755 Rattus norvegicus (Norway 1..1844 231..1844 31 leukotriene A4 hydrolas NM_008517 Mus musculus (house mouse) 1..2039 81..1916 32 ribosomal protein S2 BC002186 Mus musculus (house mouse) 1..954 19..900 33 tumor necrosis factor induced protein NM_182950 Rattus norvegicus (Norway 1..2123 172..1122 34 interleukin 4 receptor, alpha NM_010557 Mus musculus (house mouse) 1..3697 237..929 35 glycerol kinase NM_024381 Rattus norvegicus (Norway 1..2989 100..1674 36 insulin-like growth factor binding protein 4 XM_340897 Rattus norvegicus (Norway 1..1390 245..1009 37 cytochrome P450, 2d9 NM_010000 Mus musculus (house mouse) 1..1877 26..1501
38 glutathione-S-transferase, alpha NM_017013 Rattus norvegicus (Norway 1..831 64..732 39 histocompatibility 2, L regio BC023409 Mus musculus (house mouse) <1..151 <1..1001 40 transforming growth factor, beta receptor NM_031132 Rattus norvegicus (Norway 1..2080 252..1955 41 oncostatin M receptor NM_011019 Mus musculus (house mouse) 1..4792 96..3011 42 ubiquitin-conjugating enzyme E2H NM_009459 Mus musculus (house mouse) 1..2828 242..793 43 histocompatibility 2, class II, locus DM NM_010386 Mus musculus (house mouse) 1..1135 44..829 44 small inducible cytokine A2 NM_031530 Rattus norvegicus (Norway 1..780 76..522 45 galactosidase, alph NM_013463 Mus musculus (house mouse) 1..2801 25..1284 46 RAS, guanyl releasing protein AF060819 Rattus norvegicus (Norway 1..2883 7..2394 47 fumarylacetoacetate hydrolase NM_017181 Rattus norvegicus (Norway 1..1386 23..1282 48 TNF-related apoptosis inducing ligan AY115578 Rattus norvegicus (Norway - 10..873 49 lipase, hepatic (Lipc) NM_008280 Mus musculus (house mouse) 1..1934 249..1781 50 RNA polymerase 1-3 (16 kDa subunit) NM_181730 Mus musculus (house mouse) 1..914 102..482 51 platelet derived growth factor receptor, alpha NM_011058 Mus musculus (house mouse) 1..6576 190..3459 52 interferon regulatory factor 3 NM_016849 Mus musculus (house mouse) 1..1915 132..1391 53 Jun oncogene NM_010591 Mus musculus (house mouse) 1..3135 917..1921 54 CD4 antigen BC039137 Mus musculus (house mouse) 1..3082 149..1522 55 CD36 antigen (collagen type I receptor, NM_054001 Rattus norvegicus (Norway 1..1938 231..1667 56 interleukin 7 receptor NM_008372 Mus musculus (house mouse) 1..3116 48..1427 57 peptidase 4 NM_008820 Mus musculus (house mouse) 1..1876 55..1536 58 C-src tyrosine kinase NM_007783 Mus musculus (house mouse) 1..2292 249..1601 59 Large multifunctional protease 7 NM_010724 Mus musculus (house mouse) 1..828 1..828 60 interleukin 15 receptor, alpha chain NM_133836 Mus musculus (house mouse) 1..1465 367..738 61 protein kinase, DNA activated catalytic polypeptide NM_011159 Mus musculus (house mouse) 1..1265 1..12387 62 carbonyl reductase 4 NM_182672 Rattus norvegicus (Norway 1..1188 106..816 63 FK506 binding protein 1a NM_008019 Mus musculus (house mouse) 1..1556 98..424 64 transforming growth factor, beta 2 BC011170 Mus musculus (house mouse) 1..1741 127..1371 65 xanthine dehydrogenas NM_017154 Rattus norvegicus (Norway 1..4198 27..4022 66 tumor susceptibility gene 101 NM_181628 Rattus norvegicus (Norway 1..1176 1..1176 67 guanine nucleotide binding protein (G protein), NM_025278 Mus musculus (house mouse) 1..1151 235..453 68 squalene epoxidase NM_009270 Mus musculus (house mouse) 1..2611 620..2338 69 transforming growth factor beta regulated gene 3 NM_178871 Mus musculus (house mouse) 1..2822 244..660 70 casein kinase II, alpha 2, polypeptide NM_009974 Mus musculus (house mouse) 1..1877 344..1396 71 macrophage activation 2 NM_008620 Mus musculus (house mouse) 1..3295 90..1961 NCBI, national Center for Biotechnology Information; *, only one representative was listed in each pattern which may contained 40 to 218 identified proto-oncogenes
24-HR
Fig 1. The mRNA extracted from the remnant liver showed a qualified picture by agarose gel electrophoresis.
Fig 2. The colorimetric image of cDNA microarray hybridization chips with 6,144 genes showed uneven changed patterns before (0-HR), and 6, 24, 72 hours after partial hepatectomy.
Fig 3. Seventy-two different patterns of genes expressions in the remnant liver during liver regeneration according the time sequence of 0, 2, 4, 6, 12, 48, 72 hours and 5, 7 days after partial hepatectomy (C= sequence number of categories; the following number= numbers of genes included in this category; the longitudinal line is the intensity of gene expression; the longitudinal line is the intensity of gene expression).
0-HR 6-HR
72-HR 24-HR
Immune
1 2 3 4 5 6 7 8 9
histocompatibility 2, O region alpha locu
early B-cell facto B-cell receptor-associated protein 3 Macrophage migration inhibitory factor histocompatibility 2, Q region locus IMMUNOGLOBULIN J CHAIN PRECURSO histocompatibility 2, complement component factor histocompatibility 2, L regio mast cell growth facto Interferon gamma receptor 2 Fig 4. The names and their changing patterns of immune related genes.
Hormone 1 2 3 4 5 6 7 8 9 Somatostatin somatostati cholecystokini neuropeptide nociceptin proteolipid protein (myelin GATA-binding protein growth hormone releasing hormon secretogranin II
Fig 5. The names and their changing patterns of hormone related genes.
Growth Factor
1 2 3 4 5 6 7 8 9
growth factor independent 1 epidermal growth factor recepto
insulin-like growth factor fibroblast growth factor Fibroblast growth factor 12
Fibroblast growth factor receptor 3 fibroblast growth factor receptor transforming growth factor, beta receptor II
Fig 6. The names and their changing patterns of growth factor related genes.
Enzyme 1 2 3 4 5 6 7 8 9 ubiquitin c-terminal hydrolase L acetylcholinesteras glutathione synthetas glutathione peroxidase carnitine palmitoyltransferase 1, live protein kinase, cAMP dependent regulatory, type I bet
deoxyribonuclease I Amine oxidase, copper containing 3 pyruvate dehydrogenase
Fig 7. The names and their changing patterns of enzyme related genes.
Angiogenesis
1 2 3 4 5 6 7 8 9
Mus musculus mRNA for vascular cadherin-Cadherin 16 Zinc finger protein 46 angiotensinoge transforming growth factor beta 1 induced transcript cadherin Calreticulin fibronectin
Fig 8. The names and their changing patterns of angiogenesis related genes