The Complete Genome Sequence of Perina nuda Picorna-like Virus, An Insect-Infecting RNA
Virus with a Genome Organization Similar to That of the Mammalian Picornaviruses
Chih-Yu Wu,* Chu-Fang Lo,† Chang-Jen Huang,‡ Hon-Tsen Yu,† and Chung-Hsiung Wang*,1
*Department of Entomology, †Department of Zoology, National Taiwan University, Taipei, Taiwan, Republic of China; and ‡Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan, Republic of China
Received September 18, 2001; returned to author for revision November 12, 2001; accepted January 3, 2002 Perina nuda picorna-like virus (PnPV) is an insect-infecting RNA virus with morphological and physicochemical characters
similar to the Picornaviridae. In this article, we determine the complete genome sequence and analyze the gene organization of PnPV. The genome of PnPV consists of 9476 nucleotides (nts) excluding the poly(A) tail and contains a single large open reading frame (ORF) of 8958 nts (2986 codons) flanked by 473 and 45 nt noncoding regions on the 5⬘ and 3⬘ ends, respectively. Northern blotting did not detect the presence of any subgenomic RNA. The PnPV genome codes for four structural proteins (CP1–4), and determination of their N-terminal sequences by Edman degradation, showed that all four are located in the 5⬘ region of the genome. The 3⬘ part of the PnPV genome contains the consensus sequence motifs for picornavirus RNA helicase, cysteine protease, and RNA-dependent RNA polymerase (RdRp) in that order from the 5⬘ to the 3⬘ end. In all of these characters, the genome organization of PnPV resembles the mammalian picornaviruses and two other insect picorna-like viruses, infectious flacherie virus (IFV) of the silkworm and Sacbrood virus (SBV) of the honeybee. In a phylogenetic tree based on the eight conserved domains in the RdRp sequence, PnPV formed a separate cluster with IFV and SBV, which suggests that these three insect picorna-like viruses might constitute a novel group of insect-infecting RNA viruses. © 2002 Elsevier Science (USA)
Key Words: Perina nuda; PnPV; picornavirus; insect; complete genome sequence.
INTRODUCTION
Positive-strand RNA viruses are assigned to super-groupI, II, or III on the basis of sequence alignments of their RNA-dependent RNA polymerase (RdRp) (see Strauss et al., 1996). The picorna-like viruses, all of which share a conserved array of replicative proteins, i.e., H-P-Rep(Helicase-Protease-Replicase) (Koonin and Dolja, 1993), represent a single lineage within RdRp super-group I, and presently comprise the five established families Picornaviridae, Caliciviridae, Sequiviridae,
Co-moviridae, and Potyviridae (Goldbach and Haan, 1994;
Gromeier et al., 1999). Four of these families
(Picornaviri-dae, Sequiviri(Picornaviri-dae, Comoviri(Picornaviri-dae, and Potyviridae) form the
picornavirus “superfamily,” all the members of which uti-lize their genomic RNA as an exclusive message for a single polyprotein with all proteins being produced as the result of processing. By contrast, although the
Cali-civiridae are also picorna-like viruses, they are excluded
from the picornavirus superfamily because they produce subgenomic RNA. In addition, many small RNA viruses from various insect species have picornavirus-like bio-physical properties (Moore et al., 1985; Minor et al.,
1995), but their relationships with established members of the picorna-like virus lineage have only recently begun to be established. Surprisingly, the insect-infecting vi-ruses appear to form distinct taxonomic clusters outside of the family Picornaviridae (Christian et al., 1999), and the Seventh Report of the International Committee on Taxonomy of Viruses (ICTV VII) assigns five of these insect picorna-like viruses [Cricket paralysis virus (CrPV),
Drosophila C virus (DCV), Rhopalosiphum padi virus
(RhPV), Plautia stali intestine virus (PSIV), Himetobi P
virus (HiPV)] to a new genus, the “Cricket Paralysis-like Viruses ” (or “CrPV-like viruses”; Christian et al., 1999). In
this genus, the gene order of the nonstructural proteins is the same as in the picorna-like viruses, but unlike the picornavirus superfamily, CrPV-like viruses produce their capsid proteins through internal initiation of translation from the genomic length RNA. In the case of PSIV and CrPV, this phenomenon has been demonstrated to be dependent on an internal ribosome entry site (IRES) (Sasaki and Nakashima, 1999; Wilson et al., 2000). Nu-cleotide sequences highly similar to the IRES of PSIV were also found in RhPV and DCV (Sasaki and Na-kashima, 1999), suggesting the presence of an IRES for these viruses as well. Furthermore, the CrPV-like viruses resemble the caliciviruses in that their nonstructural pro-teins are encoded in the 5⬘ part of the genome, and their capsid proteins are encoded in the 3⬘ part (Johnson and
1To whom correspondence and reprint requests should be addressed
at Department of Entomology, National Taiwan University, Taipei 107, Taiwan R.O.C. Fax: 886-2-27364329. E-mail: wangch@ccms.ntu.edu.tw. Virology 294, 312–323 (2002)
doi:10.1006/viro.2001.1344, available online at http://www.idealibrary.com on
0042-6822/02 $35.00
© 2002 Elsevier Science (USA) All rights reserved.
Christian, 1998; Sasaki et al., 1998; Moon et al., 1998; Nakashima et al., 1999; Wilson et al., 2000). The CrPV-like viruses, which have two ORFs, are also bicistronic. In addition to the CrPV-like viruses, there are also other insect picorna-like viruses that are not included in the
Picornaviridae. Similar to the CrPV-like viruses,
Acyrtho-siphon pisum virus (APV) is both monopartite and bicis-tronic, and the order of the ORFs for its nonstructural and structural proteins is the same. However, in APV, the two ORFs overlapslightly, with the 3⬘-proximal ORF thought to be translated by a⫺1 ribosomal frameshift (van der Wilk et al., 1997). APV is also unlike the CrPV-like viruses in that it produces a large amount of subgenomic RNA, which was detected in purified virus particles and APV-infected aphids (van der Wilk et al., 1997). By contrast, the genomes of two other insect picorna-like viruses, Infec-tious flacherie virus (IFV) and Sacbrood virus (SBV), are both monopartite and monocistronic, and they resemble the mammalian picornaviruses in that their structural proteins are encoded in the 5⬘ part of the genome and their nonstructural proteins are encoded in the 3⬘ part (Isawa et al., 1998; Ghosh et al., 1999).
