Effects of Insulin-Like Growth Factor-1 and Donor Age on
Transplantation of Porcine Neonatal Pancreatic Cell Clusters
J.-H. Juang, C.-H. Kuo, and N.-K. Yao
ABSTRACT
Porcine neonatal pancreatic cell clusters (NPCCs) isolated from 1- to 3-day-old pigs cured
diabetic nude mice more than 14 weeks after transplantation. To shorten the latent period
between transplantation and reversal of hyperglycemia, we investigated the effects of
insulin-like growth factor-1 (IGF-1) and NPCCs isolated from 1-month-old pigs after
transplantation. Pig pancreata were cut into fragments, collagenase digested, and then
cultured. Three hundred and 2000 NPCCs were transplanted under the kidney capsule of
nondiabetic and diabetic nude mice, respectively. After transplantation, the graft-bearing
kidneys were removed to measure insulin content. NPCCs isolated from 1- to 3-day-old
pigs were cultured with or without IGF-1 for 6 days. The stimulation index was not
significantly different between the 2 groups at 1, 2, or 4 weeks. Moreover, at 4 weeks after
transplantation of 300 NPCCs to nondiabetic nude mice yielded comparable graft insulin
content as the recipients of NPCCs precultured with or without IGF-1. Two thousand
cultured NPCCs isolated from 1-to 3-day-old pigs or 1-month-old pigs were transplanted
into diabetic nude mice. The blood glucose levels of diabetic recipients in both groups
decreased at the same rate after transplantation, achieving normoglycemia at 8 weeks. The
graft insulin content at 12 weeks was not different between the 2 groups. Our data
indicated that isolated NPCCs cultured with IGF-1 showed no beneficial effects on insulin
secretion and transplantation; NPCCs isolated from 1-to 3-day-old and 1-month-old pigs
displayed similar effects on transplantation.
S
INCE 1990, islet transplantation has led to insulin independence in humans with type 1 diabetes melli-tus.1 However, a large number of islets— usually ⬎6000islet equivalents/kg body weight—is needed to achieve normoglycemia.2 Unfortunately, the number of available
organs has leveled despite the ever-growing number of patients on the transplant waiting list. To overcome this supply problem, islet tissues from animal sources have been considered for xenotransplantation. One potential source of tissue for the treatment of diabetes is porcine neonatal pancreatic cell clusters (NPCCs) isolated from 1- to 3-day-old pigs, which are easily isolated, capable of secreting significant quantities of insulin in response to an in vitro glucose challenge, and show growth potential.3–5Moreover,
recent studies have shown that NPCCs can cure diabetes in pancreatecomized pigs6 and nonhuman primates.7
How-ever, it requires more than 14 weeks to reach adequate beta-cell mass and maturity to cure diabetic nude mice after transplantation of NPCCs.4Several beta-cell growth factors
have been shown to increase beta-cell proliferation and
expand beta-cell mass in vitro and/or in vivo.8Among them,
insulin-like growth factors (IGFs) play an essential role in DNA synthesis and proliferation of beta-cells, acting as islet survival factors.9 –11 In addition, NPCCs isolated from
1-month-old pigs were larger and contained more insulin
From the Division of Endocrinology and Metabolism, Department of Internal Medicine, Chang Gung Memorial Hospital, Taoyuan (J.-H.J.), Department of Biological Science and Technology, National Chiao Tung University, Hsinchu (C.-H.K.), and Industrial Technology Research Institute, Hsinchu (N.-K.Y.), Taiwan.
J.-H. Juang and C.-H. Kuo made equal contributions to this work.
Supported by grants from Chang Gung Memorial Hospital (CMRPG33109), Industrial Technology Research Institute (PMRPG3035), and the National Science Council (NSC 94-2314-B-182A-124 and NSC95-2314-B-182A-183) of Taiwan.
