A D VA N C E S I N C L I N I C A L P R A C T I C Ejgh_6695628..638
Natural history of chronic hepatitis B REVEALed
Chien-Jen Chen*,† and Hwai-I Yang‡
*Genomics Research Center, Academia Sinica, and†Graduate Institute of Epidemiology, College of Public Health, National Taiwan University, Taipei, and‡Molecular and Genomic Epidemiology Center, China Medical University Hospital, Taichung, Taiwan
Abstract
Chronic hepatitis B is a worldwide public health challenge. Knowledge of natural history of chronic hepatitis B is important for the management of the disease. A community-based prospective cohort study was carried out to evaluate the risk predictors of progression of chronic hepatitis B in Taiwan. A total of 23 820 participants were enrolled in 1991–1992 from seven townships in Taiwan. Their serum samples were collected at study entry and tested for hepatitis B surface antigen (HBsAg) and e antigen (HBeAg), antibodies against hepatitis C virus (anti-HCV), alanine aminotransferase (ALT), anda-fetoprotein (AFP). A subcohort of 3653 male and female participants who were seropositive for HBsAg and seronegative for anti-HCV was included in the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) study. Newly devel-oped cases of cirrhosis and hepatocellular carcinoma (HCC) were ascertained through follow-up examination and data linkage with profiles of the National Cancer Registry, National Health Insurance Database and Death Certification System. The incidence of both HCC and cirrhosis were significantly associated with serum HBV DNA levels in a dose-response relationship from< 300 (undetectable) to ⱖ 1 000 000 copies/mL. The biological gradients remained significant (P< 0.001) after adjustment for age, sex, habits of cigarette smoking and alcohol drinking, HBeAg serostatus, and serum ALT level at cohort entry. A significant association with risk of cirrhosis and HCC was also observed for HBV geno-type, precore G1896A mutant and basal core promoter A1762T/G1764A double mutant. Nomograms have been developed for the long-term risk prediction of cirrhosis and HCC for patients with chronic hepatitis B. Inactive carriers of HBV have an increased HCC incidence and liver-related mortality than HBsAg-seronegative controls. Serum HBV DNA level at study entry is a major predictor of spontaneous seroclearance of HBeAg, HBV DNA and HBsAg. These findings may inform the effective and efficient management of chronic hepatitis B.
Key words
chronic hepatitis B, cirrhosis, hepatitis B virus DNA and hepatitis B surface antigen, hepatocellular carcinoma, liver disease progression, seroclearance of hepatitis B virus e antigen, viral load.
Accepted for publication 9 February 2011. Correspondence
Chien-Jen Chen, Genomics Research Center, Academia Sinica, 128 Academia Road Section 2, Nankang, Taipei 115, Taiwan. Email: cjchen@ntu.edu.tw
The REVEAL-HBV Study was supported by grants from the Academia Sinica, National Health Research Institutes, and Bristol-Myers Squibb Co., USA.
Global burden and natural history of
chronic hepatitis B
Chronic hepatitis B virus (HBV) infection is a worldwide public health challenge, which accounts for at least half a million deaths annually (World Health Organization and Centers for Disease Control and Prevention fact sheets are available at http:// www.who.int and http://www.cdc.gov).1 The implementation of
nation-wide vaccination programs has significantly reduced chronic HBV infection rate, infant mortality from acute hepatitis, and incidence of hepatocellular carcinoma (HCC) among vacci-nated birth cohorts in Taiwan2–6and several countries. Globally,
there are 350–400 million people with chronic HBV infection, which parallels the mode of infection.7It is particularly prevalent
in the Asia-Pacific and sub-Saharan Africa regions,7,8where
infec-tion is predominantly acquired either during the perinatal period or in the early childhood years. Perinatal infection results in over
90% of infected newborns becoming chronically infected as com-pared to 20–40% with infection in early childhood and 0–10% with infection in adolescence and adult life.9,10A significant
pro-portion of chronic HBV infection progresses to liver cirrhosis and hepatocellular carcinoma (HCC).11
The natural history of chronic HBV infection varies signifi-cantly at an individual level and also varies with age of infection.12
The progression of chronic HBV infection has been postulated to be separated into three periods including an immune tolerance phase, immune detection/clearance phase, and residual phase char-acterized by serum biomarkers of alanine aminotransferase (ALT), HBV surface antigen (HBsAg), HBV e antigen (HBeAg) and antibody against HBeAg (anti-HBe). Most studies on the natural history of chronic HBV infection are based on patients enrolled in clinics or hospitals. These patients represent only the tip of the iceberg of those who are chronically infected with HBV. The incidence and predictor of liver disease progression for chronic
hepatitis B may thus be overestimated and neglected. The role of serum HBV DNA level and HBV genotype and mutant types have not been examined in most previous studies.13The incidence rates
and determinants of spontaneous seroclearance of HBeAg, HBV DNA and HBeAg also remain to be carefully elucidated. Community-based long-term follow-up studies with repeated mea-surements of biomarkers are considered essential for the more precise description of the natural history of chronic hepatitis B and the better prediction of risk of developing liver cirrhosis and hepa-tocellular carcinoma.14The study design, major findings and
limi-tations of the REVEAL-HBV (Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus) study are briefly summarized in this review.
REVEAL-HBV cohort study
In 1991–1992, a community-based cohort study on virus-related cancers was carried out in seven townships in Taiwan. A total of 89 293 male and female residents aged 30 to 65 years were invited to participate, and 23 820 were enrolled with an informed consent. They agreed to participate in questionnaire interview, health examination and blood collection for serological and biochemical assays at study entry and regular follow-up. They also gave their consent for confirming their health and vital status through medical record review and computerized data linkage with national health insurance, cancer registry and death certification profiles. The health examination at study entry included abdominal ultrasonography and serological tests of HBsAg, HBeAg, antibod-ies against hepatitis C virus (anti-HCV), and serum levels of ALT anda-fetoprotein (AFP). Participants with a seropositive status of HBsAg or anti-HCV, an elevated serum level of ALT, AST or AFP, or a family history of HCC at study entry were followed by regular health examination using abdominal ultrasonography and sero-logical tests until 30 June 2004. Serum samples collected and frozen at cohort entry and follow-up examinations were tested for HBV DNA levels.
