Note
Leukemia Inhibitory Factor in Follicular Fluid Is
Not Related to the Number and Quality of Embryos
as Well as Implantation and Pregnancy Rates
Yao-Yuan Hsieh,1,2Chi-Chen Chang,1Horng-Der Tsai,1
and Chich-Sheng Lin2,3
Received 9 August 2004—Final 7 December 2004
INTRODUCTION
Follicular growth and maturation is a complex process regulated by autocrine and paracrine factors. Follicular fluids may affect fertilization and early embryonic development (Ledee-Bataille et al., 2001). Follicular fluid may play an important role in the endocrine balance between the theca and granulosa cells (Ledee-Bataille
et al., 2001). Cytokines have been shown to be present in human follicular fluid
and have regulatory functions in follicular maturation and subsequent embryo development (Coskun et al., 1998).
Leukemia inhibitory factor (LIF) is a pleiotropic cytokine of the interleukin-6 family and has different biological actions in various tissue systems. LIF regulates the growth and differentiation of embryonic stem cells, primordial germ cells, peripheral neurons, osteoblasts, adipocytes, and endothelial cells (Murray and Edgar, 2001). It also plays an important role in the physiology of ovulation, estrogen production, and early embryonic development (Arici et al., 1997). LIF is one of the key determinants of successful implantation (Arici et al., 1997). Follicular fluid LIF also enhances the rate of blastocyst formation (Dunglison
et al., 1996).
Follicular fluid LIF is a useful marker of oocyte quality (Arici et al., 1997). The rising level of follicular fluid LIF around the time of ovulation indicates that LIF may play a role in ovulatory events, early embryonic development, and
1Department of Obstetrics and Gynecology, China Medical College Hospital, Taichung, Taiwan. 2Department of Biological Science and Technology, National Chiao Tung University, 1001 Ta Hsueh
Road, Hsinchu, Taiwan.
3To whom correspondence should be addressed; e-mail: lincs@cc.nctu.edu.tw.
501
implantation (Senturk and Arici, 1998). Ovarian granulosa, stromal cells, and macrophages all express LIF mRNA and secrete the protein (Loukides et al., 1990). Ledee-Bataille et al. (2001) demonstrated that follicular fluid LIF appears to function as an embryotrophic agent. They also noted that the follicular fluid LIF concentration of the PCOS group was lower than that of normal controls.
In our previous studies, we noted that application of LIF enhances preim-plantative embryogenesis, including blastocyst, expanded blastocyst, and hatching blastocyst states (Tsai et al., 1999). In contrast, LIF has nonsignificant influence on embryo development during the early 2- to 8-cell stage (Tsai et al., 2000a). We noted that expression of endometrial LIF is dependent on menstrual stages and cellular localizations (Hsieh et al., 2002). LIF expression is stronger in the endometrial epithelium during the luteal phase (Tsai et al., 2000b). Herein we evaluate further the influence of LIF in follicular fluid on embryo development.
MATERIALS AND METHODS
Patients at the China Medical University Hospital receiving in vitro fertilization and embryo transfer were included in this series. All individuals accepted the long protocol for pituitary down-regulation. The COH protocol, ovarian follicle retrieval, insemination, culture, and transfer were performed as previously de-scribed (Hsieh et al., 1999). Follicular fluid was collected from the dominant follicle without the use of flushing media. After removal of cumulus-oocyte com-plexes, the fluid was centrifuged at 500 × g for 10 min to remove any blood or cellular debris. The cell-free supernatants were then divided into aliquots and stored at−80◦C until assay.
All embryos were scored (according to the number of blastomeres and the degree of fragmentation) prior to transfer (Hsieh et al., 1999). Immunoreactive LIF in follicular fluid samples was quantified using an ELISA from R&D sys-tems (Quantikine, Minneapolis; Cat. No. DLF00), following the steps reported by Arici et al. (1997). According to the manufacturer, there is no measurable cross-reactivity with other known cytokines in this assay. The minimum detectable dose of LIF is typically less than 8 pg/ml. Sensitivity of the LIF measurement was confirmed by our pilot test, and the experimental result showed that the intra-assay and inter-intra-assay coefficients of variation were 7 and 9%, respectively, at 8 pg/ml concentration. Follicular fluid samples were evaluated in duplicate assays. Validation of its use for human follicular fluid was also performed: human recom-binant LIF was diluted in assay buffer and pooled follicular fluid, and parallelism was observed between the standard curve of buffer and follicular fluid dilutions. Each experiment was done using three replicate wells for each condition, and supernatant from each well was tested in a single ELISA assay.
All cases were divided into two groups for further comparison (namely, Group 1, pregnancy, and Group 2, nonpregnancy) after embryo transfer. The LIF
expression, number and quality of embryos, and implantation rate in both groups were compared. The relationship of LIF expression to the number and quality of embryos, as well as pregnancy rate, was evaluated. Further statistical analyses were performed using the SAS statistics package withχ2and simple linear regression tests.
