Fan-Yi Hsu
Department of Materials Science and Engineering, National Tsing Hua University, Hsinchu 30013, Taiwan, Republic of China
Ching-Chich Leu
Department of Chemical and Materials Engineering, National University of Kaohsiung, Kaohsiung 811, Taiwan, Republic of China
Chao-Hsin Chien
Department of Electronics Engineering, National Chiao Tung University, Hsinchu 30050, Taiwan, Republic of China
Chen-Ti Hua)
Department of Materials Science and Engineering, National Tsing Hua University, Hsinchu 30013, Taiwan, Republic of China
(Received 17 March 2006; accepted 16 August 2006)
We have investigated the effect that the Ta content has on the ferroelectric properties of strontium bismuth tantalate (SBT) thin films synthesized using metalorganic decomposition (MOD) and spin coating techniques. The physical properties of these SBT samples were strongly dependent upon the Ta ratio. Polarization measurements revealed that Ta-deficient SBT exhibited a relatively low coercive field (2Ec ∼ 87
kV/cm) and a high remanent polarization (2Pr∼ 15 C/cm 2
). The value of 2Pr
decreased as the Ta ratio in SBT increased. The improved ferroelectric properties of the Ta-deficient SBT samples may have resulted from the uniformly well-grown bismuth-layered-structured (BLS) phases of the films and their highly preferential orientation along the a and b axes. We suggest that the incorporation of Ta vacancies plays an important role in enhancing the crystallinities and microstructures of
Ta-deficient SBT films.
I. INTRODUCTION
Although strontium bismuth tantalate (SrBi2Ta2O9,
SBT) is one of the most promising materials for appli-cation in nonvolatile random access memories (it dis-plays several excellent ferroelectric properties, such as a low leakage current, low operating voltage, and fatigue endurance for up to 1012 switching cycles),1 its high crystallization temperature (>750 °C), which is obvi-ously incompatible with conventional very large scale integrated (VLSI) processes, and the relatively low re-manent polarization of SBT thin films remain major ob-stacles for its deployment.
Ferroelectric SBT thin films have been grown using many techniques, including metalorganic decomposition (MOD),2 pulsed laser deposition (PLD),3 and metalor-ganic chemical vapor deposition (MOCVD).4
The MOD method in particular offers a number of advantages, such
as good homogeneity, long shelf-life, precise control over the composition, low cost, and simplicity.5It is well known that the ferroelectric properties of SBT thin films along the a and b axes are much better than those along the c axis.6 Thus, the texture of SBT thin film is an important factor for determining its electrical properties. MOD is not, however, an effective method when at-tempting to control the crystalline orientation. A number of techniques are used to manipulate the film orientation during wet chemical depositions such as the MOD and sol-gel methods, for example, utilizing the appropriate substrates with either a matching lattice or suitable buffer layers.7,8 Alternatively, modifications of the precursor solutions, film compositions, and heat treatment process may also be used to affect the film’s crystallinity and texture.9,10
SBT thin films containing Sr and Bi contents deviating from stoichiometry have been studied for many years.11
The crystalline structures and ferroelectric prop-erties are influenced markedly through small composi-tional changes. In Bi-excess and Sr-deficient SBT samples, Sr sites are inhabited by Bi ions.12,13
Shi-makawa et al. reported that the substitution of small Bi3+ a)
Address all correspondence to this author. e-mail: cthu@mx.nthu.edu.tw
ions for large Sr2+
ions causes pronounced compressive stress in the perovskite-like unit. This mismatch between the perovskite-like unit and the Bi2O2interlayer leads to
great distortion of the TaO6octahedra, resulting,
conse-quently, in a SBT sample displaying a large spontaneous polarization and a high Curie temperature.14Chen et al. have attributed the enhancement in Prto the large
aver-age grain sizes of Sr-deficient SBT.15 The value of Pr
decreases, however, when the Sr ratio becomes too low, presumably because of the formation of Sr vacancies and the appearance of a second phase (BiTaO4).
15,16
Recently, we reported that the film properties of SBT are improved when using an ultrathin Ta (<1 nm) buffer layer.17The Ta buffer layer causes the SBT composition to depart from stoichiometry to form a Ta-enriched re-gion near the SBT–Pt interface. The excess Ta atoms in local regions readily react with O atoms to form TaOx species that serve as nucleation sites for the crystalliza-tion process. This unwanted reaccrystalliza-tion can, therefore, sig-nificantly affect the microstructures and ferroelectric properties of SBT thin films. In this present study, we investigated the effect that the Ta ratio had on the physi-cal and electriphysi-cal properties of SBT thin films, and herein we suggest possible mechanisms to explain this behavior.