Personal interview using a structured questionnaire was carried out by well trained public health nurses to obtain information on sociodemographic characteristics, personal and family history of major diseases, dietary intake, as well as habits of cigarette smoking, alcohol consumption and betel nut chewing. Blood samples collected from each participant at study entry and follow-up examinations were fractionated on the day of collection and stored at-70°C until assayed. Serological tests performed at baseline included HBsAg and HBeAg by radioimmunoassay (Abbott Laboratories, North Chicago, IL, USA); anti-HCV by enzyme immunoassay using second-generation kits (Abbott Labo-ratories); and ALT by serum chemistry autoanalyzer (Model 736, Hitachi, Tokyo, Japan) using commercial reagents (Biomerieux, Marcy L’Etoile, France). The serum HBV DNA levels were tested on frozen samples using the COBAS Amplicor HBV monitor test kit (Roche Diagnostics, Indianapolis, IN, USA). The assay has been certified at a lower limit of detection of 300 copies/mL of HBV DNA. The HBV genotype was determined by melting curve analysis for 2405 participants with detectable serum HBV DNA levels and adequate serum samples for HBV genotyping. HBV mutant types were also tested by direct sequencing for 1619 par-ticipants with serum HBV DNA levelsⱖ 10 000 copies/mL.
As shown in Figure 1, there were 4155 HBsAg-seropositive and 19 665 HBsAg-seronegative participants included in this community-based cohort. Their incidence of HCC and mortality from liver diseases were compared. Among HBsAg-seropositive participants, 3653 were seronegative for anti-HCV with adequate serum samples for the measurement of HBV DNA at study entry.
There were 565 HBeAg-seropositive and 3088
HBeAg-seronegative participants with data of serum HBV DNA levels. Among them, 2780 had detectable serum HBV DNA levels and 873 had undetectable levels. Among 2405 participants with detect-able serum HBV DNA levels and adequate serum samples for genotyping of HBV, 1520 were infected by genotype B, 801 by genotype C, and 84 by both genotype A and B. For the HBV precore G1896A mutant type, there were 543 participants with wild type, 750 with mutant type, and 326 with type indetermin-able. For the HBV basal core promoter A1762T/G1764A double mutant type, there were 705 participants with wild type, 578 with mutant type, and 336 with type indeterminable.
Both HCC and cirrhosis were the major liver disease outcomes in the REVEAL-HBV study. Newly developed HCC cases were detected by follow-up health examinations, which included ultra-sound and AFP testing, or by computerized data linkage with the National Cancer Registry in Taiwan. Data linkage with the profiles on the National Death Certification System was also performed to ensure complete ascertainment. Cirrhosis was detected by abdomi-nal ultrasonography using high-resolution, real-time ultrasound scanners (Toshibee SSA-240A, Toshiba Co., Tokyo, Japan) with 3.75 MHz convex transducers, based on a quantitative scoring system involving the features of liver surface (normal, irregular, undulated), liver parenchymal texture (normal, heterogeneous, coarse), size of intrahepatic blood vessel (normal, obscure, nar-rowing), and splenic size (normal, enlarged). All examinations were performed by certified gastroenterologists and interpreted according to a standardized protocol set by a specialist panel. Participants were examined at study entry and every 6–12 months during the follow-up. Computerized data linkage to the National Health Insurance profiles in Taiwan was also carried out for the complete ascertainment of cirrhosis cases. The medical records of identified HCC and cirrhosis cases were reviewed by gastroenter-ologists using a standard Case Abstraction Form. Clinical infor-mation in the Case Abstraction Form was used to confirm HCC cases according to established criteria,15and to assist in diagnosis
of cirrhosis cases using a previously published and validated quan-titative scoring algorithm to improve the accuracy.16
Age-sex-specific seroprevalence of
HBsAg, detectable HBV DNA, HBeAg
Figure 2 shows the seroprevalence of HBsAg, detectable HBV DNA, and HBeAg at study entry for the REVEAL-HBV cohort by age in male and female participants, respectively. Men had higher seroprevalence of HBsAg, detectable HBV DNA, and HBeAg than women in all age groups. There was a decreasing trend in prevalence of HBsAg, detectable HBV DNA and HBeAg with increasing age. Men had a more rapid decrease in prevalence than women, which resulted in the similar prevalence in age group of 60–65 years. The seroprevalence of HBsAg, detectable HBV DNA and HBeAg, respectively, was 26.7%, 21.7% and 7.2% for men and 16.1%, 10.4% and 3.7% for women at ages of 30–34 years; aswell as 13.4%, 8.4% and 0.6% for men and 12.1%, 7.3% and 0.9% for women at ages of 60–65 years. The gender difference in age-specific seroprevalence of HBsAg, detectable HBV DNA, and HBeAg may be the result of differences in seroclearance of these HBV biomarkers, and/or mortality from end-stage liver disease.
