International Journal of Antimicrobial Agents 35 (2010) 261–264
Contents lists available atScienceDirect
International Journal of Antimicrobial Agents
j o u r n a l h o m e p a g e :h t t p : / / w w w . e l s e v i e r . c o m / l o c a t e / i j a n t i m i c a gShort communication
Fluoroquinolone resistance of Pseudomonas aeruginosa isolates causing
nosocomial infection is correlated with levofloxacin but not ciprofloxacin use
Yuarn-Jang Lee
a,b, Hsin-Yi Liu
a, Yi-Chun Lin
a,b, Kuo-Lun Sun
c, Chi-Li Chun
d, Po-Ren Hsueh
e,∗ aDepartment of Internal Medicine, Taipei Medical University Hospital, Taipei, TaiwanbDepartment of Infection Control, Taipei Medical University Hospital, Taipei, Taiwan cDepartment of Pharmacy, Taipei Medical University Hospital, Taipei, Taiwan
dDepartment of Chest Medicine, Taipei Medical University Hospital and School of Respiratory Therapy, College of Medicine, Taipei Medical University, Taipei, Taiwan eDepartments of Laboratory Medicine and Internal Medicine, National Taiwan University Hospital, National Taiwan University College of Medicine, Taipei, Taiwan
a r t i c l e i n f o
Article history: Received 27 August 2009 Accepted 11 November 2009 Keywords: Fluoroquinolones Ciprofloxacin Levofloxacin Resistance Pseudomonas aeruginosaa b s t r a c t
This study investigated the correlation between fluoroquinolone (ciprofloxacin or levofloxacin) use and rates of fluoroquinolone resistance in Pseudomonas aeruginosa isolates from patients with nosocomial infection at a medical centre in Taiwan. Antibiotic utilisation data were extracted on a monthly basis from the inpatient pharmacy computer system records from January 2003 to December 2008. Fluo-roquinolone use was expressed as defined daily dose per 1000 patient-days and was correlated with rates of fluoroquinolone-resistant P. aeruginosa every 6 months. Regression analysis was performed to explore the relationship between ciprofloxacin and levofloxacin use (both parenteral and oral forms) and resistance of P. aeruginosa isolates. During the study period, the susceptibility of P. aeruginosa to fluo-roquinolones decreased after increasing use of fluofluo-roquinolones, and increased after decreasing use of levofloxacin. Parenteral levofloxacin use was significantly positively correlated with resistance of P. aerug-inosa to ciprofloxacin (P = 0.015) and fluoroquinolones (either ciprofloxacin or levofloxacin, P = 0.014). Use of both parenteral and oral forms of levofloxacin was also significantly positively correlated with resis-tance of P. aeruginosa isolates to ciprofloxacin (P = 0.029), levofloxacin (P = 0.031) and fluoroquinolones (P = 0.010). The total amount of ciprofloxacin (oral and parenteral) and parenteral ciprofloxacin use were negatively correlated with resistance of P. aeruginosa isolates to fluoroquinolones. However, the amounts of oral ciprofloxacin, parenteral levofloxacin, oral levofloxacin and total levofloxacin use were each pos-itively correlated with resistance of P. aeruginosa to fluoroquinolones. Levofloxacin use was associated with increased resistance of P. aeruginosa to fluoroquinolones, whereas ciprofloxacin use did not have a significant impact on fluoroquinolone resistance rates.
© 2009 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved.
1. Introduction
Pseudomonas aeruginosa is a leading cause of healthcare-associated infections worldwide [1]. This organism ranks third among all organisms causing hospital-acquired infections in Tai-wan[2]. Recently, P. aeruginosa has become increasingly resistant to various antimicrobial agents[3]. Previous studies showed that P. aeruginosa-infected patients who were treated empirically with inappropriate antimicrobial agents had a significantly higher mor-tality rate[4].