The present study investigates the Perina nuda pi-corna-like virus (PnPV), an insect pipi-corna-like virus that was originally isolated from flacherie-infected larvae of the ficus transparent wing moth, P. nuda Fabricius (Lep-idoptera: Lymantriidae). Flacherie is a lethal disease that occurs frequently from spring to early summer every year in Taiwan, and it is considered a mixed infection of P.
nuda nucleopolyhedrovirus (PenuNPV) and PnPV (Wang et al., 1998, 1999). The PnPV viral genome is composed
of one single-strand RNA molecule with a length of 10 kb and a poly(A) tract, and the virus particle consists of three major and three minor structural proteins (Wang et
al., 1999). The viral particles of PnPV exhibit icosahedral
symmetry, are approximately 30 nm in diameter, have no envelope and no distinct surface structure, and have a buoyant density of 1.381 g/ml in cesium chloride, and it was on the basis of these biophysical properties that PnPV was first tentatively identified as an insect picorna-like virus (Wang et al., 1999). Here we report the com-plete nucleotide sequence and gene organization of the PnPV genome, including the coding regions of the capsid proteins, which were mapped by determining their N-terminal sequences. The transcript species were deter-mined by Northern blotting, and the phylogenetic rela-tionships between PnPV and other picornaviruses are also explored.
RESULTS AND DISCUSSION Nucleotide sequence and open reading frame analysis
The PnPV virion contains a single-stranded RNA mol-ecule, about 10 kb in length (Wang et al., 1999). Except for the 5⬘ end, the sequence of the genomic RNA from PnPV was constructed by compiling sequences from a series of seven overlapping cDNA clones (see Fig. 1). Both strands of each of the cDNA clones were completely sequenced. The 5⬘ end of the viral genome was cloned by 5⬘ rapid amplification of cDNA ends (RACE), and the 5⬘-terminal nucleotides were determined by comparison
FIG. 1. (A) Schematic diagram of the PnPV genome. The ORF corresponds to the entire open box. Numbers indicate nucleotide positions. The approximate positions of the nonstructural proteins were identified by sequence similarity as follows: F, helicase; f, protease; Œ, RdRp. L? denotes the probable location of the leader peptide, and the open circle of the 5⬘ end represents the VPg (if present). Solid lines indicate where cleavages are known to occur in the polyprotein, and solid rectangles indicate the positions of 2A-like sequences. CP1–4 denote the four capsid proteins of PnPV as described in Table 2. (B) The alignment of PnPV cDNA clones produced by genomic cDNA cloning (p6, p19, p51, p68, p69, p141, and p179) and 5⬘-RACE analysis (see Fig. 2). Numbers indicate nucleotide positions. The horizontal scale is the same in both parts of this figure.
of the sequences of 10 clones (see Fig. 2). Analysis of the sequences of the cDNA clones confirmed the presence of a poly(A) tail on the 3⬘ end of the PnPV genome. The PnPV genome was found to have 9476 nucleotides (nts), excluding the 3⬘ poly(A) tail. Similar to other insect picor-naviruses, the sequence is A/U rich (28.29% A, 27.55% U, 19.17% C, and 24.98% G). A computer-aided analysis of the PnPV nucleotide sequence showed that the genomic RNA contains a single, large open reading frame (ORF) oriented from the 5⬘ to the 3⬘ end. This large ORF ac-counts for 94.5% of the PnPV genome (8958 nts), while the other 5.5% consists of untranslated regions (UTR) (518 nts). The 5⬘UTR (473 nts) is considerably shorter than those of other picornaviruses (610–1200 nts) (Stan-way, 1990). No large ORFs were found in the inverse
orientation of the PnPV genome, and this finding con-firmed that PnPV is a positive-strand RNA virus.
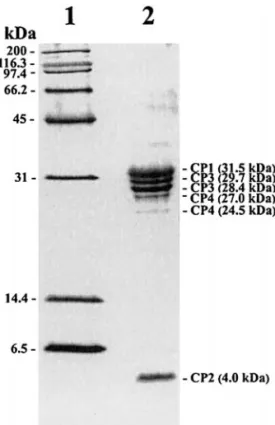
Mapping of the coding region of the capsid proteins The three major (31.5, 29.7, 28.4 kDa) and three minor (27.0, 24.5, 4.0 kDa) structural proteins of the purified PnPV particles are shown in Fig. 3. The N-terminal se-quences of these proteins were GDEDTPAGELSIEQDTH-KNT (31.5 kDa), DRPQNIIEPTNFYLQQNTSL (29.7 and 28.4 kDa), GDERREPHTV (27.0 and 24.5 kDa), and PFLS-GLLGTV (4.0 kDa). The N-terminal sequence of the 29.7-kDa protein was the same as that of the 28.4-29.7-kDa pro-tein, indicating that they had the same origin, with the smaller protein probably being formed via degradation of the C-terminal part of the larger protein. A similar
expla-FIG. 2. (A) Determination of the 5⬘-terminus of the PnPV genome. GSP1, GSP2, and GSP3 are three antisense primers used for 5⬘-RACE analysis. The bent arrows indicate the location of the 5⬘-termini of 10 5⬘-RACE clones (1–10). A complementary region (double underlines, nucleotide positions 11–38) of the PnPV genome appears to form a secondary structure that may interfere with the cDNA synthesis during reverse transcription. (B) The product of 5⬘-RACE analysis in agarose gel. M, pGEM DNA Markers (Promega).
nation applies for the 27.0- and 24.5-kDa proteins. The sequences for the 31.5-, 29.7- (or 28.4 kDa), 27.0- (or 24.5 kDa), and 4.0-kDa proteins were serially found encoded from the 5⬘ end of the PnPV genome at predicted amino acid residues 320–339, 638–657, 907–916, and 574–583,
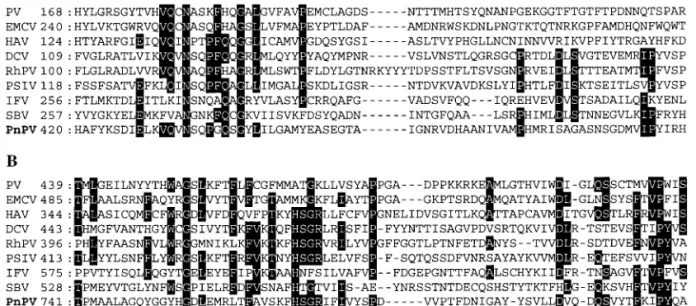
respectively, and the structural proteins were therefore named CP1 (31.5 kDa), CP2 (4.0 kDa), CP3 (29.7 or 28.4 kDa), and CP4 (27.0 or 24.5 kDa) based on their proximity to the N-terminus of the deduced amino acid sequence of PnPV’s ORF (Table 1). Their locations are shown in Fig. 1. The deduced amino acid sequence of the capsid pro-teins of PnPV was compared with entries in protein sequence databases by using BLAST. The greatest over-all similarity was observed against the structural pro-teins of insect-infecting RNA viruses: RhPV (15% identity, ID; 31% similarity, SI), PSIV (14% ID; 31% SI), DCV (14% ID; 31% SI), IFV (13% ID; 35% SI), SBV (12% ID, 31% SI), HiPV (12% ID; 30% SI), and CrPV (12% ID; 29% SI). Interestingly, although PnPV, IFV, and SBV share a picornavirus-like gene order, they have low homology to each other. Fur-thermore, the structural proteins of PnPV share relatively low overall similarity to those of mammalian picornavi-ruses listed in Table 2 (8–11% ID; 22–26% SI), and also to those of the plant picorna-like viruses PYFV (8% ID; 25% SI) and CPMV (5% ID; 23% SI). Multiple alignments (Fig. 4A) showed that the amino acid residues 420–492, which are part of the coding region of PnPV’s CP1, exhibit not only a high similarity to the VP2 structural protein of several mammalian picornaviruses (Table 3), but also to the VP1 of DCV (38-kDa protein) and PSIV (33-kDa pro-tein), the VP2 of RhPV (28-kDa propro-tein), the VP3 of IFV (31-kDa protein), and the amino acid residues 257–326 of SBV polyprotein. A second region, located at the amino acid positions 741–812 (i.e., part of the coding region of PnPV’s CP3), is similar to the VP3-like proteins of mam-malian picornaviruses (Table 3) and also to the structural
FIG. 3. Structural proteins of PnPV in a 16% SDS–polyacrylamide gel. Lane 1, broad-range protein marker (Bio-Rad); lane 2, structural pro-teins of PnPV.