II. EXPERIMENTAL
SBT thin films were prepared using a MOD method. To evaluate the influence of Ta, the molar compositions used in these studies were SrBi2Ta1.8O9 (Ta-deficient),
SrBi2Ta2O9 (stoichiometric Ta), and SrBi2Ta2.2O9
(Ta-rich); for the sake of convenience, these samples are denoted as Ta18, Ta20, and Ta22, respectively. SBT thin films were deposited in multiple layers on top of Pt (150 nm)/TiO2 (20 nm)/SiO2/Si substrates. Each layer
was spin-coated at 4000 rpm and dried for 10 min at 150 °C, and then the sample was baked at 400 °C for 10 min. After rapid thermal annealing (RTA) at 750 °C under an oxygen atmosphere for 10–60 s, the five-layered SBT exhibited a film thickness of about 200 nm. For electrical measurements, Pt top electrodes were sput-tered onto the SBT films through a shadow mask having a diameter of 0.2 mm. The ferroelectric properties of the SBT thin films were measured at room temperature using a RT66A ferroelectric testing system (Radiant Technolo-gies, Albuquerque, NM). The leakage current character-istics of the SBT thin films were studied using a semi-conductor parameter analyzer (HP 4156A, Agilent Tech-nologies, Japan). To analyze the physical characteristics, an x-ray diffractometer (XRD; M18XHF-SRA, MAC Science Co., Yokohama, Japan), field-emission scanning electron microscope (FESEM; JEOL JSM-6500F, To-kyo, Japan), and x-ray photoemission spectrometer (XPS; PHI 1600, Physical Electronics USA) were used. The XPS analysis was performed using the Mg K␣line (1253.6 eV). In addition, the C 1s peak (284.8 eV) was
used to calibrate the measured spectrum. To identify the film compositions of the SBT thin films after crystalli-zation at high temperature, the films were dissolved in an acidic solution and analyzed using inductively coupled plasma (ICP) mass spectrometry. The results indicated the composition ratios were close to those of the precur-sor solutions. This result implies that the short duration (10–60 s) of the RTA process did not lead to evaporation of any of the elements in the SBT thin film.
III. RESULTS AND DISCUSSIONS
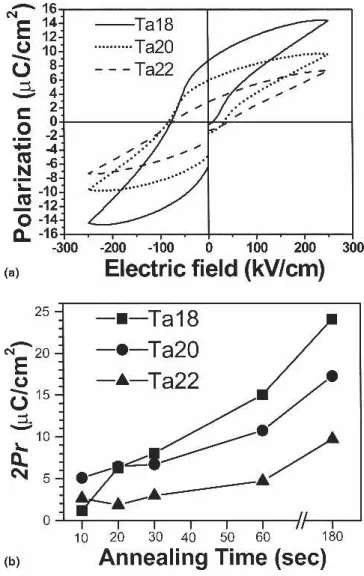
Figure 1(a) displays the polarization–electric field (P–E) curves, measured at ±5 V, of various Pt/SBT/Pt capacitors that had been annealed at 750 °C for 60 s in an oxygen ambient. All the samples exhibited well-defined hysteresis loops, suggesting that each SBT film (with different Ta content) exhibited good ferroelectric prop-erties after high-temperature annealing. Among these
FIG. 1. (a) P–E hysteresis loops of SBT thin films deposited on Pt/ TiO2/SiO2/Si substrates and crystallized at 750 °C for 60 s through RTA in an oxygen ambient. (b) Remanent polarizations of various compositions as a function of the crystallization time.
samples, Ta18 seemed to exhibit the best properties. The remanent polarization (2Pr) values of Ta18, Ta20, and
Ta22 specimens were 15.01, 10.75, and 5.55 C/cm2
, respectively, and the coercive fields (2Ec) were 87, 115,
and 117 kV/cm, respectively. These values confirm that the Ta composition had a dramatic impact on the SBT properties: a reduction of the Ta ratio led to an increase in the value of 2Prof the SBT and a decrease in the value
of 2Ec. Figure 1(b) illustrates the values of the remanent
polarization of SBT as a function of the crystallization time (i.e., the duration of RTA at 750 °C). It is evident that, regardless of the Ta ratio, the value of 2Princreased
after the annealing time was increased. The tendencies in the values of 2Pras a function of the annealing time were
similar for Ta20 and Ta22, but with Ta20 always exhib-iting relatively higher values of 2Pr. Although Ta18
ex-hibited the best properties in Fig. 1(a), it did not always provide the highest values of 2Pr under each set of
an-nealing conditions. After the shortest anan-nealing time (10 s), Ta18 exhibited the worst ferroelectric property. Nevertheless, the rate of increase of 2Pr for Ta18 as a
function of annealing time was greater than those of the other two specimens (Ta20 and Ta22). As a result, the largest remanent polarizations were observed for Ta18 when the annealing times were longer than 30 s. Al-though the values of 2Pr increased when the annealing
time was increased, the leakage current density in SBT rose significantly when the annealing time was increased from 60 to 180 s, possibly because of the larger surface roughness and greater degree of diffusion between SBT and the substrate when crystallization was performed over a longer time. The leakage current might contribute to the higher value of 2Prof SBT; it would be detrimental
to the application of SBT to nonvolatile random access memories.