Among 3653 HBsAg-seropositive REVEAL-HBV cohort members, 873 (23.9%), 372 (10.2%), 789 (21.6%), 643 (17.6%), 349 (9.6%), 254 (6.9%), and 373 (10.2%) had serum HBV DNA levels (in copies/mL)< 300 (undetectable), 300–999, 1000–9999,
10 000–99 999, 100 000–999 999, 1 000 000–99 999 999,
ⱖ 100 000 000, respectively. HBeAg-seronegatives had a signifi-cantly lower serum level of HBV DNA than HBeAg-seropostives (P< 0.001). There were 523 (92.6%) of 565 HBeAg-seropositive participants with a levelⱖ 100 000 copies/mL, and only 14.7% (453/3088) of HBeAg-seronegative participants had the same high levels. In addition to HBeAg serostatus, elevated serum HBV DNA levels were also significantly associated with male gender, younger ages, cirrhosis status, elevated serum ALT level, cigarette smoking, and HBV genotype C, but not with alcohol consumption habit.13
Biological gradient of HCC and
cirrhosis risk across serum HBV
DNA levels
In a case-control study of 44 HBeAg-seronegative patients with newly developed HCC and 86 matched controls selected from the REVEAL-HBV cohort, a significant dose-response relationship between serum HBV DNA levels at study entry and HCC risk was observed.17Compared with serum HBV DNA levels< 2.5 pg/mL
as the referent group, the multivariate-adjusted odds ratio of devel-oping HCC was 2.3 and 6.0, respectively, for serum HBV DNA levels of 2.5–13.0 and > 13.0 pg/mL. In the further analysis of HCC incidence by serum HBV DNA levels in the REVEAL-HBV study, the incidence of newly developed HCC (per 100 000 person-years) increased with serum HBV DNA levels (in copies/ mL) at study entry ranging from 108 (< 300), 111 (300–9999),
297 (10 000–99 999), 962 (100 000–999 999), to 1152 89293 individuals invited to participate in 1991–1992 23820 enrolled in the cohort 4155 HBsAg (+) 19665 HBsAg (–) 3653 HBV DNA available and anti-HCV (–) 565 HBeAg (+) 3088 HBeAg (–)
2780 HBV DNA detectable 873 HBV DNA undetectable
1520 genotype B 801 genotype C 84 genotype B+C
1619 HBV DNA 104copies/mL
543 precore 1896 wild-type 750 precore 1896 mutant
705 BCP 1762/2764 wild-type 578 BCP 1762/2764 mutant
Figure 1 Flow of the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/ Cancer-Hepatitis B Virus (REVEAL-HBV) study participants. 0 5 10 15 20 25 30 Age (years) Seroprevalence, % 30–34 35–39 40–44 45–49 50–54 55–59 60–65
Figure 2 Prevalence of hepatitis B surface antigen (HBsAg), detect-able hepatitis B virus (HBV) DNA, hepatitis B virus e antigen (HBeAg) by age and sex. , HBsAg+ in women; , HBsAg+ in men; , HBV DNA detectable in women; , HBV DNA detectable in men;
(ⱖ 1 000 000).18The corresponding hazard ratio (95% confidence
interval) was 1.0 (referent), 1.1 (0.5–2.3), 2.3 (1.1–4.9), 6.6 (3.3– 13.1) and 6.1 (2.9–12.7), respectively, after adjustment for sex, age, habit of cigarette smoking and alcohol drinking, HBeAg serostatus, serum ALT level, and cirrhosis status at entry in the Cox regression analysis. Both cirrhosis status and serum HBV DNA level were the strongest risk predictors of HCC. In the further stratification analysis, the dose-response relationship between serum HBV DNA level and HCC risk remained statistically sig-nificant for participants who were HBeAg-seronegative with normal ALT levels and without cirrhosis at study entry; showing higher multivariate-adjusted hazard ratios (95% confidence inter-vals) of 1.0 (referent), 1.4 (0.5–3.8), 4.5 (1.8–11.4), 11.3 (4.5– 28.4) and 17.7 (6.8–46.3), respectively,
In the REVEAL-HBV study, the incidence of cirrhosis (per 100 000 person-years) increased with increasing serum HBV DNA levels (copies/mL) at study entry ranging from 339 (< 300), 430 (300–9999), 774 (10 000–99 999), 1879 (100 000–999 999) to 2498 (ⱖ 1 000 000).19 The corresponding hazard ratio (95%
confidence interval) was 1.0 (referent), 1.4 (0.9–2.2), 2.5 (1.6– 3.8), 5.9 (3.0–9.0) and 9.8 (6.7–14.4), respectively, after adjust-ment for sex, age, habit of cigarette smoking and alcohol drinking, HBeAg serostatus, and serum ALT level at entry in the Cox regres-sion analysis. Increasing HBV DNA level was the strongest inde-pendent risk predictor of cirrhosis. In the further stratification analysis, the dose-response relationship between serum HBV DNA level and HCC risk remained statistically significant for participants who were HBeAg-seronegative with normal ALT levels at study entry.
Associations of HCC and cirrhosis risk
with HBV genotype and mutant type
In recent analyses of REVEAL-HBV study, the associations of the risk of HCC and cirrhosis with the HBV genotype and mutant type were assessed.20–22HBV genotype was identifiable only forpar-ticipants with detectable serum HBV DNA levels at study entry (n= 2762), and HBV mutants including precore G1896A mutant and basal core promoter A1762T/G1764A double mutant were tested only for participants with serum HBV DNA levelsⱖ 10 000 copies/mL (n= 1526). HBV genotype C infection was associated with a higher risk of HCC than HBV genotype B, showing a multivariate-adjusted hazard ratio (95% confidence interval) of 1.76 (1.19–2.61). The precore G1896A mutant was associated with a lower risk of HCC than the wild-type (multivariate-adjusted hazard ratio, 0.34; 95% confidence interval, 0.21–0.57), while the basal core promoter A1762T/G1764A double mutant was associ-ated with a higher risk than the wild-type (multivariate-adjusted hazard ratio, 1.73; 95% confidence interval, 1.13–2.67).20Elevated
serum HBV DNA levels at study entry were still significantly associated with an increased risk of HCC after adjustment for multiple risk predictors including HBV genotype and mutant types.