TABLE 1
Summary of PnPV’s Structural Proteins
Designation
N-terminal sequencea
Position of
N-terminusb Cleavage sitesc Size (aa)d
Molecular mass (kDa)
From deduced amino acid sequence From SDS– PAGE CP1 GDEDTPAGEL 320–339 VTAQ/GD 254 28.1 31.5 SIEQDTHKNT (L/CP1) CP2 PFLSGLLGTV 574–583 NPG/P 64 6.0 4.0 (CP1/CP2) CP3 DRPQNIIEPTN 638–657 KKDM/DR 269 29.3 29.7 or 28.4 FYLQQNTSL (CP2/CP3) CP4 GDERREPHTV 907–916 VTAM/GD 284 31.6 27.0 or 24.5 (CP3/CP4) NPG/P (CP4 C-terminus)d aDetermined by Edman degradation.
bRelative to the deduced amino acid sequence of PnPV’s ORF. cThe scissile bond at cleavage site is represented by “/”.
dDetermined from the deduced amino acid sequence of PnPV’s ORF and based on the assumption that each structural protein sequence is terminated at the amino acid just before the N-terminus of the following structural protein. CP4’s sequence was assumed to terminate at the cleavage site of the 2A-like sequence.
proteins of insect picorna-like viruses such as the VP2 of DCV (33.3-kDa protein) and PSIV (30-kDa protein), the VP3 of RhPV (29-kDa protein), the VP1 of IFV (35-kDa protein), and the amino acid residues 528–602 of SBV polyprotein (Fig. 4B).
The order of PnPV’s capsid proteins more closely resembles the other insect picorna-like viruses (IFV, CrPV, PSIV, and BQCV) in that the smallest capsid protein (i.e., CP2 in PnPV and the VP4-like proteins in IFV, CrPV, PSIV, and BQCV) is in all cases located near the center of the capsid protein precursor. This is in contrast to the mammalian picornaviruses, in which VP4 is located at the N-terminus of the capsid protein precursor. There is also increasing evidence that in deriving VP4 and VP3 from VP0, the insect picorna-like viruses might follow a different posttranslational processing pathway from the mammalian picornaviruses (Choi et al., 1992; Isawa et
al., 1998; Tate et al., 1999); if so, then PnPV would also
presumably process VP0 similar to the other insect pi-corna-like viruses. However, the correspondence be-tween PnPV’s CP2 and the VP4-like proteins of the insect picorna-like viruses has not been firmly established and it should be noted that PnPV’s CP2 shows only a low similarity to these VP4-like proteins. Furthermore, no conserved sequence (GF/SKP in CrPV-like viruses) has been found at the CP2/CP3 cleavage site of PnPV. Leader polypeptide
The coding region for the structural proteins (CP1– CP2–CP3–CP4) starts at deduced amino acid position 320, which suggests that the PnPV genome may code for a leader polypeptide (L) of 36.7 kDa prior to the coding region. At the N-terminus of IFV, SBV, aphthoviruses (a
TABLE 2
Summary of the Viruses Used in the Phylogenetic Analysis
Groupa
Viral strain/abbreviation
(GenBank Accession No.) Host
Number of ORFs/sgRNAb
Genomic organization
Reference Locations of the capsid
proteins/nonstructural proteins
I Human rhinovirus 1B/HRV (D00239) Mammals 1/A 5⬘ part/3⬘ part Hughes et al., 1998 Human poliovirus 1, strain Mahoney/PV (V01149) Mammals 1/A 5⬘ part/3⬘ part Racaniello and Baltimore,
1981 Foot-and-mouth disease virus, strain 01/FMDV
(AF189157)
Mammals 1/A 5⬘ part/3⬘ part c
Encephalomyocarditis virus/EMCV (X87335) Mammals 1/A 5⬘ part/3⬘ part Nelsen-Salz et al., 1996 Hepatitis A virus, strain HM-175, wild-type/HAV
(M14707)
Mammals 1/A 5⬘ part/3⬘ part Cohen et al., 1987 Echovirus 22/HPeV (S45208) Mammals 1/A 5⬘ part/3⬘ part Hypia¨ et al., 1992 II Feline calicivirus, strain F9/FCV (P27409) Mammals 3/P 3⬘ part/5⬘ part Carter et al., 1992
Rabbit haemorrhagic disease virus/RHDV (AAB02225)
Mammals 2/P 3⬘ part/5⬘ part Gould et al., 1997 III Cowpea mosaic virus/CPMV (X00206) Plant 2d/A RNA 2/RNA 1c Lomonossoff and Shanks,
1983
IV Parsnipyellow fleck virus/PYFV (D14066) Plant 1/A 5⬘ part/3⬘ part Turnbull-Ross et al., 1992 V Potato virus A/PVA (AJ131403) Plant 1/A 3⬘ part/5⬘ part Rajamaeki et al., 1998 VI Southern cowpea mosaic virus/SCPMV (M23021) Plant 4/P 3⬘ part/5⬘ part Wu et al., 1987
VII Drosophila C virus, strain EB/DCV (AF014388) Insect 2/A 3⬘ part/5⬘ part Johnson and Christian, 1998 Himetobi P virus/HiPV (AB017037) Insect 2/A 3⬘ part/5⬘ part Nakashima et al., 1999 Triatoma virus/TrV (AF178440) Insect 2/A 3⬘ part/5⬘ part Czibener et al., 2000 Rhopalosiphum padi virus/RhPV (AF022937) Insect 2/A 3⬘ part/5⬘ part Moon et al., 1998 Plautia stali intestine virus/PSIV (AB006531) Insect 2/A 3⬘ part/5⬘ part Sasaki et al., 1998 Black queen-cell virus/BQCV (AF183905) Insect 2/A 3⬘ part/5⬘ part Leat et al., 2000 Acute bee paralysis virus/ABPV (AF150629) Insect 2/A 3⬘ part/5⬘ part Govan et al., 2000 Cricket paralysis virus/CrPV (AF218039) Insect 2/A 3⬘ part/5⬘ part Wilson et al., 2000 VIII Infectious flacherie virus/IFV (AB000906) Insect 1/A 5⬘ part/3⬘ part Isawa et al., 1998
Sacbrood virus/SBV (AF092924) Insect 1/A 5⬘ part/3⬘ part Ghosh et al., 1999 IX Acyrthosiphon pisum virus/APV (AF024514) Insect 2/P 3⬘ part/5⬘ part van der Wilk et al., 1997
aI: Picornaviridae; II: Caliciviridae; III: Comoviridae; IV: Sequiviridae; V: Potyviridae; VI: Sebomovirus (genus; no assigned family); VII: “Cricket Paralysis-like Viruses” (genus; no assigned family); VIII: unassigned “Insect Picorna-like” viruses; IX: unassigned insect picorna-like viruses.