Fluoroquinolones show potency against a broad range of pathogens responsible for community- and hospital-acquired infections[5]. Owing to its potent activity against P. aeruginosa, ciprofloxacin is most frequently used for treatment of infections
∗ Corresponding author. Tel.: +886 2 2312 3456x5355; fax: +886 2 2322 4263. E-mail address:hsporen@ntu.edu.tw(P.-R. Hsueh).
due to this organism [5]. Levofloxacin, a respiratory quinolone with activity against P. aeruginosa, has also been widely used in recent years. Increasing levofloxacin use was associated with a ris-ing incidence of fluoroquinolone-resistant P. aeruginosa, whereas ciprofloxacin use did not share this association[5–8]. However, no previous studies have demonstrated a change in fluoroquinolone resistance of P. aeruginosa following a reduction in the use of lev-ofloxacin.
The present study evaluated the impact of ciprofloxacin and lev-ofloxacin use on the susceptibility of P. aeruginosa during a strict antimicrobial management programme from 2003–2008 at a uni-versity hospital in Taipei, Taiwan.
2. Materials and methods
2.1. Setting
Taipei Medical University Hospital is a private, tertiary care, university-affiliated teaching hospital located in Taipei, Taiwan.
0924-8579/$ – see front matter © 2009 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved. doi:10.1016/j.ijantimicag.2009.11.007
262 Y.-J. Lee et al. / International Journal of Antimicrobial Agents 35 (2010) 261–264
The number of beds increased from 350 to 560 between 2003 and 2008. Specialty intensive care units in the hospital include medical, surgical/trauma and neonatal.
2.2. Bacterial isolates and susceptibility testing
Susceptibility data for P. aeruginosa isolates associated with hospital-acquired infections were obtained from the infection con-trol department. The broth microdilution method (Phoenix; Becton Dickinson, Sparks, MD) was used for susceptibility testing during the study period. Breakpoints for determining susceptibility were ≤1 mg/L for ciprofloxacin and ≤2 mg/L for levofloxacin[9]and were consistently employed throughout the study period. Susceptibility data were maintained in a laboratory information system database. Duplicate isolates, defined as isolation of the same bacterial species from the same patient with the same antibiogram, were excluded. Fluoroquinolone-resistant P. aeruginosa was defined as a P. aerug-inosa isolate intermediate or resistant to either ciprofloxacin or levofloxacin.
2.3. Antibiotic consumption
Two fluoroquinolones, ciprofloxacin (parenteral and oral forms) and levofloxacin (oral form), were available on the hospital for-mulary throughout the study period. Parenteral levofloxacin was available on the hospital formulary since January 2005. Antibiotic utilisation data were collected from January 2003 to December 2008. Data were extracted on a monthly basis from the inpatient pharmacy computer system. Data on the use of fluoroquinolones (both parenteral and oral forms) were expressed as defined daily dose per 1000 patient-days (DDD/1000PD) and were correlated with rates of fluoroquinolone-resistant P. aeruginosa in the hospi-tal every 6 months from 2003 to 2008. Restrictions on levofloxacin use were implemented by the Department of Infection Control and Pharmacy in July 2007. During the study period, the rec-ommended ciprofloxacin dosage for hospitalised patients without renal impairment was 400 mg every 12 h (q12h) intravenously and 500 mg q12h orally. The recommended levofloxacin dosage for hospitalised patients without renal impairment was 500 mg daily intravenously and 500 mg daily orally between 2003 and 2006 and 750 mg daily for both oral and intravenous administration after 2007.
2.4. Correlation of fluoroquinolone use with fluoroquinolone resistance rates of Pseudomonas aeruginosa
Least-squares linear regression was used to examine the uni-variate relationship between fluoroquinolone (ciprofloxacin and levofloxacin) use and fluoroquinolone resistance rates of P. aerugi-nosa isolates associated with nosocomial infections. The correlation
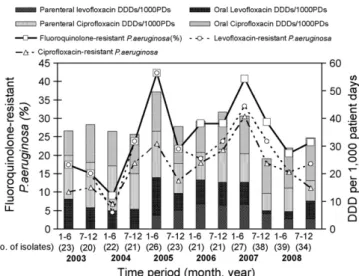
Fig. 1. Trends in resistance rates of Pseudomonas aeruginosa isolates associated with
nosocomial infections to ciprofloxacin, levofloxacin and fluoroquinolones (resistant to either ciprofloxacin or levofloxacin) and fluoroquinolone use expressed as defined daily doses per 1000 patient-days (DDD/1000PDs) in each half-year from 2003 to 2008.
coefficient values (r or r2) were determined. A P-value of <0.05 was
considered to be statistically significant.