Both HBV genotype and mutant types were also significantly associated with the risk of cirrhosis. The multivariate-adjusted hazard ratio of developing cirrhosis (95% confidence interval) was 1.9 (1.5–2.3) for HBV genotype C (vs genotype B), 0.5 (0.3–0.6) for precore G1896A mutant (vs wild type), and 1.9 (1.4–2.5) for basal core promoter A1762T/G1764A double mutant (vs wild
type). Increasing serum HBV DNA level remained an important risk predictor of cirrhosis after multivariate adjustment for risk predictors including HBV genotype and mutant types.21,22
Cumulative lifetime incidence of HCC
and LC by age, sex, HBV viral load
and genotype
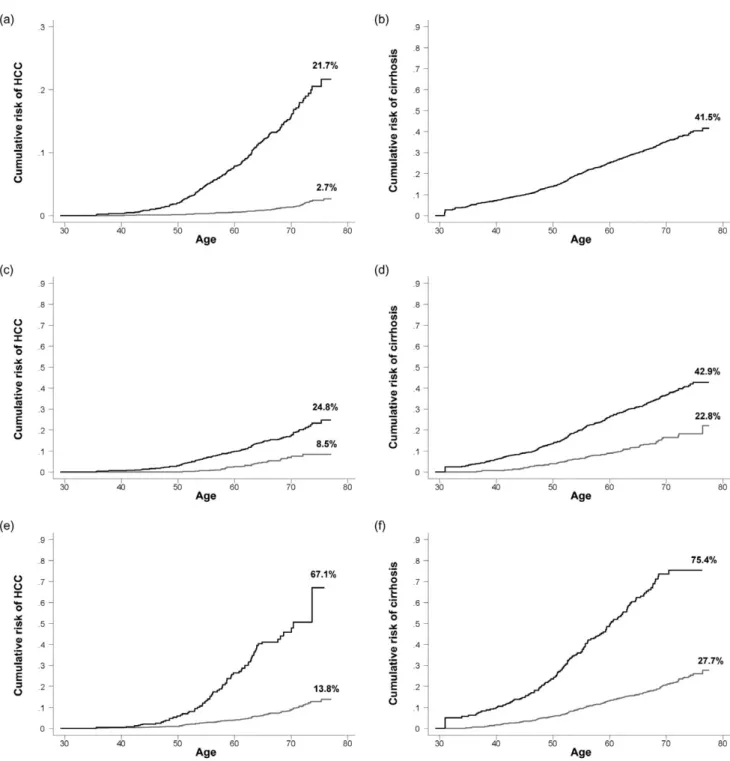
Few studies have compared the cumulative lifetime incidence of HCC in chronic HBV carriers and non-carriers. In the REVEAL-HBV study, the cumulative lifetime incidence (from 30 to 78 years old) of HCC was 21.7% for chronic carriers and 2.7% for non-carriers; and the cumulative lifetime incidence of cirrhosis was 41.5% for chronic carriers as shown in Figure 3a,b. The cumula-tive lifetime incidence of liver cirrhosis was not available for non-carriers. In previous reports of the REVEAL-HBV study,18–20
the risk of developing HCC and cirrhosis was significantly asso-ciated with increasing age, male gender, HBeAg serostatus, increasing serum levels of HBV DNA and ALT, and HBV geno-type C. Figure 3 also shows the cumulative lifetime incidence of HCC and cirrhosis by sex (panels c and d), HBeAg serostatus (panels e and f), serum HBV DNA level (panels g and h), HBV genotype (panels i and j), and serum ALT level (panels k and l) at study entry. A significant higher cumulative lifetime incidence of HCC and cirrhosis was observed for males (vs females), HBeAg-seropositives (vs HBeAg-seronegatives), HBV genotype C (vs genotype B), family history of HCC (vs no family history of HCC) and alcohol drinking habit (vs no habit of alcohol drinking). There was a biological gradient of cumulative lifetime incidence of HCC and cirrhosis across the serum HBV DNA and ALT levels. In the multivariate analysis, these risk predictors remained significantly associated with both end-stage liver diseases. In other words, they are mutually independent risk predictors of HCC and cirrhosis among patients with chronic HBV infection. Serum HBV DNA level was the strongest risk predictor.
Nomograms and risk calculators of
HCC and LC for patients with chronic
HBV infection
Counseling patients with chronic HBV infection on their indi-vidual risk of liver disease progression is challenging due to the existence of multiple risk predictors. REVEAL-HBV study has developed easy-to-use nomograms for predicting HCC and cirrho-sis risk in patients with chronic HBV infection.23,24Two thirds of
the REVEAL-HBV study cohort was allocated for model deriva-tion (n= 2435), and the remaining third was allocated for model validation (n= 1218). Sex, age, family history of hepatocellular carcinoma, alcohol consumption habit, serum ALT level, HBeAg serostatus, serum HBV DNA level, and HBV genotype were included in Cox proportional hazards regression models. Regres-sion coefficients were rounded into integer risk scores, and pre-dicted risk over 5- and 10-year periods for each risk score was calculated and depicted in nomograms. These easy-to-use nomo-grams based on noninvasive clinical characteristics can accurately predict the HCC and cirrhosis risk in patients with chronic hepa-titis B. They may facilitate risk communication between patients and clinicians.