RESULTS
Of the 102 cycles from 102 couples enrolled, there were 32 (31.4%) pregnant cycles in Group 1 and 70 (68.6%) nonpregnant cycles in Group 2. The two groups differed significantly in age (31.73 ± 4.69 vs. 34.2 ± 5.10, P = 0.02) and grade I/II embryo number (5.80 ± 2.71 vs. 4.16 ± 2.86, P = 0.01). The difference in embryo number and follicular LIF concentration (52.45 ± 47.92 vs. 51.28 ± 35.99 pg/ml) was not significant between the two groups.
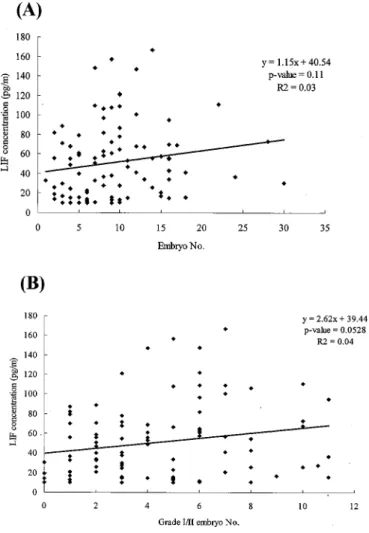
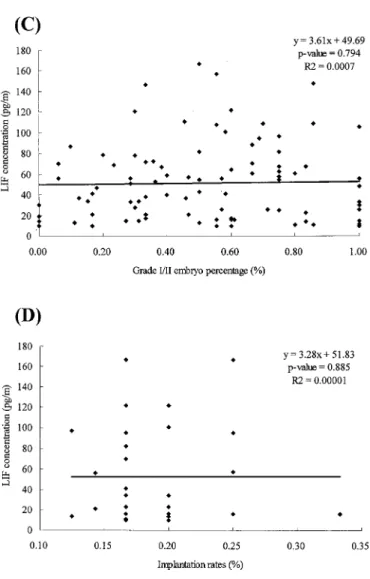
The follicular concentration of LIF was positively but insignificantly cor-related with the number and quality of embryos as well as the implantation rate (Fig. 1). The linear regression formula of embryo number (x) and LIF concentration (y) was y= 1.15x + 40.54 (P = 0.11). The linear regression of grade I/II embryo number (x) and LIF concentration (y) was y= 2.62x + 39.44 (P = 0.0528). The regression formula of grade I/II embryo percentage (x) and LIF concentration (y) was y= 3.61x + 49.69 (P = 0.794). The linear regression of embryo implanta-tion rate (x) and LIF concentraimplanta-tion (y) was y= 3.28x + 51.83 (P = 0.885).
DISCUSSION
LIF, a 43 kD glycoprotein, plays a remarkable role in the development of different tissues, including embryogenesis (Arici et al., 1997; Tsai et al., 1999). The addi-tion of LIF might enhance blastocyst development (Stewart et al., 1992), blastocyst hatching, and pregnancy rates (Fry et al., 1992). Female mice lacking a functional LIF gene are sterile because the blastocysts cannot implant (Stewart et al., 1992). Preimplantation human embryos express LIF and LIF receptor messenger RNA. It is suggested that LIF may affect embryo development by acting as an “embry-otrophin” before implantation (Chen et al., 1999). LIF plays an important role in the physiology of ovulation, estrogen production, and early embryonic develop-ment (Arici et al., 1997). Furthermore, both embryo and endometrium contain LIF receptors, suggesting a possible signaling interaction between the endometrium and the embryo (Charnok-Jones et al., 1994).
Follicular fluid LIF was correlated with embryo quality (Arici et al., 1997), and it significantly increases the quality and number of human blastocysts (Dunglison et al., 1996). It is also correlated with follicular fluid estradiol (Senturk and Arici, 1998) and progesterone (Ledee-Bataille et al., 2001; Ozornek et al.,
1999). During the normal menstruation cycle, the highest level of LIF expression is in the midluteal phase (Charnok-Jones et al., 1994).
The presence of LIF in ovaries, follicles, embryos, and endometrium might influence embryo implantation. Both granulosa-lutein and ovarian stromal cells express LIF mRNA. Modulation of LIF in these cells may play an important role in the physiology of ovulation and early embryo development (Arici et al., 1997). LIF and progesterone concentrations were significantly lower in the follicular fluid of PCOS patients compared with the controls (Ledee-Bataille et al., 2001). LIF and progesterone concentrations in follicular fluid were correlated with each
Fig. 1. Linear regression of LIF concentration with (A) embryo number,
(B) grade I/II embryo number, (C) grade I/II embryo percentage, and (D) implantation rate.
Fig. 1. Continued.
other (Ledee-Bataille et al., 2001). In contrast, some investigators demonstrated a negative association between LIF and embryo development/implantation. Ozornek
et al. (1999) demonstrated that concentrations of follicular LIF were similar in
fertilized and unfertilized oocytes. LIF concentration in the uterine flushing fluid at day 26 was negatively correlated with subsequent implantation (Ledee-Bataille
et al., 2002).