Address reprint requests to Dr Jyuhn-Huarng Juang, Division of Endocrinology and Metabolism, Department of Internal Med-icine, Chang Gung Memorial Hospital, 5 Fu-Shin Street, Kweis-han, Taoyuan, Taiwan. E-mail:jjuang@adm.cgmh.org.tw
0041-1345/09/$–see front matter © 2009 by Elsevier Inc. All rights reserved.
doi:10.1016/j.transproceed.2009.03.057 360 Park Avenue South, New York, NY 10010-1710
than those from 1- to 3-day-old pigs.12Thus, in the present
study, we tested whether NPCCs isolated from 1-month-old pigs and NPCCs precultured with IGF-1 were beneficial for transplantation.
MATERIALS AND METHODS Animals5,12
Donor pancreata were obtained from 1- to 3-day-old and 1-month-old neonatal pigs of either gender. Male athymic nude Balb/c mice, age 8 –12 weeks, were used as recipients of the NPCCs. Mice were rendered diabetic by intravenous injection of 90 mg/kg alloxan (Sigma Chemical Co, St Louis, Mo, United States) which was freshly dissolved in 1 mmol/L hydrochloric acid 14 days before transplantation.
Preparation and Culture of NPCCs5,12
Each pancreas was cut into⬃1–2 mm3fragments before digestion with collagenase (type V, Sigma Immunochemicals) in a water bath at 37°C.5,12 Filtered and washed digest was placed in RPMI maintained at 37°C (5% CO2, 95% air) in humidified air.
NPCCs Culture With IGF-1 Treatment
Isolated NPCCs were cultured in RPMI medium containing IGF-1 (100 ng/mL) for measurement of insulin content at 1, 2, and 4 weeks.5,12,13NPCCs secretory response to glucose was determined at 2 and 4 weeks using static incubation.5,12NPCCs were incubated in RPMI medium supplemented with 100 mg/dL glucose and then 500 mg/dL glucose for 120 minutes, respectively. The media were collected at 120 and 240 minutes for insulin measurement. Stimu-lation indices were calculated by dividing the amount of insulin release at 500 mg/dL glucose by that released at 100 mg/dL glucose.
Transplantation of NPCCs
After 6 days of culture, 300 and 2000 NPCCs were transplanted into nondiabetic and diabetic nude mice, respectively.5,12NPCCs were centrifuged in PE-50 tubing connected to a 200-L pipette tip. A capsulotomy was performed in the lower pole of the mouse kidney. The tip of the tubing was advanced under the capsule of the upper pole, the site of final injection.
Insulin Content of NPCCs and the Graft
One hundred fifty NPCCs were sonicated. The removed graft-bearing kidneys were homogenized in acid ethanol.5,12,13After sonica-tion or homogenizasonica-tion, the samples were extracted overnight at 4°C. On the following day, they were centrifuged at 2400 rpm for 10 minutes (NPCCs) or 30 minutes (kidney), and the supernatant stored at⫺20°C. The pellet was again sonicated or homogenized in acid ethanol, and insulin extracted overnight. After centrifugation, this second was added to the first extraction sample. Insulin was measured using radioimmunoassay with an INSI-PR kit (CIS US Inc, Bedford, Mass, United States).
Statistical Analysis
Results were expressed as mean values and standard errors of the mean (M⫾ SEM). We used unpaired Student t test for compari-sons between the 2 groups. A value of P⬍ .05 was significant.
