236
T
he early administration of methylprednisolone (MP) is believed to improve the neurologic recovery of patients sustaining an acute traumatic spinal cord injury (SCI). In the National Acute Spinal Cord Injury Study (NASCIS)-2 Bracken et al. reported that patients who were treated with large dosage of MP (bolus of 30 mg per kilogram of body weight, maintained at 5.4 mg perkilogram per hour for 23 hours) within 8 hours of their injuries had significant neurologic improvement after the periods of 6 weeks, 6 months and one year.1In 1997 they
even recommended to maintain the treatment for 48 hours if steroid therapy was initiated 3 to 8 hours after injury.2
Since the releases of NASCIS-2 and NASCIS-3, there have been several commentaries about the
Formos J Surg 2006;39:236-245
Reappraisal of Methylprednisolone Treatment for Acute
Traumatic Cord Injury
Carlos Lam*,***, Ming-Fu Chiang****, Shin-Han Tsai*,**,***, Hung-Yi Chiou***,
Cheuk-Sing Choy*,***, Mau-Roung Lin***, Wen-Ta Chiu**,***
Objective: The value of methylprednisolone (MP) treatment in acute traumatic
spinal cord injury (SCI) remains controversial. We attempted to evaluate the
utilization of resources, motor function recovery and adverse effect after the use of
MP.
Methods: We compared 13 demographic and clinical characteristics between MP
and non-MP treatment groups in 110 patients with acute traumatic SCI treated in
hospitals between June 1st, 2000 and May 31st, 2001, and analyzed 10 short-term
outcome variables. These demographic and clinical characteristics included age,
cause of injury, number of associated injuries, Revised Trauma Score, Glasgow Coma
Scale, mean blood pressure, level/completeness and pattern of SCI, number and types
of spinal surgeries, frequency of rehabilitation therapy and the time interval between
trauma and admission.
Results: The MP (64.5%) and non-MP (35.5%) treatment groups showed no
significant differences in all characteristics except the time interval between trauma
and admission (P=0.024). MP treatment was associated with a higher frequency of
infectious complications (P=0.038), but there was no difference between the two
treatment groups in other outcome parameters. The results of analysis stratified by
dosage of MP showed that the length of ICU stay (P=0.021) and the number of
tracheostomies (P=0.005) and pneumonia cases (P=0.004) were increased significantly
in the standard dose group.
Conclusions: Although the rate of infection had risen in patients receiving MP, the
steroid treatment did not significantly increase utilization of resources during
hospitalization and appeared safe in terms of mortality. However, it had not been
proven to improve motor function recovery.
Key words: methylprednisolone, spinal cord injury, outcome, infection
From the Department of Critical and Emergency Medicine*, Division of Neurosurgery, Wanfang Hospital, Taipei Medical
University**, Graduated Institute of Injury Prevention and Control, Taipei Medical University***, Division of Neurosurgery, Macky
methodology of the trials and the risk-benefit of MP treatment.3,4,5 The results of MP treatment remain
controversial. The debate regarding its efficacy ranges between two polarized thoughts. At one end, clinicians, who strongly maintain the convictions of NASCIS-2 and NASCIS-3, use large doses of MP within 8 hours of injury.6,7At the other extreme are those who think that
large dosage of steroid has no benefit on neurologic recovery, sometimes even harmful for the patient.8,9,10The
theoretical advantages supporting the early use of MP in the acute phase are prevention of lipid peroxidation, inhibition of free radical injury and early stabilization of the damaged cell membrane, which can maximize the potential for recovery and result in improvement of long-term motor and sensory function.11
Owing to their widespread application in North America, the major difficulty in assessing the results of MP treatment is the identification of a suitable control group for statistical analysis. A prospective controlled trial has recently been completed in France and no benefit in neurologic recovery was found; instead, more infectious complications occurred.12There were also
reports about its septic consequences.13,14
We performed a retrospective study to evaluate the utilization of resources, motor function recovery and adverse effect after the use of MP in patients of acute traumatic SCI.