The electrical properties of ferroelectrics generally correspond to their crystallinities and microstructures. To understand the causes of the distinct behavior displayed in Fig. 1 for the SBT samples containing different Ta ratios, we performed physical analyses of these speci-mens. The x-ray diffraction (XRD) patterns of SBT films after crystallization at 750 °C (Fig. 2) clearly illustrated their polycrystalline structures. In Fig. 2(a), each of the three SBT films, after annealing for 10 s, possessed a mixture of bismuth-layered-structured (BLS) and fluorite phases. Because fluorite is an intermediate structure, its presence implies that the phase transformation in SBT was incomplete. When the annealing time was increased from 10 to 30 s [Figs. 2(a)–2(c)], the peak intensity of the fluorite phase decreased significantly while the BLS peaks became stronger and sharper. Regardless of the annealing duration, Ta18 possessed the lowest amount of fluorite among the three samples; the volume fraction of fluorite in Ta22 was always greater than that in Ta20. By further extending the annealing time, the amount of BLS
continued to increase, with the accompanying decrease of the signal for fluorite. Figure 3(a) presents the ten-dency of the integrated intensity ratios of the fluorite (111) to BLS (115) reflections {IF(111)/[IB(115)+ IF(111)]}
as a function of annealing time; here, IF(111)is the
inte-grated intensity of the (111) reflection of the fluorite phase. After annealing for 60 s, Ta18 possessed a nearly pure BLS phase, but slight and considerable amounts of fluorite phases were still detectable for Ta20 and Ta22, respectively. Because the fluorite phase is non-ferroelectric, the existence of fluorite degrades the ferro-electric properties of SBT. Furthermore, it is worth not-ing that not only the crystallinities changed with respect to the Ta ratio but so did the textures of the SBT samples. Figure 3(b) indicates that the intensity ratios of the (200) peaks (I(200)/[I(115) + I(200)]) of the BLS phases varied
with respect to the annealing time. [In Fig. 3, we ex-cluded the BLS (220) orientation from the calculations because the overlap between the Pt (200) and BLS (220) peaks would possibly obstruct the results.] Because the ferroelectric properties are much better along the a-b axes of preferentially oriented SBT, this intensity ratio should indicate the degree of preferential orientation along the a and b axes as well as the ferroelectric prop-erties. As indicated in Fig. 3(b), Ta18 exhibited the high-est BLS (200) intensity ratios among all the specimens, regardless of the annealing time. In addition, it seems that the texture of the SBT sample depended strongly on its Ta content and slightly on the annealing duration. To summarize the XRD analyses, Ta18 displayed superior preferential orientation and a higher extent of crystalli-zation. Based on these results, Ta18 should exhibit the greatest ferroelectric properties among all of these speci-mens under all of the annealing conditions. This hypoth-esis holds true for the samples that had been annealed for longer than 30 s, but it was clearly not the case for shorter crystallization times. Thus, there must be other factors that affect the ferroelectric properties of the SBT samples, such as the microstructures of the SBT films.