Table 1 shows the integer scores assigned to each risk predic-tor, which was significantly associated with cirrhosis and HCC in the REVEAL-HBV study. There were six risk predictors of cir-rhosis including gender, age, serum ALT level, HBeAg serosta-tus, serum HBV DNA level, and HBV genotype; and two
additional risk predictors for HCC including family HCC history and alcohol consumption habit. Table 2 shows 5- and 10-year risk of developing HCC and cirrhosis by summary risk scores, respectively. The information in Tables 1 and 2 may be used to calculate the long-term risk of developing HCC and cirrhosis for Figure 3 Cumulative lifetime (30–78 years old) risk of hepatocellular carcinoma (HCC) and cirrhosis by sex, hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) e antigen (HBeAg) serostatus, serum HBV and alanine aminotransferase (ALT) level, HBV genotype, family history of hepatocellular carcinoma (HCC), and alcohol drinking habit. (a,b) ——, HBsAg (+); , HBsAg (-); (c,d) ——, Male; , Female; (e,f) ——, HBeAg (+); , HBeAg (-); (g,h) ——, HBV DNAⱖ 106copies/mL; - - -, HBV DNA 105–< 106copies/mL; , HBV DNA 300–< 105copies/mL; , HBV DNA < 300 copies/mL; (i,j) ——, Genotype C; , Genotype B; (k,l) ——, ALTⱖ 45 U/L; , ALT 15–44 U/L; , ALT< 15 U/L.
a patient with available information on risk predictors. For example, for a 50-year-old man who had a family history of HCC, no habit of alcohol consumption, a serum ALT level of 15–44 U/L, an HBeAg-seronegative status, a serum HBV DNA level of 100 000–999 999 copies/mL, and a genotype C HBV infection, his summary risk scores for predicting cirrhosis and HCC would be 17 and 16, respectively. According to the risk of end-stage liver diseases shown in Table 2, his predicted 5- and 10-year risk was 19.23% and 44.83%, respectively, for cirrhosis; and 36.54% and 71.96%, respectively, for HCC.
HCC incidence and liver-related
mortality in inactive HBV carriers
In order to assess the risk of liver disease progression in carriers of inactive HBV, a total of 1932 HBsAg-seropositive and HBeAg-seronegative participants with low serum levels of HBV DNA (< 10 000 copies/mL) and 18 137 HBsAg-seronegative and anti-HCV-seronegative participants were compared in the REVEAL-HBV study.25All of them had serum ALT levels< 45 U/L and no
HCC or cirrhosis diagnosed before and within one year after study Figure 3 Continued.
entry. Liver-related death and newly diagnosed HCC cases were ascertained through computerized data linkage with National Cancer Registry and Death Certification profiles. The annual inci-dence rates of HCC and liver-related death were 0.06% and 0.04%, respectively, for inactive HBV carriers; and 0.02% and 0.02% for controls, respectively. The multivariate-adjusted hazard ratio (95% confidence interval) was 4.6 (2.5–8.3) for HCC incidence and 2.1 (1.1–4.1) for liver-related death for carriers of inactive HBV com-pared to controls. Older age and alcohol drinking habit were inde-pendent risk predictors of HCC for carriers of inactive HBV. Thus, carriers of inactive HBV still have a substantial risk of HCC and liver-related death compared with individuals without chronic HBV infection.
HCC risk predicted by serum HBV
DNA and ALT levels at follow-up
examinations
The HCC risk associated with serum HBV DNA levels at last follow-up has been assessed in the REVEAL-HBV study.18Serum
samples collected at last follow-up examination from participants with serum HBV DNA levelsⱖ 10 000 copies/mL at study entry were tested for HBV DNA levels. The median time between study entry and last follow-up was around 10 years. After adjustment for all HCC risk predictors at study entry, participants with an elevated serum HBV DNA level at last follow-up had an increased risk of HCC. Among participants starting with HBV DNA level of ⱖ 100 000 copies/mL, a significant HCC risk gradient (P < 0.01) by last follow-up HBV DNA level was observed. Compared with participants with serum HBV DNA levels< 10 000 copies/mL at study entry as the referent group, the multivariate-adjusted hazard (95% confidence interval) of developing HCC was 1.9 (0.8–4.4), 4.3 (2.0–9.3), and 5.3 (2.9–9.7) for participants with baseline serum HBV DNA levelsⱖ 100 000 copies/mL and last follow-up
serum HBV DNA levels of < 10 000, 10 000–99 999, and
ⱖ 100 000 copies/mL, respectively.
Time-dependent analyses were also carried out to elucidate the importance of serial measurements of serum HBV DNA and ALT levels in the development of newly-developed HCC and cirrho-sis.26Serum HBV DNA and ALT levels at both study entry and
regular follow-up examinations were all significantly associated with the risk of HCC and cirrhosis showing a dose-response rela-tionship. In other words, it is important to monitor the changes in serum HBV DNA and ALT levels for the clinical management of chronic hepatitis B.