Between June 1st, 2000 and May 31st, 2001, we studied 110 patients that were diagnosed to have traumatic SCI and admitted to the medical centers or regional hospitals in the northern and eastern parts of the island, and these medical institutions had the capacity to provide comprehensive care for patients with SCI. To be included in our study, the patients had to be no less than 16 years old and admitted within 30 days after injury without definitive treatment in other hospitals. Patients sustaining spinal column injury without cord involvement or with nerve root injury only or merely the cauda equina syndrome were not included in the study. There was no penetrating injury found in this series.
Moreover, patients with incomplete records about the treatment method or in-hospital course were not included. Using a chart review, we recorded the patients' data on demographic and admission characteristics, treatment methods, clinical course of hospitalization, complications and outcome parameters by predetermined form.
Ten parameters of short-term outcome were used in this study for the evaluation of the utilization of
resources, neurologic recovery and adverse effects after the use of MP. Because the adverse effects on the patient after steroid treatment would be apparent shortly after administration, we only chose the in-hospital parameters of short-term outcome for evaluation in this study. To evaluate the utilization of resources, we looked at the length of hospitalization, ICU stay, number of ventilated days, number of tracheostomy cases and duration between admission and start of rehabilitation; for the neurologic recovery, the scores of motor function change during hospitalization and 6 weeks after admission were used; and for the evaluation of adverse effects, the number and types of complications and in-hospital mortality were used.
In this study, the patients received MP treatment only once and no additional peri-operative prophylactic use. Halos or skull tongs were rarely applied and were not included as spinal surgery.
Only motor function was analyzed for neurologic recovery. In a previous study of cord injury, the patients who were treated with high-dose MP had significant motor functional improvement after 6 weeks.1Therefore;
this shorter follow-up interval (6 weeks) was used in the present study.
The 5-point scale of muscle power of each extremity was used for motor function assessment. A score of 0 indicated no contraction, 1 indicated reduced contraction, 2 indicated active movement without antigravity, 3 indicated active movement with antigravity, 4 indicated reduced function but active movement against resistance, and 5 indicated normal motor function. A normal motor scale was described as 20 and the lowest was 0. The change in motor scale was calculated between admission and examination done at discharge and 6 weeks after admission.
The pattern of SCI was classified as quadriplegic, paraplegic, quadriparetic, and paraparetic. The classification is as follows: quadriplegic, if most cephalad muscle with no contraction was the first dorsal interosseous (C-8 to T-1) or higher with no contraction in any distal muscle; paraplegic, if most cephalad muscle with no contraction was below the first dorsal interosseous with no contraction in any distal muscle; quadriparetic, if most cephalad muscle with a trace of contraction or active movement without antigravity was the first dorsal interosseous or higher; and paraparetic, if most cephalad muscle with a trace of contraction or active movement without antigravity was below the first dorsal interosseous. The completeness of SCI was recorded according to the description of the admission diagnosis.
We hypothesized that the effect of treatment would be influenced by how fast and in how large dosage MP
Formos J Surg 2006 Vol 39 No 4
238 Methylprednisolone for Spinal Cord Injury in Taiwan
was given to the patient. Hence, stratified analysis was done by timing ( 8 hours or 8 hours) and dosage (standard or lesser dose) according to the NASCIS-2 protocol. At present, the NASCIS-3 protocol is rarely applied in Taiwan.
Details of 13 demographic and clinical characteristics, including age, cause of injury, time interval between trauma and admission, number of associated injuries, Revised Trauma Score (RTS), Glasgow Coma Scale (GCS) and mean blood pressure (MBP) at the emergency room (ER), level/completeness and pattern of SCI, number and types of spinal surgeries, and frequency of rehabilitation therapy were recorded to compare the entrance features between the two groups and to minimize the opportunity of confounding.
The information was entered into the computer for processing by 8.0 SPSS. Standard statistical methods, such as the Chi-square test and Fisher test, were utilized properly for data analysis. P<0.05 was considered significant.
Of the 110 patients, 71 patients received MP treatment (MP group), and 39 patients had no such treatment (non-MP group). There was no significant
difference between the two treatment groups concerning the level of hospitals providing treatment, either medical centers or regional hospitals.