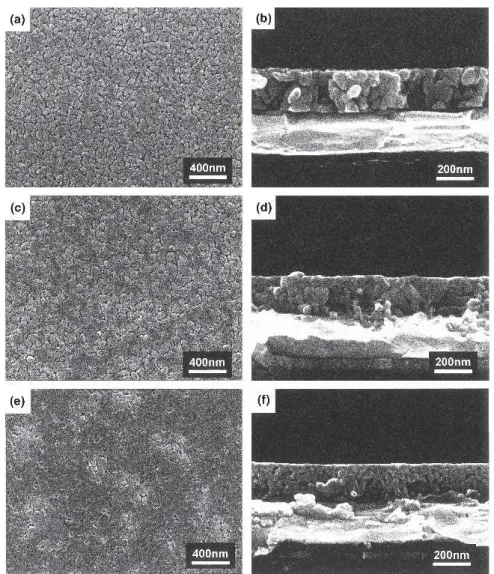
We used the FESEM to examine the surface mor-phologies of the SBT thin films (Fig. 4). The SBT films that had been crystallized for 10 s were composed of mostly nano-sized grains [Figs. 4(a)–4(c)], although we observed some elliptical grains with sizes greater than 70 nm for Ta20 [Fig. 4(b)]. These large grains are the typical BLS grain structure.18,19We believe that the ap-pearance of these large grains is the reason for the rela-tively high value of 2Pr in Ta20. Although the Ta18
sample possessed the greatest volume fraction of the fer-roelectric BLS phase, its BLS grains were not suffi-ciently large. It has been reported that the electrical prop-erties of ferroelectrics depend strongly on their grain sizes.20
When the grains are smaller than a critical size, the ferroelectrics exhibit only weak ferroelectric behav-ior. In Ta18, each grain had a size smaller than 40 nm
[Fig. 4(a)]. For this reason, we speculate that Ta18 will display poor ferroelectric properties if the annealing time is insufficient to produce large grains. In contrast, an abundance of large grains was produced in Ta18 when the annealing time was greater than 30 s [Fig. 4(d)]. Under these conditions, the volume fraction of large grains (∼90 nm) in Ta18 exceeded that in Ta20 [Fig. 4(e)]. When we extended the annealing time further to 60 s, uniformly large grain structures developed in Ta18 [Figs. 5(a) and 5(b)], whereas Ta20 [Figs. 5(c) and 5(d)] retained a considerable volume of nano-sized grains. Moreover, the growth of such large grains was considerably slower in Ta22; only a few large grains emerged in the nano-grain matrix even after the sample was subjected to a relatively long annealing time of 60 s [Figs. 5(e) and 5(f)]. The changes in the microstructures
of the SBT samples reflect the fact that the growth rates of the BLS phase depend strongly on the Ta ratio in SBT; indeed, the growth rate increased upon decreasing the Ta content from Ta-rich (Ta22) to Ta-deficient (Ta18) SBT. To summarize the physical analyses from the XRD and SEM results, we found that the microstructures, textures, and phase constitutions were all closely related to the Ta ratios in the SBT samples. The fast transformation of fluorite to the BLS phase and the rapid BLS growth rate were the major factors contributing to the ultimately uni-form and large grain structures in Ta18. This result im-plies that the nucleation and growth processes of the BLS grains were more pronounced in Ta18 than they were in either Ta20 or Ta22.
Because the ionic radii of Sr (1.16 Å) and Bi (1.03 Å) are much greater than that of Ta (0.64 Å),11,20a deficiency in FIG. 2. XRD patterns of the SBT thin films that had been crystallized at 750 °C for (a) 10 s, (b) 20 s, (c) 30 s, and (d) 60 s. The Si peak originated from the silicon substrate.
Ta cannot be compensated well by an excess of Sr or Bi. Based on defect chemistry, the deficiency of Ta atoms in the SrBi2Ta1.8O9film would be accompanied by the
oc-currence of cation vacancies and holes: 5
2O2→ 2V⬘⬙⬙Ta+ 5OO
x + 10h° , (1)
where VTa⬘⬙⬙ indicates a negatively charged vacancy
(rela-tive to a perfect lattice) on the Ta site, OxO denotes an
oxygen atom on the oxygen site, and h° represents a hole. The existence of cation vacancies and holes can be proved by the variations of conductivity in SBT. The measured conductivity of the Ta18 sample annealed for 60 s was greater than those of the other two samples (Table I). This result may be due to the induction of
p-type charge carriers (holes) in Ta18.21,22
Whereas a higher conductivity might lead to apparently higher po-larization, the leakage current density of each of these three samples (Ta18, Ta20, and Ta22) was lower than
3.7 × 10−7
A/cm2
under an applied electric field of 130 kV/cm. The current densities of these SBT samples are comparable to those (10−6
–10−7
A/cm−2
) reported previously.23–25If our hypothesis is correct, a large num-ber of Ta+5 vacancies would be generated within the Ta-deficient SBT. Because the Ta–O bonding strength is greater than those of Sr–O and Bi–O bonds, the diffusion coefficient of Ta (<10−17 cm2/s) in SBT is much lower than those of the Sr (10−15cm2/s) and Bi (10−14cm2/s) atoms, as measured from the SBT species in a SiO2layer
at 800 °C.26 We believe that the diffusion behavior of SBT in our present study is controlled by the constituted Ta ions and is accelerated significantly by the generation of Ta+5 vacancies. Therefore, both the nucleation and growth of the BLS grains in Ta18 would be promoted by the deficiency of Ta ions in the material. The existence of Ta+5 vacancies also affects the polarization-switching properties of ferroelectrics.27Because the defects can act as nuclei,28 the energy barrier for the nucleation of fer-roelectric domains was significantly lowered by the ex-istence of Ta+5vacancies, leading to a lower value of Ec.