3. Results
During the study period, a total of 315 P. aeruginosa isolates caus-ing nosocomial infections were recovered from 299 patients. The number of P. aeruginosa isolates for each half-year period ranged from 20 to 39. The trends in resistance rates of P. aeruginosa to fluoroquinolones as well as fluoroquinolone usage (parenteral and oral) in each half-year period are shown inFig. 1. Ciprofloxacin use decreased after July 2005, whereas levofloxacin use increased to 18.54 DDD/1000PD during the first half of 2005, more than dou-ble its average use for 2003 and 2004 (8.05 DDD/1000PD). This may have been partially attributable to the listing of parenteral levofloxacin on the hospital formulary since January 2005. Lev-ofloxacin use decreased to 6.67 DDD/1000PD during the second half of 2007, and further to 4.99 DDD/1000PD during the first half of 2008.
The fluoroquinolone resistance rate increased to 42.3% in the first half of 2005 from the relatively low resistance rates of <20% from 2003 to 2004. The rate of fluoroquinolone-resistant P. aerug-inosa decreased from 40.7% in the first half of 2007 to 20.5% in the first half of 2008 and was sustained at 23.5% in the following half year.
The correlation between fluoroquinolone (ciprofloxacin or lev-ofloxacin) use and rates of fluoroquinolone-resistant P. aeruginosa
Table 1
Correlation between fluoroquinolone (ciprofloxacin and levofloxacin) use and fluoroquinolone resistance in Pseudomonas aeruginosa isolates causing nosocomial infections.
Ciprofloxacin resistance Levofloxacin resistance Fluoroquinolone (ciprofloxacin or levofloxacin) resistance
Coefficient (r2) Coefficient (r) P-value Coefficient (r2) Coefficient (r) P-value Coefficient (r2) Coefficient (r) P-value
Ciprofloxacin use
Oral 0.076 0.277 0.384 0.056 0.237 0.458 0.072 0.269 0.398
Parenteral 0.041 0.203 0.527 <0.001 0.002 0.994 0.047 0.216 0.500
Parenteral and oral 0.006 0.078 0.810 0.014 0.119 0.713 0.009 0.095 0.769
Levofloxacin use
Oral 0.049 0.222 0.489 0.179 0.423 0.171 0.114 0.337 0.284
Parenteral 0.466 0.682 0.015* 0.235 0.484 0.111 0.470 0.685 0.014*
Parenteral and oral 0.393 0.627 0.029* 0.385 0.620 0.031* 0.497 0.705 0.010* *Statistically significant (P < 0.05).
Y.-J. Lee et al. / International Journal of Antimicrobial Agents 35 (2010) 261–264 263
isolates causing nosocomial infections is shown inTable 1andFig. 2. Parenteral levofloxacin use was significantly positively correlated with resistance of P. aeruginosa to ciprofloxacin (P = 0.015) and to fluoroquinolones (either ciprofloxacin or levofloxacin, P = 0.014). Total use of parenteral and oral forms of levofloxacin was also significantly positively correlated with resistance of P. aeruginosa to ciprofloxacin (P = 0.029), levofloxacin (P = 0.031) and fluoro-quinolones (P = 0.010). The total amount of ciprofloxacin (oral and parenteral) use and parenteral ciprofloxacin use alone were negatively correlated with resistance of P. aeruginosa to fluoro-quinolones. However, the amount of oral ciprofloxacin use as well as oral only, parenteral only and total levofloxacin use were all pos-itively correlated with P. aeruginosa resistance to fluoroquinolones (Fig. 2).