bPresent (P) or absent (A) of subgenomic RNA (sgRNA). cL. Benvenisti and Y. Stram, 1999, direct submission.
dCPMV has a bipartite genome; the other viruses in the table have a monopartite genome.
genus under Picornaviridae), and cardioviruses (a genus under Picornaviridae), a (sometimes putative) leader pro-tein (L), precedes the structural propro-tein (Isawa et al., 1998; Ghosh et al., 1999; Stanway, 1990). In aphthovi-ruses, the L protein amino acid sequence contains the conserved cysteine-tryptophan motif, which includes the histidine moiety required for protease activity in the L protein of FMDV (foot-and-mouth disease virus, which is also a member of the Picornaviridae) (Gorbalenya et al., 1991; Piccione et al., 1995; Roberts and Belsham, 1995). However, this motif was not found in the L protein of PnPV nor in the L proteins of IFV and SBV (Isawa et al., 1998; Ghosh et al., 1999), which suggests that these proteins are not subject to proteinase.
“2A-like” sequences
The conserved “2A-like” “cleavage” motif -DxExNPGP-was originally identified by alignment of cardio- and aphthovirus 2A sequences (Hahn and Palmenberg, 1996; Donnelly et al., 1997). Interestingly, there were two 2A-like sequences found within the PnPV polyprotein at amino acid residues 555–574 (AQGWVPDLTVDGDVES-NPGP) and 1172–1191 (IGGGQKDLTQDGDIES(AQGWVPDLTVDGDVES-NPGP). As Fig. 1 shows, the first sequence is located inside the structural protein-coding region and spans the CP1/CP2 junction. Its activity was confirmed by N-terminal se-quencing of the CP2 protein (Table 1). The second motif is located near the downstream end of the PnPV
struc-FIG. 4. Comparison of the deduced amino acid sequence of capsid proteins between PnPV and other viruses. (A) Alignment of the amino acid sequences of the CP1 of PnPV with VP2 of PV, EMCV, and HAV, VP1 of DCV (38-kDa protein) and PSIV (33-kDa protein), VP2 of RhPV (28-kDa protein), VP3 of IFV (31-kDa protein), and the amino acid residues 257–326 of SBV polyprotein. (B) Alignment of the amino acid sequences of CP3 of PnPV with VP3 of PV, EMCV, and HAV; VP2 of DCV (33.3-kDa protein) and PSIV (30-kDa protein), VP3 of RHPV (29-kDa protein), VP1 of IFV (35-kDa protein), and the amino acid residues 741–812 of SBV polyprotein. Numbers on the left indicate residue number for the N-terminus. Residues identical in at least half of the viruses are shown in inverse typeface.
TABLE 3
Pairwise Comparisons of Amino Acid Sequences of PnPV’s CP1 (Upper Right Triangle) and PnPV’s CP3 (Lower Left Triangle) with the Corresponding Regions of Picornaviruses and Picorna-Like Virusesa
PnPV SBV IFV PSIV RhPV DCV HAV EMCV PV
PnPV — 21 (53) 17 (42) 22 (50) 18 (39) 24 (41) 16 (32) 14 (32) 13 (29) SBV 24 (48) — 22 (52) 21 (40) 17 (41) 21 (45) 9 (31) 6 (24) 9 (22) IFV 18 (42) 23 (52) — 21 (45) 21 (41) 21 (47) 14 (31) 13 (32) 6 (25) PSIV 29 (58) 20 (53) 20 (49) — 29 (58) 35 (58) 20 (37) 13 (31) 16 (29) RhPV 25 (51) 20 (44) 23 (46) 34 (65) — 46 (65) 15 (34) 15 (34) 16 (30) DCV 24 (47) 28 (42) 23 (48) 45 (67) 39 (61) — 21 (32) 10 (33) 13 (32) HAV 21 (48) 16 (44) 26 (53) 31 (54) 25 (49) 21 (51) — 21 (37) 20 (28) EMCV 25 (52) 21 (46) 33 (52) 25 (55) 21 (43) 25 (47) 30 (56) — 31 (44) PV 19 (42) 18 (41) 17 (41) 25 (49) 15 (40) 22 (44) 30 (46) 41 (59) —
aThe percentages of amino acid sequence identity (or similarity) were calculated using GeneDoc (score table: Blosum 35) for the alignments shown in Fig. 4.
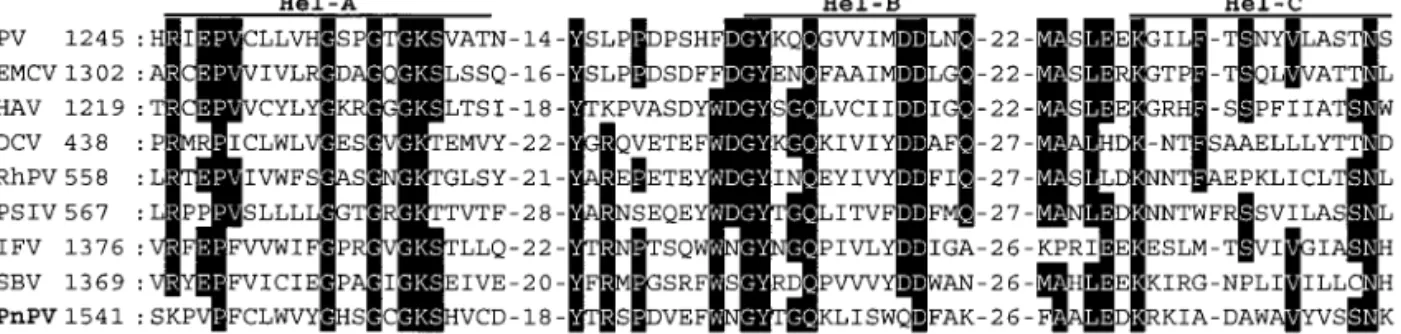
FIG. 5. Comparison of deduced amino acid sequences of nonstructural proteins of PnPV and other picorna-like viruses. (A) Alignment of the conserved regions of the putative RNA helicase regions from PnPV with PV, EMCV, HAV, DCV, RhPV, PSIV, IFV, and SBV (full names and references for these viruses are given in Table 2). Residues identical in at least half of the viruses are shown in inverse typeface. The motifs identified by Koonin and Dolja (1993) are labeled Hel-A, Hel-B, and Hel-C. Numbers on the left show the amino acid position of the aligned sequences. (B) Alignment of the putative protease domain of PnPV with those of other viruses. The residues that should form the catalytic triad (Koonin and Dolja, 1993) are marked with asterisks (*). (C) Alignment of the putative RNA-dependent RNA polymerase domain of PnPV with those of other viruses. The motifs identified by Koonin and Dolja (1993) are labeled I–VIII.
tural protein-coding region, and its proximity to the pre-dicted C-terminal end of the CP4 protein suggests that it might also be functionally active.