This result corresponds well with the low value of Ecof
Ta18 observed in our P–E analysis (Fig. 1). On the other hand, a Ta deficiency can also be regarded as an excess of Sr and Bi in SBT. Although an excess of Bi in Sr-deficient SBT has been proven to improve the material’s properties, few studies describe the variations of the properties of SBT when Bi and Sr are both present in excess.13,15The only example of which we are aware is that of a study of Sr1.2Bi2.3Ta2O9 films by Chen et al.;
their experimental results—poor ferroelectric proper-ties—are in contrast to ours.15Although similar to Ta18, the Ta/Sr and Ta/Bi ratios in Chen’s samples were lower than stoichiometric; the starting compositions of the SBT samples were quite different in the two sets of experi-ments. Further examinations are necessary if we are to understand the roles of Sr, Bi, and Ta and their ratios in SBT samples.
It was possible that the SBT films possessed the fol-lowing three types of defects: O vacancies, Ta vacancies, and Bi vacancies. The ferroelectric Aurivillius phases of SrBi2Ta2O9 consist of layers of perovskite-like blocks
[(SrTa2O7) 2−
] sandwiched between two consecutive (Bi2O2)
2+
layers. The oxygen ions in the SrTa2O7
perovskite layers are much more stable than those within the Bi2O2layers. Therefore, for SBT thin films, oxygen
atom vacancies occur preferentially in the Bi2O2layers. 29
We speculated that the deficiency of Ta in Ta18 films contributed to Ta vacancies, and the redundant oxygen atoms would stabilize (or compensate) the oxygen ions (vacancies) in the SrTa2O7octahedra or Bi2O2layers. In
contrast, the excess of Ta atoms would contribute to the oxygen atom vacancies in SrTa2O7octahedra. This extra
amount of Ta in Ta22 would cause oxygen ions to be-come unstable, either at the SrTa2O7octahedra or Bi2O2
FIG. 3. (a) Intensity ratios {IF(111)/[IB(115)+ IF(111)]} of the fluorite phase and (b) texturing ratios {I(200)/[I(115) + I(200)]} of SBT films containing different Ta contents as a function of annealing time.
layers. As discussed above, a deficiency of Ta could introduce extra Ta vacancies into the SBT film. The in-corporation of such vacancies would also affect the chemical bonding conditions of SBT. XPS is a sensitive technique that can distinguish between local electronic structures when the chemical nature of a specimen is probed. We conducted XPS analyses of the SBT film surfaces to study the changes in the chemical states of Ta at various Ta contents. Figure 6 displays the XPS spectra of the Ta 4f core levels for the samples before [Figs. 6(a)–6(c)] and after [Figs. 6(d)–6(f)] argon-ion sputtering for 60 s. To identify the contributions of the different oxidation states, it was necessary to perform a deconvolution (peak fitting) of the measured spectra with respect to the different chemical shifts. The ratio of the intensities of the doublet components (Ta 4f core levels) was determined using the respective ratio of the occupa-tion number of the subshell states [i.e., (Ta 4f7/2)/(Ta
4f5/2) ⳱ 4/3]. After correction for the spectral
back-ground using Shirley’s method,30 we decomposed the
spectra into the Gaussian–Lorentzian profiles associated with metallic Ta and Ta in Ta2O5and suboxides,
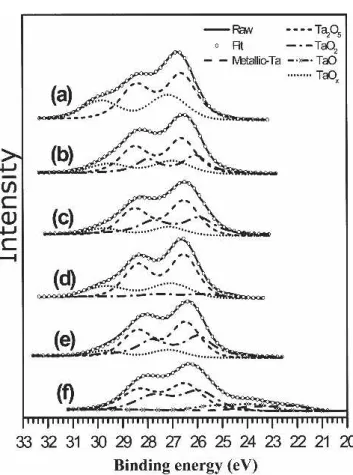
respec-tively. Table II lists the fitting peak positions for metallic Ta and its associated oxides; Table III summarizes the entire measured chemical bonding states of Ta atoms in the different SBT samples. The sequences illustrate the relative amounts of the various chemical states in the specimens (high to low). In Fig. 6, the solid line repre-sents the real spectrum, while the curve of circular sym-bols represents the fitted one. The Ta 4f spectrum ob-tained from the Ta18 sample [Fig. 6(a)] was deconvo-luted into two 4f7/2 peaks at 26.6 and 27.4 eV. We
attribute the binding energy of 26.6 eV to Ta2O5, the
highest oxidation state of tantalum. The other peak (at 27.4 eV) might be related to the TaOx complex. The binding energy in TaOx is 0.8 eV higher than that in Ta2O5, implying that more electronegative species are
involved in the bonds.31 This species was possibly a negatively charged Ta vacancy or oxygen ion. Figures 6(b) and 6(c) depict the deconvoluted XPS peaks from FIG. 4. FESEM planar images of SBT thin films: (a) Ta18, (b) Ta20, and (c) Ta22 annealed at 750 °C for 10 s,; (d) Ta18, (e) Ta20, and (f) Ta22 annealed at 750 °C for 30 s.