In conclusion, our results do not suggest that LIF concentration in follicular fluid can predict the IVF outcome. Follicular LIF concentration is not a useful marker for the prediction of number and quality of embryos, as well as implantation
and pregnancy rates. This could provide a basis for further studies of LIF in follicular fluid.
REFERENCES
Arici, A., Oral, E., Bahtiyar, O., Engin, O., Seli, E., and Jones, E. E. (1997). Leukaemia inhibitory factor expression in human follicular fluid and ovarian cells. Hum. Reprod. 12:1233–1239. Charnok-Jones, D. S., Sharkey, A. M., Fenwick, P., and Smith, S. K. (1994). Leukemia inhibitory
factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at the time. J. Reprod. Fertil. 101:421–426. Chen, H. F., Shew, J. Y., Ho, H. N., Hsu, W. L., and Yang, Y. S. (1999). Expression of leukemia
inhibitory factor and its receptor in preimplantation embryos. Fertil. Steril. 72:713–719. Coskun, S., Uzumcu, M., Jaroudi, K., Hollanders, J. M., Parhar, R. S., and al-Sedairy, S. T. (1998).
Presence of leukemia inhibitory factor and interleukin-12 in human follicular fluid during follicular growth. Am. J. Reprod. Immunol. 40:13–18.
Dunglison, G. F., Barlow, D. H., and Sargent, I. L. (1996). Leukaemia inhibitory factor significantly enhances the blastocyst formation rates of human embryos cultured in serum-free medium. Hum.
Reprod. 11:191–196.
Fry, R. C., Batt, P. A., Fairclough, R. J., and Parr, R. A. (1992). Human leukemia inhibitory factor improves the viability of ovine embryos. Biol. Reprod. 46:470–474.
Hsieh, Y. Y., Tsai, H. D., Chang, C. C., Chang, S. C., Lo, H. Y., and Lai, A. C. (1999). Ultrarapid cryopreservation of human embryos: Experience with 1,582 embryos. Fertil. Steril. 72:253–256. Hsieh, Y. Y., Lin, C. S., Sun, Y. L., Chang, C. C., Tsai, H. D., and Wu, J. C. (2002). In vivo gene transfer of leukemia inhibitory factor (LIF) into mouse endometrium. J. Assist. Reprod. Genet.
19:79–83.
Ledee-Bataille, N., Lapree-Delage, G., Taupin, J. L., Dubanchet, S., Taieb, J., Moreau, J. F., and Chaouat, G. (2001). Follicular fluid concentration of leukaemia inhibitory factor is decreased among women with polycystic ovarian syndrome during assisted reproduction cycles. Hum.
Reprod. 16:2073–2078.
Ledee-Bataille, N., Lapree-Delage, G., Taupin, J. L., Dubanchet, S., Frydman, R., and Chaouat, G. (2002). Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum. Reprod. 17:213–218.
Loukides, J. A., Loy, R. A., Edwards, R., Honig, J., Visintin, I., and Polan, M. L. (1990). Human follicular fluids contain tissue macrophages. J. Clin. Endocrinol. Metab. 71:1363–1367. Murray, P., and Edgar, D. (2001). The regulation of embryonic stem cell differentiation by leukaemia
inhibitory factor (LIF). Differentiation 68:227–234.
Ozornek, M. H., Bielfeld, P., Krussel, J. S., Hirchenhain, J., Jeyendran, R. S., and Koldovsky, U. (1999). Epidermal growth factor and leukemia inhibitory factor levels in follicular fluid: Association with in vitro fertilization outcome. J. Reprod. Med. 44:367–369.
Senturk, L. M., and Arici, A. (1998). Leukemia inhibitory factor in human reproduction. Am. J. Reprod.
Immunol. 39:144–451.
Stewart, C. L., Kaspar, P., Brunet, L. J., Bhatt, H., Gadi, I., Kontgen, F., and Abbondanzo, S. J. (1992). Blastocyst implantation depends on maternal expression of leukemia inhibitory factor. Nature
359:76–79.
Tsai, H. D., Chang, C. C., Hsieh, Y. Y., Lo, H. Y., Hsu, L. W., and Chang, S. C. (1999). Recombinant human leukemia inhibitory factor enhances the development of preimplantation mouse embryo in vitro. Fertil. Steril. 71:722–725.
Tsai, H. D., Chang, C. C., Hsieh, Y. Y., Hsu, L. W., Chang, S. C., and Lo, H. Y. (2000a). Effect of different concentrations of recombinant leukemia inhibitory factor on different development stages of mouse embryo in vitro. J. Assist. Reprod. Genet. 17:352–355.
Tsai, H. D., Chang, C. C., Hsieh, Y. Y., and Lo, H. Y. (2000b). Leukemia inhibitory factor expression in different endometrial locations between fertile and infertile women throughout different menstrual phases. J. Assist. Reprod. Genet. 17:415–418.