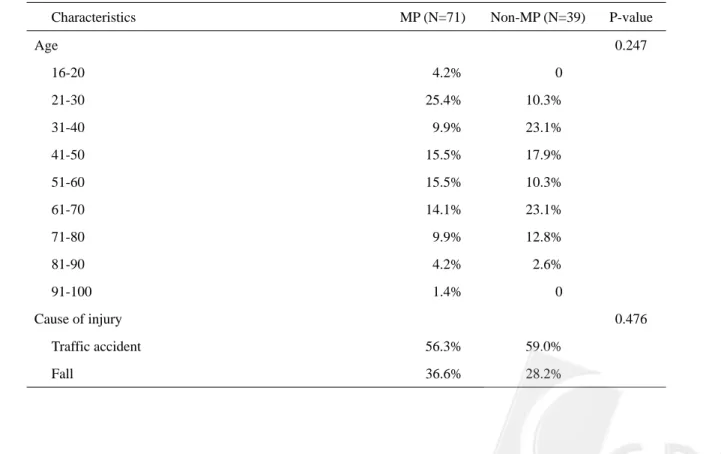
Table 1 compares the 13 characteristics of patients at entry between the two groups. There was no difference in age, cause of injury and associated non-spinal injuries. The severity of trauma, represented by the MBP, RTS and GCS at ER, was not significantly different between the two groups, nor the severity of the SCI, including level, completeness and pattern.
Approximately 82% of the patients had spinal surgery, which was performed mainly for neural decompression, and no significant difference was noted in the number or types of surgeries between the two groups. Frequency of rehabilitation therapy also showed the same results.
However, the two groups showed significant differences in the time interval between trauma and admission. The patients in the MP group arrived at the hospital for definitive treatment on an average of 4 days sooner than those without. These differences were due to treatment policy recommended by the NASCIS protocol, which selected only patients arriving at the hospital in less than 8 hours after injury.
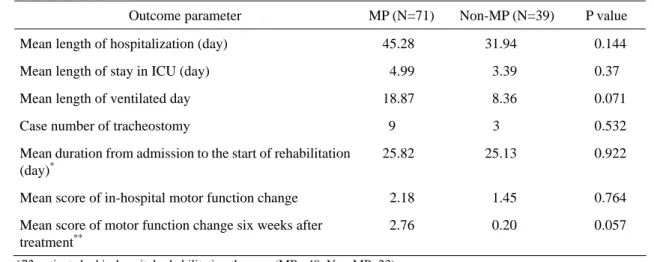
The outcome evaluated for in-hospital utilization of resources including length of hospitalization, length of intensive care, number of ventilated days, number of
R
esults
Characteristics MP (N=71) Non-MP (N=39) P-value
Age 0.247 16-20 4.2% 0 21-30 25.4% 10.3% 31-40 9.9% 23.1% 41-50 15.5% 17.9% 51-60 15.5% 10.3% 61-70 14.1% 23.1% 71-80 9.9% 12.8% 81-90 4.2% 2.6% 91-100 1.4% 0 Cause of injury 0.476
Formos J Surg 2006 Vol 39 No 4
Time between injury to admission (average day) 0.4366% 4.3889% 0.024 Mean Blood Pressure at ER (average) 91.8584% 96.4017% 0.263 Glasgow Coma Scale at ER (average) 14.4789% 13.9231% 0.234 Revised Trauma Score at ER (average) 7.6445% 7.5857% 0.604
Level of SCI 0.354 C-spine 76.1% 82.1% T-spine 9.9% 2.6% L-spine 18.3% 33.3% Completeness of SCI 0.179 Complete 18.8% 18.8% Incomplete 81.3% 81.3% Pattern of SCI 0.408 Quadriparetic 53.5% 56.4% Quadriplegic 7.0% 5.1% Paraparetic 16.9% 5.1% Paraplegic 9.9% 10.3%
Number of associated injury* 0.857
0 36.6% 38.5%
1 38.0% 30.8%
2 15.5% 20.5%
3
Њ 9.9% 0.3%
Spinal surgery following injury 0.289
Yes 63.4% 69.2%
No 36.6% 28.2%
Type of spinal surgery 0.588 Ant. Decompression 48.9% 45.7%
Post. Decompression 33.3% 17.1%
Rehabilitation therapy before discharge 0.289
Yes 69.0% 59.0%
No 31.0% 41.0%
* Associated injury included head injury, facial fracture, bony fracture of limb, thoracic injury, abdominal injury, pelvic
240 Methylprednisolone for Spinal Cord Injury in Taiwan
tracheostomy cases and length between admission and start of rehabilitation. There was no significant difference in these parameters between the two treatment groups (Table 2).