Fig. 2. Linear regression analysis of fluoroquinolone use (expressed as defined daily
doses per 1000 patient-days) and fluoroquinolone-resistant Pseudomonas aerugi-nosa causing nosocomial infections: (A) parenteral ciprofloxacin and levofloxacin use; (B) oral ciprofloxacin and levofloxacin use; and (C) total (parenteral and oral) ciprofloxacin and levofloxacin use.
4. Discussion
Resistance of P. aeruginosa to antimicrobial agents involves mul-tiple mechanisms. Mutation in the gyrA gene and activation of efflux pumps are responsible for resistance to fluoroquinolones[10]. In addition, resistance to fluoroquinolones in P. aeruginosa is linked to resistance to other antibiotics [11]. An increasing prevalence of fluoroquinolone resistance in P. aeruginosa has previously been reported[12,13]. In our hospital, an increasing resistance rate of P. aeruginosa to fluoroquinolones was noted in the first half of 2005. Despite the lack of a specific control policy, the use of both lev-ofloxacin and ciprlev-ofloxacin decreased over the following 6 months, with a concurrent decrease in the rate of fluoroquinolone-resistant P. aeruginosa. However, an increasing trend of non-susceptibility was again noted during 2006–2007 and was concurrent with an increasing use of levofloxacin but stable use of ciprofloxacin.
Bhavnani et al.[14]showed that an increase in levofloxacin use was associated with a decrease in P. aeruginosa susceptibility to ciprofloxacin. A similar finding that use of levofloxacin, but not ciprofloxacin, contributed significantly to rates of fluoroquinolone-resistant P. aeruginosa has also been reported [5]. However, whether a change in susceptibility occurs with a subsequent reduc-tion in levofloxacin use has not been documented. Taipei Medical University Hospital implemented restrictions on levofloxacin use beginning in July 2007 in an attempt to control further the resis-tance rate of P. aeruginosa. As expected, the rate of resisresis-tance of P. aeruginosa to fluoroquinolones subsequently decreased. This is the first study to demonstrate that susceptibility of P. aeruginosa to fluoroquinolones can be restored by reducing levofloxacin use.
Despite the positive correlation between oral ciprofloxacin use and the resistance rate of P. aeruginosa to fluoroquinolones in this study, a surprisingly negative correlation was found between parenteral ciprofloxacin use and P. aeruginosa resistance to fluo-roquinolones. The correlations between the resistance rate of P. aeruginosa and fluoroquinolone use were low for both parenteral ciprofloxacin and total ciprofloxacin, suggesting that ciprofloxacin had no effect on P. aeruginosa susceptibility to fluoroquinolones.
Several reasons may explain these seemingly discrepant find-ings. First, ciprofloxacin is more active in vitro against P. aeruginosa than levofloxacin owing to its lower minimum inhibitory con-centration [5–7]. The selective pressure of fluoroquinolones on the gastrointestinal flora also plays a role in fluoroquinolone-resistant Gram-negative bacilli [15]. Polk et al. [5] explained that the different effects of levofloxacin and ciprofloxacin on the resistance of P. aeruginosa were due to the higher bioavail-ability of levofloxacin compared with ciprofloxacin, resulting in a lower gastrointestinal concentration of levofloxacin possibly selecting for fluoroquinolone-resistant isolates[5–7,15]. However, this hypothesis could only partially explain the loss of susceptibil-ity in our isolates, as the linear regression of levofloxacin use and fluoroquinolone-resistant P. aeruginosa showed a lower correlation with oral levofloxacin (r2= 0.114) than with parenteral levofloxacin
(r2= 0.470).
Finally, the concept of mutation prevention concentration (MPC) may help explain this phenomenon. MPC is the mini-mum concentration that blocks growth of all single-step resistant mutants. Hansen et al.[13]found that the MPC of ciprofloxacin was four times lower than that of levofloxacin against P. aeruginosa.