Annual rate and predictors of HBeAg,
HBV DNA and HBsAg seroclearance
Seroclearance of HBeAg, HBV DNA, and HBsAg are important clinical outcomes for chronic hepatitis B treatment trials. Few studies have explored the incidence and determinants of sponta-neous seroclearance using a long-term follow-up study. The annual seroclearance rates of HBeAg, HBV DNA and HBsAg have been estimated, respectively, for 439 HBeAg-seropositiveparticipants with serum HBV DNA level ⱖ 10 000 copies/mL,
1289 participants with serum HBV DNA level ⱖ 10 000
copies/mL at enrollment, and 3087 HBsAg-seropositives in
REVEAL-HBV study.27–30 The annual seroclearance rate was
5.92% for HBeAg, 1.97% for HBV DNA, and 2.26% for HBsAg. The annual HBeAg seroclearance rate for HBeAg-seropositive
participants who had serum HBV DNA levels ⱖ 100 000 000
copies/mL was 4.44%.28,29The annual HBV DNA seroclearance
rate after the seroclearance of HBeAg was 3.20%.27The annual
HBsAg seroclearance rate after the seroclearance of HBV DNA was around 6%. In other words, the cumulative incidence of HBsAg seroclearance at 60 and 100 months after serum HBV-DNA level decreased to undetectable was 25.8% and 51.3%, respectively.30
Significantly higher HBeAg seroclearance rates were observed for female gender, elevated serum ALT level at entry (ⱖ 45 vs < 45 IU), low serum HBV DNA level (< 100 000 vs ⱖ 100 000
copies/mL), HBV genotype B/B+ C (vs genotype C), and HBV
precore G1896A mutant (vs wild type). The predictors of sero-clearance of HBV DNA included low serum HBV DNA level (< 100 000 copies/mL) and central obesity (waist circumference > 90 cm in men and > 80 cm in women). The HBsAg seroclear-ance was significantly associated with increasing age, low serum HBV DNA level (< 100 000 vs ⱖ 100 000 copies/mL), high body Table 1 Risk scores assigned to risk predictors of hepatocellular
car-cinoma (HCC) and liver cirrhosis for patients with chronic hepatitis B virus (HBV) infection
Risk predictor Risk score
for liver cirrhosis Risk score for HCC Gender Female 0 0 Male 3 2 Age (years) 30–34 0 0 35–39 1 1 40–44 2 2 45–49 3 3 50–54 4 4 55–59 5 5 60–64 6 6 Family history of HCC No – 0 Yes – 2
Alcohol consumption habit
No – 0
Yes – 2
Serum ALT level (U/L)
< 15 0 0
15–44 0 1
ⱖ 45 2 1
HBeAg/HBV DNA (copies/mL)/genotype
Negative/< 300 (Undetectable)/- 0 0 Negative/300–9999/- 2 1 Negative/10 000–99 999/B or B+ C 4 3 Negative/10 000–99 999/C 3 4 Negative/100 000–999 999/B or B+ C 6 3 Negative/100 000–999 999/C 10 7 Negative/ⱖ 106/B or B+ C 6 4 Negative/ⱖ 106/C 12 7 Positive/B or B+ C 6 6 Positive/C 9 6
mass index (ⱖ 30 vs < 30 kg/m2) and ethnicity of mainland
Chinese (vs ethnicity of Hakka and Fukkienese).
Natural history of chronic hepatitis B
based on REVEAL-HBV study findings
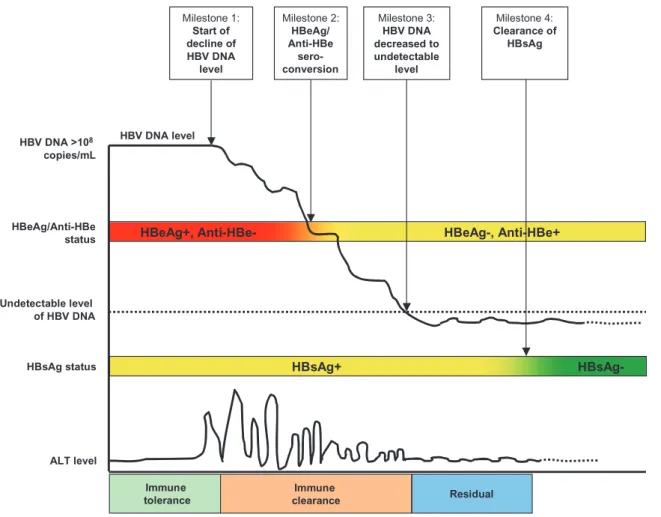
As previously mentioned, the natural history of chronic hepatitis B has been classified into three phases: immune tolerance, immune detection/clearance, and residual phase. They have previously been characterized by serum biomarkers of ALT, HBsAg, HBeAgand anti-HBe.12 According to the findings of REVEAL-HBV
study, the natural history of chronic hepatitis B is illustrated in Figure 4 using the long-term change in serum HBV DNA level as a basis. In the immune tolerance phase, HBV is highly replicated (serum level > 100 000 000 copies/mL) in the human host with detectable circulating HBsAg and HBeAg, and with normal serum ALT level. This phase may last for decades, with significant indi-vidual variation, and is thought to be associated with no evidence of liver injury. The immune tolerance phase is frequently observed in perinatal and early childhood HBV infection, as in the REVEAL-HBV study cohort. The vast majority of adult HBV infections resolve spontaneously, and the few patients (approxi-mately 5%) who do not clear the infection progress directly to the immune detection/clearance phase without experiencing an immune tolerance phase.
The phase of immune detection/clearance starts when the host immune system tries to clear HBV-infected hepatocytes; this
results in hepatic inflammation and elevation of serum ALT level. The seroconversion of HBeAg status from positive to negative and anti-HBe status from negative to positive occurs along with the decline in serum HBV DNA levels, which remain detectable with a significant individual variation at the time of HBeAg seroclear-ance. After the seroclearance of HBeAg, serum HBV DNA levels keep declining until undetectable, when the immune clearance phase ends. This phase may thus be further classified into “HBeAg-seropositive” and “HBeAg-seronegative” chronic hepa-titis B depending on the seroclearance of HBeAg. The elevation of serum ALT levels is much higher in the HBeAg-seropositive phase than in the HBeAg-seropositive phase. The immune clearance phase is highly variable in duration, and a prolonged phase with recurrent episodes of acute liver inflammation may result in repeated cycles of injury and regeneration/fibrosis and an increased risk of progression to cirrhosis and HCC.