the Ta20 and Ta22 samples, respectively. The 4f7/2peaks
at 25.9, 26.6, and 27.4 eV indicate the presence of TaO2,
Ta2O5, and TaOx, respectively; i.e., a new Ta oxidation state (TaO2) occurred in Ta20 and Ta22 that was absent
in Ta18. Because the well-bonded Ta atoms in SBT ex-hibit an oxidation state of Ta2O5, the presence of TaO2
implies that some of the Ta atoms in the perovskite layers of SBT were not fully bonded and, possibly, were asso-ciated with the oxygen vacancies. In addition, the relative amount of TaO2 in Ta22 was greater than that in Ta20,
indicating that the extra Ta in SBT caused an increase in the number of embedded oxygen vacancies. Figures 6(d)–6(f) present the XPS spectra of the Ta 4f core levels for sputtered Ta18, Ta20, and Ta22, respectively. We
performed this sputtering with argon ions to remove any residual contamination on the films’ surfaces. In addi-tion, any changes in the nature of the chemical states after ion bombardment could be useful to understanding the stability of the tantalum oxides. After sputtering, we observed no new chemical states for Ta18 [Fig. 6(d)] or Ta20 [Fig. 6(e)], but the relative intensities of the Ta states had changed slightly. The intensity ratio of TaOx decreased, accompanied by increases in the ratios of TaO2and Ta2O5; this result suggests that the TaOxbond is relatively unstable. In contrast, we observed significant changes in the XPS spectrum in Ta22 after sputtering [Fig. 6(f)]; new chemical states appeared at lower en-ergy—22.1 and 23.8 eV—which corresponded to metal-like Ta (4f7/2) and TaO (4f7/2) states, respectively.
32
Al-though we could not confirm definitively which of the bonds contributed to the formation of the metal-like Ta and TaO during ion bombardment, this result does clearly reflect the fact that the Ta–O bonds in Ta22 were less stable than those in Ta18 and Ta20. The weakness of the Ta–O bonds in Ta22 might have arisen from the FIG. 5. FESEM planar and cross-sectional images of SBT thin films after crystallization at 750 °C for 60 s: (a,b) Ta18, (c,d) Ta20, and (e,f) Ta22.
TABLE I. AC conductivities (measured at 10 Hz and room tempera-ture) of SBT films incorporating different Ta contents.
Ta18 Ta20 Ta22 Conductivity (⍀−1cm−1) 9.55 × 10−10 9.25 × 10−12 1.4852 × 10−12
excess of Ta atoms, which were difficult to fully oxidize, leading to the presence of oxygen vacancies and the ob-served poor ferroelectric properties. In our previous pa-per, we reported that the use of a Ta buffer layer enriched the Ta content near the SBT/Pt interface and improved the SBT properties.17 Because the Ta buffer layer was ultrathin (<0.3 nm), the bulk composition of the SBT film was barely influenced by the presence of the buffer layer. Therefore, it is reasonable that we have obtained distinct SBT properties for the Ta22 specimen and for the SBT sample possessing a Ta buffer layer. When the buffer layers became thicker (∼1 nm), the abundance of Ta ions resulted in a significant change in the SBT com-position near the SBT–Pt interface, which degraded the properties of the SBT sample. This finding suggested that an SBT sample possessing an excessively thick Ta film layer would exhibit poor properties. This situation is similar to the properties displayed by the Ta22 specimen in the present study.
We tested the fatigue endurance of the materials by applying 1 MHz bipolar pulses at 5 V. Figure 7 displays the normalized polarizations (PNV, P*– Pˆ ) of the SBT
capacitors (which had been prepared at 750 °C for 60 s) as a function of the number of polarization switching cycles. All of the SBT films exhibited nearly fatigue-free characteristics after 1010cycles. It has been reported that the fatigue behavior correlates with the nature of the defect traps at the domain boundaries.33,34We expected that the Ta-deficient (Ta18) and Ta-rich (Ta22) SBT TABLE II. Photospectroscopic peak positions for Ta and its oxides. Observed positions are those of Ta22; they exhibited a systematic shift of∼0.2 eV from the expected values as a result of instrumental offset.