Recently, in a study of the aphthovirus 2A/2B polypro-tein cleavage mechanism, it was proposed that the ac-tivity of this site depends not on a proteolytic reaction, but on a novel translational effect that involves a putative ribosomal “skip” from one codon to the next without the formation of a peptide bond (Donnelly et al., 2001a). Some 2A-like sequences have already been identified at various locations within the ORFs of insect virus polypro-teins (reviewed by Donnelly et al., 2001b). In IFV, a pre-sumably functional 2A-like sequence was found near the downstream end of the structural protein-coding region and probably functions as it does in mammalian picor-naviruses (Isawa et al., 1998). In DCV, ABPV, and CrPV, there are conserved 2A-like sequences in the N-terminal region of the replicative ORF1 (Johnson and Christian, 1998; Govan et al., 2000; Wilson et al., 2000). In the
Thosea asigna virus (Tetraviridae), a 2A-like sequence is
present within the capsid protein precursor (Pringle et
al., 1999). In PnPV, the N-terminal sequence of CP2
sug-gests the possible functionality of the first 2A-like motif (aa 555–574) (Table 1). The second PnPV 2A-like se-quence (aa 1172–1191) has a similar location to the 2A-like motif in IFV (i.e., at the downstream end of the structural protein-coding region) and it also appears to be functionally active. Thus PnPV appears to have two active 2A-like cleavage sites, a characteristic that has never been seen before in any picornaviruses.
Nonstructural proteins
Analysis of the 3⬘ part of the PnPV genome revealed that three distinct regions correspond to the conserved motifs of the helicase, protease, and RNA-dependent RNA polymerase (Fig. 1). These nonstructural proteins are also found in the same order in the picorna-like viruses (Koonin and Dolja, 1993). In Fig. 5, the amino acid sequences of the conserved region of PnPV’s putative helicase, protease, and RdRp protein are aligned with those of eight other viruses: mammalian picornaviruses (PV, EMCV, HAV), CrPV-like viruses (DCV, RhPV, PSIV), and insect picorna-like viruses (IFV, SBV).
Helicase. Three conserved helicase regions
recog-nized by Koonin and Dolja (1993) were found in the deduced amino acid sequence of PnPV’s ORF from 1541 to 1655 (Fig. 5A). The highly conserved consensus se-quence within the first domain, GXXGXGKS (Gorbalenya
et al., 1990), occurs in the PnPV sequence between
amino acids 1552 and 1559. The last two domains devi-ate somewhat from the consensus. The highly conserved amino acids in these two domains are QX5DD and
KGX4SX5STN, while the PnPV equivalents are QX5QD and
KRX4AX5SSN, respectively (Fig. 5A).
Protease. The deduced amino acid sequence of
Pn-PV’s ORF from 2255 to 2399 is similar to the protease sequences of other picornaviruses (Fig. 5B). The GXCG motif was found at 2380–2383, although valine was sub-stituted for glycine at the first position of this motif. Multiple alignment suggested that the H2260, D2298, and
C2382 of PnPV’s protease sequence should form the
cat-alytic triad. However, although we were unable to find any alternative putative protease site, the dependence of PnPV’s protease activity on these three amino acids has not yet been confirmed experimentally. The inclusion of aspartic acid in this triad suggests that PnPV belongs to the lineage B groupof viruses (Ryan and Flint, 1997). This lineage includes members of the cardioviruses, aphtho-viruses, and parechoviruses. By contrast, the lineage A group, which includes the enteroviruses and human rhi-noviruses, all have glutamatic acid in the triad instead of the aspartic acid residue (Ryan and Flint, 1997).
RNA-dependent RNA polymerase. Eight conserved
do-mains in RdRps corresponding to those recognized by Koonin and Dolja (1993) were found between amino acids 2565 and 2875 on the deduced amino acid se-quence of PnPV (Fig. 5C). The conserved motifs in RdRp of positive-strand RNA viruses, SGX3TX3N, YGDD, and
FLKR (Koonin, 1991), are located at amino acids 2765– 2774, 2804–2807, and 2841–2844, respectively (Fig. 5C). Northern blot analysis
Northern blot analysis of the RNA produced during PnPV infection of PN cells confirmed that PnPV does not produce subgenomic RNA (Fig. 6). No hybridization was seen in uninfected PN extracts (data not shown).
FIG. 6. Northern blot analysis of PnPV RNAs accumulated in infected PN cells (3 days postinfection) showing that no subgenomic RNA was synthesized.