RESULTS
Effects of IGF-1 on Beta-Cell Function and Transplantation
The insulin content of 150 NPCCs isolated from 1- to 3-day-old pigs cultured with versus without IGF-1 was comparable at 1 (6.6⫾ 1.9 vs 7.5 ⫾ 1.2g; P ⫽ .66), 2 (6.6 ⫾ 0.7 vs 8.2 ⫾ 0.5g; P ⫽ .10), and 4 (3.7 ⫾ 1.0 vs 4.3⫾ 1.0g; P ⫽ .21) weeks. In addition, the stimulation index of those cultured with versus without IGF-1 was not significantly different at 1 (2.6⫾ 0.2 vs 2.1 ⫾ 0.4; P ⫽ .51), 2 (5.0 ⫾ 0.7 vs 6.3 ⫾ 0.8; P ⫽ .41), and 4 (4.3 ⫾ 0.7 vs 3.7⫾ 0.5; P ⫽ .21) weeks. Furthermore, at 4 weeks after transplantation of 300 NPCCs to nondiabetic nude mice, the mean insulin content of the grafts was comparable with that of recipients of NPCCs precultured with versus without IGF-1 (18.7⫾ 5.6 vs 17.2 ⫾ 0.9g; P ⫽ .75).
Effects of NPCCs Isolated From 1- to 3-Day-Old Versus 1-Month-Old Pigs on Islet Transplantation
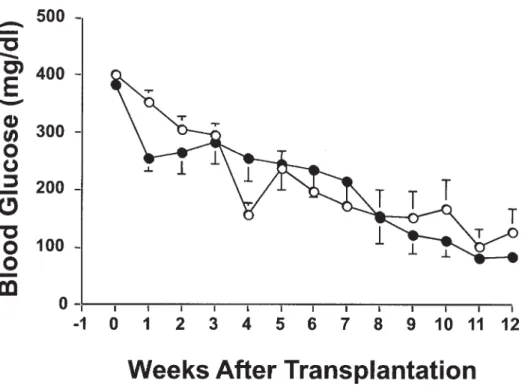
In diabetic recipients transplanted with 2000 NPCCs iso-lated from 1- to 3-day-old and 1-month-old pigs, the blood glucose in both groups decreased at the same rate after transplantation and normoglycemia was achieved at 8 weeks (Fig 1). The body weight did not significantly differ between the 2 groups. Additionally, their insulin content of the graft was comparable at 12 weeks after transplantation (7.9 ⫾ 1.5 and 5.4 ⫾ 1.1 g in 1- to 3-day-old and 1-month-old NPCCs recipients, respectively; P⫽ .055).
DISCUSSION
Previously, Korbutt et al showed that it required 8 and more than 14 weeks to reach an adequate beta-cell mass and maturity to cure diabetic nude mice after transplantation with 2000 and 1000 NPCCs, respectively.4To shorten the
latent period between transplantation and hyperglycemia reversal, we investigated the effects of IGF-1 and donor age. It had been demonstrated that IGFs stimulate DNA synthesis and proliferation as well as inhibit beta-cell apoptosis,9 –11 which may increase beta-cell mass, thereby
benefiting transplantation outcomes. However, in the present study, we observed the insulin content and stimu-lation index of NPCCs cultured with versus without IGF-1 to be not significantly different at 1, 2, and 4 weeks. Further-more, at 4 weeks after transplantation of 300 NPCCs to nondiabetic nude mice, the mean insulin content of the grafts was comparable with that of recipients of NPCCs precultured with versus without IGF-1. These findings were consistent with those of Lopez-Avalos et al who showed no significant in-crease in DNA and insulin content in NPCCs treated with growth factors, including IGF-1. In addition, although in-creased insulin content of NPCCs was achieved in vitro by addition of a combination of fetal calf serum, IGF-I, nicotin-amide, and sodium butyrate, the increase did not shorten the time to achieve normoglycemia after transplantation.14 The
dose, timing, and duration of IGF-1 treatment may partly explain why there was no response of NPCCs to growth factors that had previously been reported to induce proliferation of
beta cells and/or differentiation duct cells. However, further studies are needed to explore the underlying mechanism(s).