Peak Observed position (eV) Expected position (eV)
Ta 4 f5/2 23.9 23.8a Ta 4 f7/2 22.1 21.9a TaO4 f5/2 25.6 25.8 ± 0.4b TaO4 f7/2 23.8 24.0 ± 0.4b TaO24 f5/2 27.7 27.8 ± 0.4b TaO24 f7/2 25.9 26.0 ± 0.4b Ta2O54 f5/2 28.4 28.6 ± 0.2b Ta2O54 f7/2 26.6 26.8 ± 0.2b TaOx4 f5/2 29.9 TaOx4 f7/2 27.4 27.8 ± 0.2 c
aHandbook of X-ray Photoelectron Spectroscopy, Physical Electronics
(Perkin-Elmer Corporation, Eden Prairie, MN, 1992).
bRef. 32. cRef. 31.
C 1s⳱ 284.8 eV.
TABLE III. Relative amounts (high to low) of Ta oxidation states in different SBT films, as measured from XPS spectra.
As-deposited Sputtered Ta18 Ta2O5, TaOx Ta2O5, TaOx, TaO2
Ta20 Ta2O5, TaOx, TaO2 Ta2O5, TaO2, TaOx
Ta22 Ta2O5, TaO2, TaOx Ta2O5, TaO2, TaO, Ta
FIG. 7. Fatigue characteristics of Ta18, Ta20, and Ta22 samples an-nealed at 750 °C for 60 s. This test was performed using bipolar pulses of 1 MHz at an amplitude of 250 kV/cm.
FIG. 6. Deconvoluted Ta (4f) XPS spectra obtained from the surfaces of SBT samples before [(a) Ta18, (b) Ta20, (c) Ta22] and after [(d) Ta18, (e) Ta20, and (f) Ta22] argon ion sputtering. Each specimen was crystallized at 750 °C for 60 s. Solid lines: experimental data; dashed lines: deconvoluted components; circular symbols: sums of deconvo-luted components.
films would each exhibit an abundance of defect states (such as Ta or O vacancies) and significant fatigue be-havior. However, our present analyses indicated only slight degrees of fatigue in Ta18 and Ta22. Robertson et al. studied the electronic structures of SBT materials and reported that the electrons are shallowly trapped at the Ta5+or Bi3+ions and are readily detrapped.35 There-fore, the fatigue characteristics in SBT samples degraded slightly as a result of the appearance of these defects (Ta or O vacancies).
IV. CONCLUSIONS
We have investigated the effect that the Ta content has on the electrical and physical properties of SBT materi-als. A Ta-deficient SBT sample (Ta18) exhibited supe-rior crystallinity, texture, and microstructure relative to those properties of the stoichiometric (Ta20) or Ta-rich (Ta22) SBT species. After annealing at 750 °C for 60 s, Ta18 exhibited a uniform BLS structure, whereas we detected a considerable amount of a second-phase (fluo-rite) in the Ta-rich SBT. Because Ta18 also displayed a relatively high (200) preferential orientation, we ob-served better ferroelectric properties in this Ta-deficient SBT material. We attribute the improved properties of Ta-deficient SBT to the presence of Ta vacancies, the existence of which was supported by conductivity meas-urements and XPS analyses. The SBT thin films possess-ing a 10% Ta deficiency exhibited negligible fatigue deg-radation (<4%) at up to 1010 switching cycles, which makes such materials competitive for use in memory applications.
ACKNOWLEDGMENTS
We are grateful to the staff of the National Nano De-vice Laboratories, Hsinchu, Taiwan, for providing tech-nical support. This study was supported by the National Science Council, Republic of China, through Contract No. 93-2215-E492-010.
REFERENCES
1. C.A. Paz de Araujo, J.D. Cuchiaro, L.D. McMillan, M.C. Scott, and J.F. Scott: Fatigue-free ferroelectric capacitors with platinum electrodes. Nature 374, 627 (1995).
2. A.D. Li, D. Wu, H.Q. Ling, T. Yu, M. Wang, X.B. Yin, Z.G. Liu, and N.B. Ming: Effects of processing on the characteristics of SrBi2Ta2O9 films prepared by metalorganic decomposition. J. Appl. Phys. 88, 1035 (2000).
3. R. Dat, J.K. Lee, O. Auciello, and A.I. Kingon: Pulsed laser ab-lation synthesis and characterization of layered Pt/SrBi2Ta2O9/Pt ferroelectric capacitors with practically no polarization fatigue. Appl. Phys. Lett. 67, 572 (1995).