After the seroclearance of HBV DNA, a proportion of chronic HBV carriers are able to inactivate the HBV replication to reach the residual or non-replicative phase, sometimes referred to as the “inactive HBV carrier state.” It is characterized by HBsAg-seropositivity, HBeAg-seronegativity and/or anti-HBe-seropositivity, and normal serum ALT level. Spontaneous sero-clearance of HBsAg occurs among some individuals in this phase, while some individuals may reactivate HBV replication and return to the immune clearance phase. HBsAg-seropositive patients in the inactive carrier state may progress to end-stage liver diseases depending on the events that have already occurred during the Table 2 Five- and 10-year risk of developing hepatocellular carcinoma (HCC) and cirrhosis by summary scores of risk predicitors for patients with chronic hepatitis B virus (HBV) infection
Liver cirrhosis HCC
Summary score 5-year risk for liver cirrhosis, %
10-year risk for liver cirrhosis, %
Summary score 5-year risk for HCC, % 10-year risk for HCC, %
0 0.15 0.42 0 0.01 0.03 1 0.20 0.56 1 0.02 0.04 2 0.27 0.75 2 0.03 0.07 3 0.36 1.01 3 0.05 0.13 4 0.49 1.35 4 0.08 0.21 5 0.65 1.80 5 0.13 0.36 6 0.87 2.39 6 0.22 0.62 7 1.16 3.19 7 0.38 1.05 8 1.54 4.24 8 0.64 1.79 9 2.06 5.64 9 1.09 3.02 10 2.75 7.47 10 1.85 5.09 11 3.66 9.86 11 3.13 8.51 12 4.86 12.96 12 5.27 14.05 13 6.45 16.95 13 8.81 22.72 14 8.54 22.00 14 14.52 35.51 15 11.25 28.28 15 23.44 52.62 16 14.76 35.89 16 36.54 71.96 17 19.23 44.83 17 53.89 88.52 18 24.85 54.87 18 73.23 97.49 19 31.76 65.50 19 89.39 99.81 20 40.02 75.92 20 97.81 100.00 21 49.53 85.11 22 59.94 92.17 23 70.58 96.69
immune clearance phase and pre-existing liver fibrosis. However, individuals with seroclearance of HBsAg have a low risk of HCC and cirrhosis.
The normal serum ALT level (< 45 IU) in the immune tolerance phase deserves additional discussion. It has been generally accepted that persons in the immune tolerance phase of the infec-tion do not have any liver injury and this contributes to their being excluded from any considerations for therapy. In one study of young chronic hepatitis B patients (n= 183; mean age 24) with normal serum ALT (mean level 22 U/L) in China, all had biopsy evidence of necro-inflammation and over 60% had evidence of hepatic fibrosis.31The progression to fibrosis in these patients may
be due to a chronic low-level liver inflammation as a result of ineffective attempts at clearing HBV-infected hepatocytes result-ing in recurrent liver injury, regeneration and fibrosis over several years. The fact that a chronic state of liver inflammation is suffi-cient to cause HCC has been shown in elegant rat models in which the continued presence of HBsAg-positive hepatocytes served as a continuous trigger for T-cell recognition and ongoing liver damage.32The extent of sub-clinical liver cell injury occurring in
the immune tolerance phase may be related to the ongoing pres-ence of infected hepatocytes expressing HBV antigens and a weak
attempt by the immune system to clear such cells. In addition, the definition of upper limit of normal serum ALT level (often taken as ~45 IU) needs further scrutiny.
Limitations and future perspectives of
the REVEAL-HBV study
Taiwan is an endemic area of perinatal or early childhood infection of HBV, and almost all chronic HBV carriers in this study had an immune tolerance phase of infection. Whether patients infected by HBV in adulthood have the same natural history of chronic hepa-titis B needs further elucidation. The participants enrolled in the REVEAL-HBV study were adults aged 30–65 years, and the annual incidence of HCC and cirrhosis as well as the seroclearance rates of HBsAg, HBV DNA and HBeAg may not be applicable to chronic HBV carriers younger than 30 years old. As almost all chronic HBV carriers in the REVEAL-HBV study were infected by HBV genotype B and/or C, whether the natural history of chronic infection by HBV genotype A, D or other genotypes is the same or not remains to be assessed. The risk calculators for HCC and cirrhosis derived from the REVEAL-HBV study also need further validation in other populations.
Milestone 3: HBV DNA decreased to undetectable level HBsAg+ HBsAg-HBeAg-, Anti-HBe+ HBeAg+, Anti-HBe-Undetectable level of HBV DNA Milestone 1: Start of decline of HBV DNA level HBV DNA level HBeAg/Anti-HBe status HBsAg status Milestone 2: HBeAg/ Anti-HBe sero-conversion HBV DNA >108 copies/mL Milestone 4: Clearance of HBsAg Immune tolerance Residual Immune clearance ALT level
Figure 4 Natural history of chronic hepatitis B based on the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-Hepatitis B Virus (REVEAL-HBV) Study findings.
The serotiter of HBsAg has recently been found to be an impor-tant predictor of clinical outcome in anti-viral therapy for chronic hepatitis B. We are investigating whether HBsAg serotiter is also an important risk predictor of HCC and cirrhosis independent of serum HBV DNA level and other predictors. There exists signifi-cant individual variability in the natural history of chronic hepatitis B, and both environmental and genetic factors may be important in determining such individual variation in the natural history of chronic hepatitis B.33 Host factors documented to be associated
with HBV-related HCC include obesity and metabolic factors,34,35
sex hormones and polymorphisms of their biosynthesis and receptor genes,34,35aflatoxin, tobacco smoke, and other chemical
carcinogens and polymorphisms of genes related to their metabo-lism,36and intake of micronutrients including anti-oxidants and
selenium.37,38Roles of these genetic and environmental factors and
their interactions in the liver disease progression in patients with chronic HBV infection deserve further investigation.