With the previously mentioned method of neurologic assessment, the MP group showed no significant motor recovery at discharge. Only 37 patients were admitted for more than 6 weeks, an average motor-function change of 2.769 and 0.200 was found respectively in the MP and non-MP groups and that only approached significant difference (Table 2).
As seen in Table 3, only three patients died during
hospitalization with a mortality rate of 2.7%. When the mortality rate was examined in relation to treatment regimen, no difference was noted. We also found no difference in frequency of complications between the two groups. Sepsis was rare (1.8%) and no thrombo-embolic complication (deep vein thrombosis or pulmonary embolism) was noted in the study. Seven categories of complications were analyzed: pneumonia, respiratory failure, upper gastro-intestinal bleeding (UGIB), urinary tract infection (UTI), pressure sores, neurogenic bladder and "other infections". The category of "other infections" was defined as all in-hospital infections other than
Outcome parameter MP (N=71) Non-MP (N=39) P value Mean length of hospitalization (day) 45.28 31.94 0.144 Mean length of stay in ICU (day) 4.99 3.39 0.37 Mean length of ventilated day 18.87 8.36 0.071 Case number of tracheostomy 9 3 0.532 Mean duration from admission to the start of rehabilitation
(day)*
25.82 25.13 0.922 Mean score of in-hospital motor function change 2.18 1.45 0.764
Mean score of motor function change six weeks after treatment**
2.76 0.20 0.057
Table 2. In-Hospital Utilization of Resource and Motor Function Change
* 72 patients had in-hospital rehabilitation therapy. (MP= 49, Non-MP=23) ** 37 patients were admitted more than 6 weeks. (MP= 30, Non-MP=7)
Outcome parameter MP (N=71) Non-MP (N=39) P value In-hospital mortality 2 1 0.714 Frequency of in-hospital complications 1.29 0.87 0.099 Neurogenic bladder 25 10 0.303 Urinary tract infection 20 5 0.066
Pneumonia 12 4 0.344
Pressure ulcer 9 3 0.534
Respiratory failure 9 4 0.483
UGI bleeding 6 1 0.418
“Other infections” 8 5 0.517
Formos J Surg 2006 Vol 39 No 4
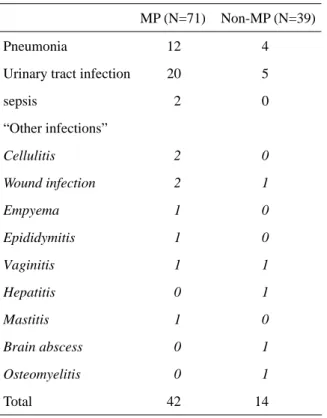
pneumonia, UTI, sepsis and wound infection after surgery. This category included cellulitis, epididymitis, vaginitis, empyema, etc. No significant difference was noted concerning these seven categories between the two groups. UTI was the most common infectious compli-cation in this study and showed a difference barely approaching the significant level (P=0.066). However, if we compared infectious complications between the two groups, which included pneumonia, UTI, sepsis and "other infections", 42 instances were found in the former and 14 instances in the later group, and the difference was significant (P=0.038). The types of infectious complications in both groups were shown in Table 4.
Forty-nine patients had spinal surgery (MP=32, Non-MP=17), mainly for spinal cord decompression, and no significant difference was found concerning post-operative complications, which included wound infection (P=0.664) and re-operation (P=0.583).
The dosages of MP administration could be traced clearly in all 71 patients of the MP group. Fifty-three patients (74.6%) received the standard dose of NASCIS-2 and 18 patients (25.4%) received an MP dose less than that assigned in the NASCIC-2 protocol. The results of the analysis stratified by doses are shown in Table 5. Owing to the lack of accurate data about the timing of MP administration in more than half of the patients (59%), no analysis stratified by timing was attempted.