Regarding donor age, we have investigated the characteris-tics of NPCCs isolated from 1-month-old pigs and their effects on transplantation.12 The mean yield of NPCCs isolated
from 1- to 3-day-old and 1-month-old pigs was comparable. However, soon after isolation the latter were larger than the former, containing more insulin after a 6-day culture. The stimulation indices of NPCCs isolated from 1- to 3-day-old and 1-month-old pigs were not significantly different. Nondi-abetic recipients of NPCCs isolated from 1- to 3-day-old versus 1-month-old pigs showed similar graft beta-cell mass and function in both groups. In the present study of diabetic recipients transplanted with 2000 NPCCs isolated from 1- to 3-day-old and 1-month-old pigs, the blood glucose in both groups decreased at the same rate after transplantation; normoglycemia was achieved at 8 weeks, which was consistent with a previous study.4Additionally, we observed their graft
insulin content was comparable at 12 weeks after transplanta-tion. In terms of growth and function, NPCCs isolated from 1-month-old pigs are not better than those from 1- to 3-day-old pigs. Presumably, significant NPCCs maturation takes more than 1 month after birth. Further studies on the characteristics of young pig islets are needed to know their maturation process. In conclusion, our data indicated that isolated NPCCs cultured with IGF-1 showed no beneficial effects on in vitro function and transplantation, and NPCCs isolated from 1- to 3-day-old and 1-month-old pigs had similar effects on transplantation.
REFERENCES
1. Robertson RP: Islet transplantation as a treatment for diabe-tes-a work in progress. N Engl J Med 350:694, 2004
2. Brendel MD, Hering B, Schultz AO, et al: International Islet Transplant Registry Newsletter 8:4, 2001
3. Korsgren O, Jansson L, Eizirik D, et al: Functional and morphological differentiation of fetal porcine islet-like clusters after transplantation into nude mice. Diabetologia 34:379, 1991
4. Korbutt GS, Elliott JF, Ao Z, et al: Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest 97:2119, 1996
5. Juang JH, Hsu BRS, Kuo CH, et al: Neonatal porcine pancreas as a source of islet transplantation. Transplant Proc 33:757, 2001
6. Kin T, Korbutt GS, Kobayashi T, et al: Reversal of diabetes in pancreatectomized pigs after transplantation of neonatal porcine islets. Diabetes 54:1032, 2005
7. Cardona K, Korbutt GS, Milas Z, et al: Long-term survival of neonatal porcine islets in nonhuman primates by targeting con-stimulation pathways. Nat Med 12:304, 2006
8. Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, et al: Growth factors and beta cell replication. Int J Biochem Cell Biol 38:931, 2006 9. Hogg J, Han VKM, Clemmons DR, et al: Interactions of nutrients, insulin-like growth factors (IGFs) and IGF-binding proteins in the regulation of DNA synthesis by isolated fetal rat islets of Langerhans. J Endocrinol 138:401, 1993
10. Sieradzki J, Fleck H, Chatterjee AK, et al: Stimulatory effect of insulin-like growth factor-I on [3H]thymidine incorporation, DNA content and insulin biosynthesis and secretion of isolated pancreatic rat islets. J Endocrinol 117:59, 1988
11. Petrik J, Arany E, McDonald TJ, et al: Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology 139:2994, 1998
12. Juang JH, Hsu BRS, Kuo CH, et al: Characteristics and transplan-tation of the porcine neonatal pancreatic cell clusters isolated from 1-to 3-day-old versus 1-month-old pigs. Transplant Proc 36:1203, 2004
13. Juang JH, Hsu BRS, Kuo CH, et al: Influence of donor age on mouse islet characteristics and transplantation. Cell Transplant 10:277, 2001
14. Lopez-Avalos MD, Tatarkiewicz K, Sharma A, et al: En-hanced maturation of porcine neonatal pancreatic cell clusters with growth factors fails to improve transplantation outcome. Trans-plantation 71:1154, 2001
Fig 1. Evolution of blood glu-cose in diabetic recipients trans-planted with 2000 NPCCs iso-lated from 1- to 3-day-old (open circle) and 1-month-old (closed circle) pigs. Data are expressed as mean⫾ SE.