References
1 World Health Organization. Fact sheet: hepatitis B. 2008.
2 Chang MH, Chen CJ, Lai MS et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N. Engl. J. Med. 1997; 336: 1855–9.
3 Chang MH, Shau WY, Chen CJ et al. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA 2000; 284: 3040–2.
4 Chang MH, Chen TH, Hsu HM et al. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin. Cancer Res. 2005; 11: 7953–7. 5 Chang MH, You SL, Chen CJ et al. Decreased incidence of
hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J. Natl Cancer Inst. 2009; 101: 1348–55. 6 Chien YC, Jan CF, Kuo HS, Chen CJ. Nationwide hepatitis B
vaccination program in Taiwan: effectiveness in the 20 years after it was launched. Epidemiol. Rev. 2006; 28: 126–35.
7 Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J. Clin.
Gastroenterol. 2004; 38: S158–68.
8 Chen CJ, Wang LY, Yu MW. Epidemiology of hepatitis B virus infection in the Asia-Pacific region. J. Gastroenterol. Hepatol. 2000; 15 (Suppl.): E3–6.
9 McMahon BJ, Alward WL, Hall DB et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J. Infect. Dis. 1985; 151: 599–603.
10 Beasley RP, Trepo C, Stevens CE, Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am. J.
Epidemiol. 1977; 105: 94–8.
11 Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin. Liver Dis. 2005; 9: 191–211, v. 12 Chen CJ, Iloeje UH, Yang HI. Long-term outcomes in hepatitis B:
the REVEAL-HBV study. Clin. Liver Dis. 2007; 11: 797–816. 13 Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels
and outcomes in chronic hepatitis B. Hepatology 2009; 49: S72–S84.
14 Chen CJ. Time-dependent events in natural history of occult hepatitis B virus infection: the importance of population-based long-term follow-up study with repeated measurements. J. Hepatol. 2005; 42: 438–40.
15 Bruix J, Sherman M, Llovet JM et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J.
Hepatol. 2001; 35: 421–30.
16 Lin DY, Sheen IS, Chiu CT, Lin SM, Kuo YC, Liaw YF. Ultrasonographic changes of early liver cirrhosis in chronic hepatitis B: a longitudinal study. J. Clin. Ultrasound 1993; 21: 303–8.
17 Yang HI, Lu SN, Liaw YF et al. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N. Engl. J. Med. 2002; 347: 168–74.
18 Chen CJ, Yang HI, Su J et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level.
JAMA 2006; 295: 65–73.
19 Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load.
Gastroenterology 2006; 130: 678–86.
20 Yang HI, Yeh SH, Chen PJ et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma.
J. Natl Cancer Inst. 2008; 100: 1134–43.
21 Chen YL. Risk of liver cirrhosis associated with genotypes and mutants of HBV [Master thesis]. Graduate Institute of Epidemiology, National Taiwan University, 2007.
22 Chen YL, Chen PJ, Yang HI et al. Risk of liver cirrhosis associated with genotype and mutants of hepatitis B virus [Abstract]. J.
Hepatol. 2007; 46: 222.
23 Yang HI. Hepatitis B virus related factors and the risks of liver cirrhosis and hepatocellular carcinoma [Doctoral dissertation]. Graduate Institute of Epidmeiology, National Taiwan University, 2006.
24 Yang HI, Sherman M, Su J et al. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J. Clin. Oncol. 2010; 28: 2437–44.
25 Chen JD, Yang HI, Iloeje UH et al. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology 2010; 138: 1747–54.
26 Yang HI, Su J, Jen CL, You SL, Chen CF, Chen CJ. Serial monitoring of viral load and serum alanine aminotranferase level and the risk of hepatocellular carcinoma (HCC): REVEAL-HBV study update [Abstract]. J. Hepatol. 2008; 48: 141.
27 Chen CJ, Yang HI, Jen CL, Su J, Iloeje UH. Incidence and determinants of spontaneous decline of HBV DNA to undetectable level in patients with high viral load [Abstract]. J. Hepatol. 2010; 52: S6.
28 Hung HL, Lee MH, Yang HI, Jen CL, Iloeje UH, Chen CJ. Incidence and determinants for hepatitis B e antigen seroclearance [Abstract]. Hepatol. Int. 2010; 4: 104.
29 Hung HL. Incidence and determinants of seroclearance of hepatitis B e antigen and its association with risk of liver cirrhosis and HCC [Master Thesis]. Graduate Institute of Epidemiology, Natioanl Taiwan University, 2010.
30 Liu J, Yang HI, Lee MH et al. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology 2010; 139: 474–82.
31 Yang LM, Xu KC, Zhao YL et al. Clinical significance of liver biopsy in chronic hepatitis B patients with persistently normal transaminase. Chin. J. Dig. Dis. 2002; 3: 150–3.
32 Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu.
Rev. Immunol. 1995; 13: 29–60.
33 Chen CJ, Chen DS. Interaction of hepatitis B virus, chemical carcinogen, and genetic susceptibility: multistage
hepatocarcinogenesis with multifactorial etiology. Hepatology 2002; 36: 1046–9.
34 Chen CL, Yang HI, Yang WS et al. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology 2008; 135: 111–21.
35 Yu MW, Shih WL, Lin CL et al. Body-mass index and progression of hepatitis B: a population-based cohort study in men. J. Clin.
Oncol. 2008; 26: 5576–82.
36 Chen CJ, Yu MW, Liaw YF et al. Chronic hepatitis B carriers with null genotypes of glutathione S-transferase M1 and T1
polymorphisms who are exposed to aflatoxin are at increased risk of hepatocellular carcinoma. Am. J. Hum. Genet. 1996; 59: 128–34. 37 Yu MW, Horng IS, Hsu KH, Chiang YC, Liaw YF, Chen CJ. Plasma
selenium levels and risk of hepatocellular carcinoma among men with chronic hepatitis virus infection. Am. J. Epidemiol. 1999; 150: 367–74.
38 Yu MW, Hsieh HH, Pan WH, Yang CS, CHen CJ. Vegetable consumption, serum retinol level, and risk of hepatocellular carcinoma. Cancer Res. 1995; 55: 1301–5.