MP (N=71) Non-MP (N=39)
Pneumonia 12 4
Urinary tract infection 20 5
sepsis 2 0 “Other infections” Cellulitis 2 0 Wound infection 2 1 Empyema 1 0 Epididymitis 1 0 Vaginitis 1 1 Hepatitis 0 1 Mastitis 1 0 Brain abscess 0 1 Osteomyelitis 0 1 Total 42 14
Table 4. Frequency and Content of Infectious
Complications in Table 3
Dose* Outcome measure
STD (N=53) Non STD (N=18) P value Mean length of hospitalization (day) 47.07 26.18 0.093 Mean length of stay in ICU (day) 4.74 1.38 0.021 Mean length of ventilated day 17.54 13.06 0.646 Case number of tracheostomy 6 1 0.005 Mean duration from admission to the start of rehabilitation (day) 23.76 14.57 0.281 Mean score of in-hospital motor function change 2.87 -0.21 0.066 Mean score of motor function change six weeks after treatment 3.13 -1.00 0.389 In-hospital mortality 2 0 0.438 Frequency of in-hospital complications 1.33 0.87 0.280
Pneumonia 9 1 0.004
Infectious complication 21 3 0.053
*dose of MP as NASCIC-2 protocol
STD: standard treatment dose as NASCIC-2 protocol Non STD: treatment dose less than NASCIC-2 protocol Table 5. Stratified Analysis of 71 Patients by dose of MP
Objective measurement of the results of different treatment regimens in acute SCI is difficult due to the complexity of the nature of injury and the lack of randomized and prospective controlled studies. It was not until the release of the NASCIC -2 results in 1990 that the medical treatment of acute SCI was standardized.1 At
present, large dosage of MP is widely accepted in North America as the routine treatment in emergency care and neurosurgical practice. Failure of administration after cord injury may become a medico-legal problem.10
Although the NASCIC reports strongly recommend that large dosage of steroids has been useful for neurologic recovery, there is still considerable controversy regarding this topic.7,15,16
About 30 years ago, the patho-physiology of SCI was evaluated and the role of glucocorticoids for SCI treatment was emphasized. Several reports on animal trial in laboratory were published.17The mechanism of cord
damage after injury involves activation of membrane lipases, which results in disturbance of the cell membrane. Since lipid peroxidation is considered as a major factor in cord damage and deterioration of the function of the central nervous system, it seems rational to apply MP because of its strong capacity of antioxidant effect and its activities as a free-radical scavenger. Braughler and Hall et al. reported using corticosteroids as a means of inhibiting free radical injury in experimental spinal cord injury, and post-injury neurologic condition was improved.18 The NASCIC-2 trial was the first
prospective, randomized, placebo-controlled trial that confirmed the efficacy of MP and justified its use as the first medication proved to improve the neurologic outcome for victims of SCI. Since then, its utility has grown rapidly and immediate administration has become the rule of care.19,20
Gerndt et al. analyzed 140 patient records with acute SCI admitted to a Level I trauma center, and compared different outcome parameters. The MP group had an increase in number of ventilated days and length of ICU stay; but also a shorter duration of rehabilitation. They concluded that a large-dose steroid increased utilization of resources during hospitalization.21According to the
results of our study; MP did not increase utilization of resources, which was represented by the length of hospitalization, ICU stay and the number of ventilated days, as well as the number of tracheostomy cases. Large dosage of steroids did not prolong in-hospital rehabilitation.
The evolution of neurologic function not only parallels biological changes in the spinal cord; it is also essential for the following rehabilitation therapy that can help the patient regain social activity. Recovery of neurologic status is not only the motive but also the cornerstone of the MP treatment. In-hospital and six-week changes in motor function were the measures of neurologic gain in this study. Although there were some improvements in these parameters, no significant difference in recovery was found between the two treatment groups.
Despite the results of NASCIC-2 and NASCIC-3, large-dose steroid treatment is still of concern to many physicians due to its well-known immunosuppressive action and its adverse effects in old-aged and trauma patients, particularly septic and gastro-intestinal complications.13,22,23 Gerndt et al. reported an increase in
pneumonia in the MP group. Although large-dose steroids induced early infection, they concluded that the steroid usage was safe and had no adverse impact on mortality.21
No significant increase in mortality and frequency of complications was noted between the two treatment groups in our study. When we divided the complications into seven categories (neurogenic bladder, UTI, pneumonia, pressure ulcer, respiratory failure, UGI bleeding, and other infections), again no significant differences were found, except UTI that appeared to occur more frequently in the MP group. When we measured infectious complications as a single parameter which included pneumonia, UTI, sepsis and "other infections", we indeed found a significant difference between the two groups. (P=0.038) The result strongly indicated that the patients were more likely to have infectious complications when receiving MP.