PnPV’s taxonomic status
PnPV’s genomic organization [5⬘NTR–putative leader protein–structural proteins–nonstructural proteins–3⬘NTR– poly(A) tail; see Fig. 1], the fact that it does not produce subgenomic RNA (see Fig. 6), and the fact that its genomic RNA contains a single large ORF, all argue that PnPV should be a member of the picorna-like su-perfamily. Accordingly, we propose that PnPV should be grouped with the unassigned “insect picorna-like” viruses IFV and SBV (groupVIII), as shown in Table 2. Phylogenetic analysis
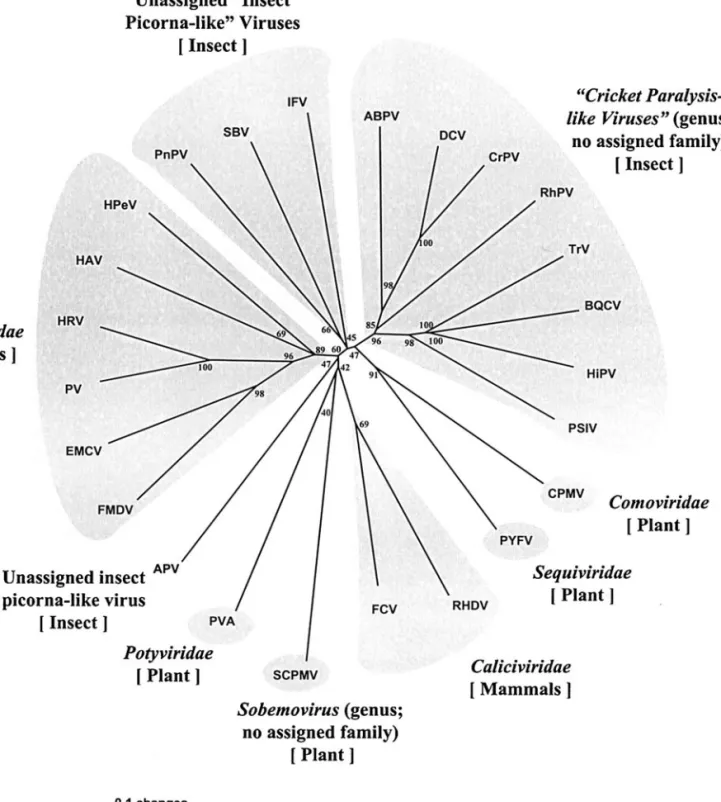
The highly conserved fragments of RdRpamino acid sequences encompassing motifs I to VIII in RdRps (ap-proximately 300 amino acid residues) of the picornavi-ruses and picorna-like vipicornavi-ruses (Koonin and Dolja, 1993) were used in the phylogenetic analysis. Both the neigh-bor-joining (NJ) and the Quartet Puzzling trees generated similar results, but since the NJ tree revealed finer struc-tures within major phylogenetic clades, only the NJ tree is shown here. The results reflect the current systematic assignment of the viruses. As Fig. 7 shows, all the mem-bers of Picornaviridae form a clade (bootstrapvalue 89) and a second clade includes all the members of Cricket
paralysis-like viruses [note that since ICTV VII, three new
members have been added to this genus: Triatoma virus (TrV; Czibener et al., 2000); Black queen-cell virus (BQCV; Leat et al., 2000); and Acute bee paralysis virus (ABPV; Govan et al., 2000)] (bootstrapvalue 96). A third clade of insect picorna-like viruses (PnPV, SBV, and IFV) was formed, though with less bootstrap support (bootstrap value 45). A fourth clade of two caliciviruses (FCV and RHDV) was also formed (bootstrapvalue 69). Notably, the insect like viruses do not belong to the picorna-virus clade (all the members of which are mammalian-infection viruses). This suggests that despite the similar-ity in genomic organization (i.e., the members of both clades contain just one ORF, they do not express sub-genomic RNAs, and their capsid and nonstructural pro-tein sequences are respectively located in the 5⬘ and 3⬘ region, see Table 2), the insect picorna-like viruses and the Picornaviridae are distinct evolutionary entities. Therefore, we suggest that PnPV, SBV, and IFV might constitute a novel groupof insect-infecting viruses.
MATERIALS AND METHODS Virus purification and viral RNA extraction
PnPV was originally isolated from its natural host, P.
nuda Fabricius (Lepidoptera: Lymantriidae) and
propa-gated in its homologous cell line, NTU-PN-HH (Wang et
al., 1996). The viral particles were purified from the
in-fected PN cells as previously described by Wang et al. (1999). Genomic PnPV RNA was extracted from purified viral particles using TRIzol reagent (Gibco BRL) based on
the acid guanidinium thiocyanate-phenol-chloroform method (Chomczynski and Sacchi, 1987) and then pre-cipitated with ethanol according to the manufacturer’s recommendations.
cDNA synthesis, cloning, and nucleotide sequencing Standard molecular biological procedures, essentially as described by Sambrook et al. (1989), were used to clone PnPV cDNAs. Briefly, cDNA synthesis was per-formed using a cDNA synthesis kit (Gibco BRL), reverse transcription of PnPV RNA being primed by the combi-nation of oligo(dT12–18), and six random residue primers.
Second-strand synthesis, EcoRI (NotI) adaptor ligation, and size fractionation of cDNA were performed accord-ing to the manufacturer’s recommendations. The synthe-sized cDNAs were ligated into the EcoRI site of pUC19 plasmid molecules and transformed into Escherichia coli JM109 cells. The large cDNA inserts (⬎1000 bp) were selected by EcoRI digestion. Seven overlapping cDNA clones containing almost the entire PnPV genome except for the extreme 5⬘ end were obtained. They were se-quenced in both directions commercially (Mission Bio-tech, Taiwan) using a Taq DyeDeoxy Terminator Cycle Sequencing Kit (ABI PRISM) and a 373A sequencing system (Perkin–Elmer).
5⬘-RACE analysis
The sequence of the extreme 5⬘ end of the PnPV genome was obtained by using a 5⬘-RACE system (Gibco BRL). Briefly, total RNA was prepared from PnPV-infected cells and treated by proteinase K (400g/ml) at 37°C for 60 min prior to use as a template for cDNA synthesis. This procedure ensured that the VPg of PnPV genomic RNA was removed to avoid interference in cloning the 5⬘ end as described by Johnson and Christian (1998). Three antisense primers, GSP1 (nt 713–694), GSP2 (nt 602– 581), and GSP3 (nt 220–200), were synthesized and 5 ⬘-RACE was performed as per the manufacturer’s instruc-tions. First-strand cDNA was tailed with dCTP using terminal deoxynucleotidyl transferase, and then the dC-tailed cDNA was amplified by polymerase chain reaction (PCR). All PCR products (approximately 260 bp) were ligated into the pGEM-T Easy vectors (Promega), pre-pared according to the manufacturer’s protocol. The in-serts of 10 clones were sequenced.
SDS–PAGE and N-terminal sequencing of viral structural proteins
Viral structure proteins were electrophoresed in 16% SDS–polyacrylamide gels using the Laemmli (1970) buffer system. Proteins were further stained by a silver stain or transferred onto a PVDF membrane as described by Moos et al. (1988). The membrane was stained with 0.1% amido black solution. The stained bands were ex-cised and analyzed directly with an automated Edman
degradation sequencer (Applied Biosystems, Model 477A/120A).
Northern blot analysis
The general procedure of Sambrook et al. (1989) was used for Northern blot hybridizations. Briefly, total RNA was extracted from PnPV-infected (3 days postinfection) and uninfected PN cells, electrophoresed in a 1% aga-rose gel containing formaldehyde, and blotted onto a nylon membrane (Hybond-N⫹, Amersham). Two species
of DIG-RNA probes were prepared from in vitro transcrip-tion with a commercial kit (DIG-RNA Labeling Kit, Roche). These probes corresponded to the coding regions of a putative leader protein (nt 581-1092) and the helicase domain (nt 4850–5463) of the PnPV sequence, and they were used simultaneously in the Northern analysis. Nucleotide sequence analysis and comparison
PnPv’s genome cDNA sequences were assembled and analyzed using three computer programs (Genework
FIG. 7. Phylogenetic analysis of putative RdRp domains. The neighbor-joining trees were produced and bootstrapped (1000 replicates) using PAUP* 4.0b software. Viruses and references are as given in Table 2.