In conclusion, increasing use of levofloxacin was positively correlated with an increase in resistance of P. aeruginosa to fluo-roquinolones. The susceptibility of P. aeruginosa was recovered by reducing levofloxacin use. Ciprofloxacin does not cause increasing P. aeruginosa resistance to fluoroquinolones.
Funding: No funding sources. Competing interests: None declared. Ethical approval: Not required.
264 Y.-J. Lee et al. / International Journal of Antimicrobial Agents 35 (2010) 261–264
References
[1] Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in med-ical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 1999;27:887–92.
[2] Hsueh PR, Chen ML, Sun CC, Chen WH, Pan HJ, Yang LS, et al. Antimicrobial drug resistance in pathogens causing nosocomial infections at a university hospital in Taiwan, 1981–1999. Emerg Infect Dis 2002;8:63–8.
[3] Harris AD, Smith D, Johnson JA, Bradham DD, Roghmann MC. Risk factors for imipenem-resistant Pseudomonas aeruginosa among hospitalized patients. Clin Infect Dis 2002;34:340–5.
[4] Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. Pseudomonas aeruginosa bloodstream infection: importance of appropriate ini-tial antimicrobial treatment. Antimicrob Agents Chemother 2005;49:1306– 11.
[5] Polk RE, Johnson CK, McClish D, Wenzel RP, Edmond MB. Predicting hospital rates of fluoroquinolone-resistant Pseudomonas aeruginosa from fluo-roquinolone use in US hospitals and their surrounding communities. Clin Infect Dis 2004;39:497–503.
[6] Mohr JF, Jones A, Ostrosky-Zeichner L, Wanger A, Tillotson G. Associations between antibiotic use and changes in susceptibility patterns of Pseudomonas aeruginosa in a private, university-affiliated teaching hospital: an 8-year-experience: 1995–2002. Int J Antimicrob Agents 2004;24:346–51.
[7] Van Eldere J. Multicentre surveillance of Pseudomonas aeruginosa susceptibil-ity patterns in nosocomial infections. J Antimicrob Chemother 2003;51:347– 52.
[8] Kaye KS, Kanafani ZA, Dodds AE, Engemann JJ, Weber SG, Carmeli Y. Dif-ferential effects of levofloxacin and ciprofloxacin on the risk for isolation of quinolone-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 2006;50:2192–6.
[9] Clinical and Laboratory Standards Institute. Performance standards for antimi-crobial susceptibility testing. Nineteenth informational supplement. Document M100-S19. Wayne, PA: CLSI; 2009.
[10] Akasaka T, Tanaka M, Yamaguchi A, Sato K. Type II topoisomerase mutations in fluoroquinolone-resistant clinical strains of Pseudomonas aeruginosa isolated in 1998 and 1999: role of target enzyme in mechanism of fluoroquinolone resistance. Antimicrob Agents Chemother 2001;45:2263–8.
[11] Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseu-domonas aeruginosa: our worst nightmare? Clin Infect Dis 2002;34:634–40. [12] Ohmagari N, Hanna H, Graviss L, Hackett B, Perego C, Gonzalez V, et al. Risk
fac-tors for infections with multidrug-resistant Pseudomonas aeruginosa in patients with cancer. Cancer 2005;104:205–12.
[13] Hansen GT, Zhao X, Drlica K, Blondeau JM. Mutant prevention concentration for ciprofloxacin and levofloxacin with Pseudomonas aeruginosa. Int J Antimicrob Agents 2006;27:120–4.
[14] Bhavnani SM, Callen WA, Forrest A, Gilliland KK, Collins DA, Paladino JA, et al. Effect of fluoroquinolone expenditures on susceptibility of Pseu-domonas aeruginosa to ciprofloxacin in U.S. hospitals. Am J Health Syst Pharm 2003;60:1962–70.
[15] Richard P, Delangle MH, Raffi F, Espaze E, Richet H. Impact of fluoroquinolone administration on the emergence of fluoroquinolone-resistant Gram-negative bacilli from gastrointestinal flora. Clin Infect Dis 2001;32:162–6.