In France, Pointillart et al. prospectively evaluated 106 patients with SCI, addressed the controversy by analyzing the results of MP versus non-MP management in concurrently treated patients. They found no difference in neurologic recovery; instead, more infectious complications occurred.12Our finding was consistent with
the observation of Pointillart et al.
There were some surprising results disclosed after analysis stratified by dosage of MP as the NASCIC-2 protocol, thus separating the 71 patients into two groups: standard dose and standard dose. The group of non-standard dosage included patients that received a smaller dosage of MP. The length of ICU stay and the number of tracheostomy cases increased significantly in the standard dose group, as well as the number of septic complications, particularly pneumonia. Interestingly both parameters of
242 Methylprednisolone for Spinal Cord Injury in Taiwan
group, although the difference was not significant. Owing to the small number in some sub-groups after stratification, the data should be verified carefully by further studies with a larger sample size. However, the stratified results might imply that the dosage of MP is crucial for its clinical consequence and impact in the patient's outcome, either positively or negatively.
Shortly after the release of NASCIC-1,24 DeMaria et
al. reported an increase in septic complications, not only in frequency but also severity, after use of corticosteroids for CNS trauma.25 Since then, the contention around the
risk and benefit of large-dose steroids persisted and many reports or reviews have been published.10,26,27Nasathurai et
al. reported more sepsis and pneumonia in the MP group in NASCIS-3.3 Gerndt et al. also reported that the
incidence of pneumonia would increase after MP treatment.21The association between steroids and some
adverse consequences, such as infection, delayed healing, gastro-intestinal complications, are widely accepted14,22,23
The reason for use of large dosage steroids is only justified by the fact that neurologic recovery is the supreme priority for the SCI patient and there is currently no alternative medication. The consequences caused by large dosage of steroids should be emphasized and evaluated seriously if the results of such treatment are still in question.
Although we reviewed the charts of each patient extensively, this observation has its limitations because of the potential shortcomings of a retrospective study and the relatively small size of the sample. The results of this study should be interpreted with caution.
The present study could not confirm that the use of steroid would increase the utilization of resources during hospitalization, including length of hospitalization and ICU stays and number of ventilated days, and it would not delay the rehabilitation therapy that was essential for functional recovery. Although the use of MP treatment appeared safe in terms of mortality, the MP treatment did not show beneficial effects in motor function recovery in our observation. We found that patients receiving MP were more likely to have infectious complications. On the contrary, large-dosage therapy could increase the opportunity of serious complications such as pneumonia and the utilization of medical resources such as the ICU care and the number of tracheostomy cases. Controversy still exists; the value of MP in the treatment of acute SCI remains unconfirmed. Further rigorously controlled, randomized, prospective study, particularly a
well-prepared stratification by the dosage and timing of MP administration, is essential for solving this puzzle.
Abbreviations used in this paper: MP=methylpredni-solone; SCI=spinal cord injury; MBP=mean blood pressure; RTS=Revised Trauma Score; GCS=Glasgow Coma Scale; ER=emergency room; NASCIS=the National Acute Spinal Cord Injury Study; UTI=urinary tract infection; UGIB=upper gastro-intestinal bleeding; ICU=intensive care unit; CNS=central nervous system
The authors thank Chih-Han Lin, M.S. for his contribution in the preparation of this manuscript.
The following hospitals had provided assistance: National Taiwan University Hospital, Tri-service General Hospital, Mackay Memorial Hospital (Taipei), Cathay General Hospital, Municipal Yang-Ming Hospital, Municipal Chung-Hsin Hospital, Buddhist Tzu-Chi General Hospital (Hualien), Mennonite Christian Hospital, Taipei Medical University Hospital, Municipal Wanfang Hospital.
1. Bracken MB, Shepard MJ, Collins WF, et al: A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: Result of the Second National Acute Spinal Injury Study. N Engl J Med 1990; 322:1405-11.