Version 2.5.1, GCG release 9.0, and Neural Network). The DNA and deduced amino acid sequences were com-pared with the latest GenBank/EMBL databases using FASTA (Pearson and Lipman, 1988) and BLAST (Altschul
et al., 1990), respectively. Multiple alignments of amino
acid sequences were obtained with CLUSTALX (Thomp-son et al., 1997) and edited in Genedoc (Nicholas and Nicholas, 1997). We used the deduced amino acid se-quences of RNA-dependent RNA polymerase to con-struct phylogenetic trees of 24 viruses (see Table 2) using the neighbor-joining method (Saitou and Nei, 1987) and the Quartet Puzzling method as implemented in the PAUP* 4.0b program (Swofford, 1998). The statistical sig-nificance of branch order was estimated by performing 1000 replications of bootstrap resampling of the original aligned amino acid sequences.
ACKNOWLEDGMENTS
This work was supported by the National Science Council under Grant NSC 89-2313-B-002-171, Republic of China. We are indebted to Paul Barlow for helpful criticism of the manuscript. The GenBank accession number of the complete genomic sequence of P. nuda picorna-like virus is AF323747.
REFERENCES
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment tool. J. Mol. Biol. 215, 403–410.
Carter, M. J., Milton, I. D., Meanger, J., Bennett, M., Gaskell, R. M., and Turner, P. C. (1992). The complete nucleotide sequence of a feline calicivirus. Virology 190, 443–448.
Chomczynski, P., and Sacchi, N. (1987). Single-stepmethod of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extrac-tion. Anal. Biochem. 162, 156–159.
Choi, H., Sasaki, T., Tomita, T., Kobayashi, M., and Kawase, S. (1992). Processing of structural polypeptides of infectious flacherie virus of silkworm, Bombyx mori: VP1 and VP4 are derived from VP0. J.
In-vertbr. Pathol. 60, 113–116.
Christian, P., Carstens, E., Domier, L., Johnson, K., Nakashima, N., Scotti, P., and van der Wilk. F. (1999). Cricket paralysis-like viruses. In “Virus Taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses” (M. H. V. Van Regenmortel, C. M. Fauquet, D. H. L. Bishop, C. H. Calisher, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle and R. B. Wickner, Eds.), pp. 678–683. Academic Press, San Diego, CA. Cohen, J. I., Ticehurst, J. R., Purcell, R. H., Buckler-White, A. J., and
Baroudy, B. M. (1987). Complete nucleotide sequence of wild-type hepatitis A virus: Comparison with different strains of hepatitis A virus and other picornaviruses. J. Virol. 61, 50–59.
Czibener, C., Torre, J. L., Muscio, O. A., Ugalde, R. A., and Scodeller, E. A. (2000). Nucleotide sequence analysis of Triatoma virus shows that it is a member of a novel groupof insect RNA viruses. J. Gen.
Virol. 81, 1149–1154.
Donnelly, M. L. L., Gani, D., Flint, M., Monoghan, S., and Ryan, M. D. (1997). The cleavage activity of aphtho- and cardiovirus 2A proteins.
J. Gen. Virol. 78, 13–21.
Donnelly, M. L. L., Luke, G., Mehrotra, A., Li, X., Hughes, L. E., Gani, D., and Ryan, M. D. (2001a). Analysis of the aphthovirus 2A/2B polypro-tein “cleavage” mechanism indicates not a proteolytic reaction, but a novel translational effect: A putative ribosomal “skip”. J. Gen. Virol. 82, 1013–1025.
Donnelly, M. L. L., Hughes, L. E., Luke, G., Mendoza, H., ten Dam, E.,
Gani, D., and Ryan, M. D. (2001b). The “cleavage” activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring “2A-like” sequences. J. Gen. Virol. 82, 1027–1041. Ghosh, R. C., Ball, B. V., Willcocks, M. M., and Carter, M. J. (1999). The
complete nucleotide sequence of honeybee sacbrood virus. J. Gen.
Virol. 80, 1541–1549.
Goldbach, R., and Haan, de P. (1994). RNA viral supergroups and evolution of RNA viruses. In “The Evolutionary Biology of Viruses” (S. S. Morse, Ed.), pp. 105–119. Raven Press, New York.
Gorbalenya, A. E., Koonin, E. V., and Lai, M. M.-C. (1991). Putative papain-related thiol proteases of positive strand RNA viruses. FEBS
Lett. 288, 201–205.
Gorbalenya, A. E., Koonin, E. V., and Wolf, Y. L. (1990). A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 262, 145–148.
Gould, A. R., Kattenbelt, J. A., Lenghaus, C., Morrisy, C., Chamberlain, T., Collins, B. J., and Westbury, H. A. (1997). The complete nucleotide sequence of rabbit haemorrhagic disease virus (Czech strain V351): Use of the polymerase chain reaction to detect replication in Aus-tralian vertebrates and analysis of viral population sequence varia-tion. Virus Res. 47, 7–17.
Govan, V. A., Leat, N., Allsopp, M., and Davison, S. (2000). Analysis of the complete genome sequence of acute bee paralysis virus shows that it belongs to the novel groupof insect-infecting RNA viruses.
Virology 277, 457–463.
Gromeier, M., Wimmer, E., and Gorbalenya, A. E. (1999). Genetics, pathogenesis and evolution of picornaviruses. In “Origin and Evolu-tion of Viruses” (E. Domingo, R. Webster, and J. Holland, Eds.), pp. 287–343. Academic Press, San Diego, CA.
Hahn, H., and Palmenberg, A. C. (1996). Mutational analysis of the encephalomyocarditis virus primary cleavage. J. Virol. 70, 6870–6875. Hughes, P. J., North, C., Jellis, C. H., Minor, P. D., and Stanway, G. (1988). The nucleotide sequence of human rhinovirus 1B: Molecular rela-tionships within the rhinovirus genus. J. Gen. Virol. 69, 49–58. Hyypia¨, T., Horsnell, C., Maaronen, M., Khan, M., Kalkkinen, N., Auvinen,
P., Kinnunen, L., and Stanway, G. (1992). A distinct picornavirus group identified by sequence analysis. Proc. Natl. Acad. Sci. USA 89, 8847–8851.
Isawa, H., Asano, S., Sahara, K., Iizuka, T., and Bando, H. (1998). Analysis of genetic information of an insect picorna-like virus, infec-tious flacherie virus of silkworm: Evidence for evolutionary relation-ships among insect, mammalian and plant picorna (-like) viruses.
Arch. Virol. 143, 127–143.
Johnson, K. N., and Christian, P. D. (1998). The novel genome organi-zation of the insect picorna-like virus Drosophila C virus suggests this virus belongs to a previously undescribed virus family. J. Gen.
Virol. 79, 191–203.
Koonin, E. V. (1991). The phylogeny of RNA-dependent polymerase of positive-strand RNA viruses. J. Gen. Virol. 72, 2197–2206.