2. Bracken MB, Shepard MJ, Holford TR, et al: Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Result of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. JAMA 1997;277:1597-604. 3. Nesathurai S: Steroids and spinal cord injury: revisiting the
NASCIS 2 and NASCIS 3 Trails. J Trauma 1998;45:1088-93. 4. Short D: Use of steroids for acute spinal cord injury must be
reassessed. BMJ 2000 (Letter);321:1224.
5. Blight AR, Zimber MP: Acute spinal cord injury: pharmacotherapy and drug development perspectives. Curr Opin Investig Drugs 2001;2:801-8.
6. Bracken MB, Holford TR: Neurological and functional status 1 year after acute spinal cord injury: estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. J Neurosurg 2002;96:259-66.
Formos J Surg 2006 Vol 39 No 4
C
onclusions
A
cknowledgment
A
bbreviations
244 Methylprednisolone for Spinal Cord Injury in Taiwan
7. Fehlings MG, Editorial: Recommendations regarding the use of methylprednisolone in acute spinal cord injury: making sense out of the controversy. Spine 2002;26:S56-7.
8. Benzel EC, Editorial: Commentary on National Acute Spinal Cord Injury Study III. J Neurosurg 2002;96:257-8.
9. Hadley MN, Walters BS, Grabb PA, et al: Guidelines for the management of acute cervical spine and spinal cord injuries. Clin Neurosurg 2002;49:407-98.
10. Hurlbert RJ: Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg 2000;93:1-7. 11. Hall ED: The effects of glucocorticoid and nonglucocorticoid
steroids on acute neuronal degeneration. Adv Neurol 1993;59: 241-8.
12. Pointillart V, Petitjean ME, Wiart L, et al: Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord 2000;38:71-6.
13. Matsumoto T, Tamaki T, Kawakami M, et al: Early complications of high-dose Methylprednisolone Sodium Succinate treatment in the follow-up of acute cervical spinal cord injury. Spine 2001;26:426-30.
14. Molano Mdel R, Broton JG, Bean JA, et al: Complications associated with the prophylactic use of methylprednisolone during surgical stabilization after spinal cord injury. J Neurosurg 2002;96:267-72.
15. Short DJ, El Masry WS, Jones PW: High dose methylpredni-solone in the management of acute spinal cord injury-a systematic review from a clinical perspective. Spinal Cord 2000;38:273-86.
16. Hurlbert RJ, Moulton R: Why do you prescribe methylpredni-solone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci 2002;29:236-9. 17. Hansebout RR, Kuchner EF, Romero-Sierra C: Effects of
local hypothermia and of steroids upon recovery from
experimental spinal cord compression injury. Surg Neurol 1975;4:531-6.
18. Hall ED, Braughler JM: Glucocorticoid mechanisms in acute spinal cord injury: a review and therapeutic rationale. Surg Neurol 1982;18:320-7.
19. Amar PA, Levy ML: Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery 1999;44:1027-40.
20. Chappell ET: Pharmacological therapy after acute cervical spinal cord injury. Neurosurgery 2002 (Letter);51:855-6. 21. Gerndt SJ, Rodriguez JL, Pawlik JW, et al: Consequences of
high-dose steroid therapy for acute spinal cord injury. J Trauma 1997;42:279-84.
22. Epstein N, Hood DC, Ransohoff J: Gastro-intestinal bleeding in patients with spinal cord trauma: Effects of steroids, cimitidine, and mini-dose heparin. J Neurosurg 1981;54:16-20.
23. Weiner HL, Rezai AR, Cooper PR: Sigmoid diverticular perforation in neurosurgical patients receiving high-dose corticosteroids. Neurosurgery 1993;33: 40-3.
24. Bracken MB, Collins WF, Freeman DF, et al: Efficacy of methylprednisolone in acute spinal cord injury. JAMA 1984;251:45-52.
25. DeMaria EJ, Reichman W, Kenney PR, et al: Septic complications of corticosteroid administration after central nervous system trauma. Ann Surg 1985;202:248-52.
26. Hall ED: Pharmacological treatment of acute spinal cord injury: how do we build on past success? J Spinal Cord Med 2001;24:142-6.
27. Otani K, Abe H, Kadoya S: Beneficial effect of Methylprednisolone Sodium Succinate in the treatment of acute spinal cord injury. Sekitsui Sekizui J 1994 (Jpn);7:633-47.