Koonin, E. V., and Dolja, V. V. (1993). Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 28, 375–430. Laemmli, U. K. (1970). Cleavage of structural proteins during assembly
of the head of bacteriophage T4. Nature (London) 227, 680–685. Leat, N., Ball, B., Govan, V., and Davison, S. (2000). Analysis of the
complete genome sequence of black queen-cell virus, a picorna-like virus of honey bees. J. Gen. Virol. 81, 2111–2119.
Lomonossoff, G. P., and Shanks, M. (1983). The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 2, 2253–2258.
Minor, P. D., Brown, F., Domingo, E., Hoey, E., King, A., Knowles, N., Lemon, S., Palmenberg, A., Rueckert, R. R., Stanway, G., Wimmer, E., and Yin-Murphy, M. (1995). In “Virus Taxonomy: Sixth Report of the International Committee on Taxonomy of Virus” (F. A. Murphy, C. M. Fanquet, D. H. L. Bishop, S. A. Ghabrial, A. W. Jarvis, G. P. Martelli, M. A. Mayo and M. D. Summers, Eds.), pp. 335. Springer-Verlag, New York.
Moon, J. S., Domier, L. L., McCoppin, N. K., D’Ary, C. J., and Jin, H. (1998). Nucleotide sequence analysis shows that Rhopalosiphum padi virus
is a member of a novel groupof insect-infecting RNA viruses.
Virol-ogy 243, 54–65.
Moore, N. F., Reavy, B., and King, L. A. (1985). General characteristics, gene organization and expression of small RNA virus of insects.
J. Gen. Virol. 66, 647–659.
Moos, M., Nguyen, N. Y., and Liu, T.-Y. (1988). Reproducible, high-yield sequencing of proteins electrophoretically separated and transferred to an insert support. J. Biol. Chem. 263, 6005–6008.
Nakashima, N., Sasaki, J., and Toriyama, S. (1999). Determining the nucleotide sequence and capsid-coding region of Himetobi P virus: A member of a novel groupof RNA viruses that infect insects. Arch.
Virol. 144, 2051–2058.
Nelsen-Salz, B., Zimmermann, A., Wickert, S., Arnold, G., Botta, A., Eggers, H. J., and Kruppenbacher, J. P. (1996). Analysis of sequence and pathogenic properties of two variants of encephalomyocarditis virus differing in a single amino acid in VP1. Virus Res. 41, 109–122. Nicholas, K. B., Nicholas, H. B., Jr., and Deerfield, D. W., II (1997). GeneDoc: Analysis and visualization of genetic variation. EMBNEW.
News 4, 14.
Pearson, W. R., and Lipman, D. J. (1988). Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85, 2444–2448. Piccione, M. E., Zellner, M., Kumosinski, T. F., Mason, P. W., and
Grubman, M. (1995). Identification of the active-site residues of foot-and-mouth disease virus leader proteinase. J. Virol. 69, 4950–4956. Pringle, F. M., Gordon, K. H. J., Hanzlik, T. N., Kalmakoff, J., Scotti, P. D., and Ward, V. K. (1999). A novel capsid expression strategy for Thosea
asigna virus (Tetraviridae). J. Gen. Virol. 80, 1855–1863.
Racaniello, V. R., and Baltimore, D. (1981). Molecular cloning of polio-virus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc. Natl. Acad. Sci. USA 78, 4887–4891. Rajamaeki, M., Merits, A., Rabenstein, F., Andrejeva, J., Paulin, L.,
Kekarainen, T., Kreuze, J. F., Forster, R. L. S., and Valkonen, J. P. T. (1998). Biological, serological and molecular differences among iso-lates of potato A potyvirus. Phytopathology 88, 311–321.
Roberts, P. J., and Belsham, G. J. (1995). Identification of critical amino acids with in the foot-and-mouth disease virus leader protein, a cysteine proteinase. Virology 213, 140–146.
Ryan, M. D., and Flint, M. (1997). Virus-encoded proteinases of the picornavirus super-group. J. Gen. Virol. 78, 699–723.
Saitou, N., and Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4, 406–425.
Sambrook, J., Fritsch, E. F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
Sasaki, J., and Nakashima, N. (1999). Translation initiation at the CUU codon is mediated by the internal ribosome entry site of an insect picorna-like virus in vitro. J. Virol. 73, 1219–1226.
Sasaki, J., Nakashima, N., Saito, H., and Noda, H. (1998). An insect picorna-like virus, Plautia stali intestine virus, has genes of capsid proteins in it’s 3⬘ part of the genome. Virology 244, 50–58. Stanway, G. (1990). Structure, function and evolution of picornaviruses.
J. Gen. Virol. 71, 2483–2501.
Strauss, E. G., Strauss, J. H., and Levine, A. J. (1996). Virus evolution. In “Fields Virology” (B. N. Fields, D. M. Knipe, and P. M. Howley, Eds.), 3rd ed., pp. 153–171. Lippincott-Raven, New York.
Swofford, D. L. (1998). PAUP*: Phylogenetic analysis using parsimony (* and other methods). Version 4. Sinauer, Sunderland, MA.
Tate, J., Liljas, L., Scotti, P., Christian, P., Lin, T., and Johnson, J. E. (1999). The crystal structure of cricket paralysis virus: The first view of a new virus family. Nat. Struct. Biol. 6, 765–773.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., and Higgins, D. G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools.
Nucleic Acids Res. 25, 4876–4882.
Turnbull-Ross, A. D., Reavy, B., Mayo, M. A., and Murant, A. F. (1992). The nucleotide sequence of parsnip yellow fleck virus: A plant picorna-like virus. J. Gen. Virol. 73, 3203–3211.
van der Wilk, F., Dullemans, A. M., Verbeek, M., and Van den Heuvel, J. F. (1997). Nucleotide sequence and genomic organization of
Acyrtho-siphon pisum virus. Virology 238, 353–362.
Wang, C. H., Chou, C. M., Liu, H. C., Kau, S. L., Kou, G. H., and Lo, C. F. (1996). Continuous cell line from pupal ovary of Perina nuda (Lepi-doptera: Lymantriidae) that is permissive to nuclear polyhedrosis virus from P. nuda. J. Invertebr. Pathol. 67, 199–204.
Wang, C. H., Wu, C. Y., Chen, W. Y., and Chen, C. W. (1998). Studies on the infectious flacherie of Perina nuda (Fabricius). Chin. J. Entomol. 18, 259–271.
Wang, C. H., Wu, C. Y., and Lo, C. F. (1999). A new picorna-like virus, PnPV, isolated from ficus transparent moth, Perina nuda (Fabcicius).
J. Invertebr. Pathol. 74, 62–68.
Wilson, J. E., Powell, M. J., Hoover, S. E., and Sarnow, P. (2000). Naturally occurring dicistronic cricket paralysis virus RNA is reg-ulated by two internal ribosome entry sites. Mol. Cell. Biol. 20, 4990–4999.
Wu, S., Rinehart, C. A., and Kaesberg, P. (1987). Sequence and organi-zation of Southern bean mosaic virus genomic RNA. Virology 161, 73–80.