4. N.J. Seony, C.H. Yang, W.C. Shin, and S.G. Yoon: Oxide inter-facial phases and the electrical properties of SrBi2Ta2O9thin films prepared by plasma-enhanced metalorganic chemical vapor depo-sition. Appl. Phys. Lett. 72, 1374 (1998).
5. R.W. Vest: Metallo-organic decomposition (MOD) processing of ferroelectric and electrooptic films: A review. Ferroelectrics 102, 53 (1990).
6. K. Franke, G. Matin, M. Weihnacht, and A.V. Sotnikov: SrBi2Ta2O9has only two polar axes—A problem for high density ferroelectric memory devices. Solid State Commun. 119, 117 (2001).
7. T.A. Derouin, C.D.E. Lakeman, X.H. Wu, J.S. Speak, and F.F. Lange: Effect of lattice mismatch on the epitaxy of sol-gel LiNbO3thin films. J. Mater. Res. 12, 1391 (1997).
8. Y.H. Xu, C.H. Cheng, Y.D. Lou, and J.D. Mackenzie: Epitaxial ferroelectric thin films prepared by the sol-gel technique. Ferroelectrics 195, 283 (1998).
9. R. Ramesh, J. Lee, T. Sands, V.G. Keramidas, and O. Auciello: O r i e n t e d f e r r o e l e c t r i c L a – S r – C o – O / P b – L a – Z r – T i – O / La–Sr–Co–O heterostructures on [001] Pt/SiO2Si substrates using a bismuth titanate template layer. Appl. Phys. Lett. 64, 2511 (1994).
10. T. Hayashi, H. Takahashi, and T. Hara: Chemical processing and dielectric properties of ferroelectric SrBi2Ta2O9thin films. Jpn. J. Appl. Phys. 35, 4952 (1996).
11. T. Atsuki, N. Soyama, T. Yonezawa, and K. Ogi: Preparation of Bi-based ferroelectric thin films by sol-gel method. Jpn. J. Appl. Phys., Part 1 34, 5096 (1995).
12. K. Miura and M. Tanaka: The effect of Bi ions substituting at the Sr site in SrBi2Ta2O9. Jpn. J. Appl. Phys., Part 1 37, 2554 (1998). 13. T. Noguchi, T. Hase, and Y. Miyasaka: Analysis of the depend-ence of ferroelectric properties of strontium bismuth tantalate (SBT) thin films on the composition and process temperature. Jpn. J. Appl. Phys., Part 1 35, 4900 (1996).
14. Y. Shimakawa, Y. Kubo, Y. Nakagowa, T. Kamiyama, H. Asano, and F. Izumi: Crystal structures and ferroelectric properties of SrBi2Ta2O9 and Sr0.8Bi2.2Ta2O9. Appl. Phys. Lett. 74, 1904 (1999).
15. S-Y. Chen and V-C. Lee: Effect of lead additive on the ferroelec-tric properties and microstructure of SrxPbyBi2zTa2O9thin films. J. Appl. Phys. 87, 8024 (2000).
16. T. Hase, T. Noguchi, K. Amanuma, and Y. Miyasaka: Sr content dependence of ferroelectric properties in SrBi2Ta2O9thin films. Integr. Ferroelectr. 15, 127 (1997).
17. C.C. Leu, C.H. Chien, F.Y. Hsu, H-T. Lin, and C.T. Hu: Influence of ultrathin tantalum buffer layers on microstructure and ferro-electric properties of SrBi2Ta2O9thin films. J. Electrochem. Soc.
151,F167 (2004).
18. S.B. Ren, C.J. Lu, H.M. Shen, and Y.N. Wang: Size-related fer-roelectric-domain-structure transition in a polycrystalline PbTiO3 thin film. Phys. Rev. B. 54, R14337 (1996).
19. S.B. Ren, C.J. Lu, H.M. Shen, and Y.N. Wang: In situ study of the evolution of domain structure in free-standing polycrystalline Pb-TiO3 thin films under external stress. Phys. Rev. B. 55, 3485 (1997).
20. W.C. Kwak and Y-M. Sung: Crystallization kinetics of sol-gel-derived (1−x)SrBi2Ta2O9–xBi3TiTaO9 ferroelectric thin films. J. Mater. Res. 17, 1463 (2002).
21. S. Bhattacharyya, A. Laha, and S.B. Krupanidhi: Impact of Sr content on dielectric and electrical properties of pulsed laser ab-lated SrBi2Ta2O9thin films. J. Appl. Phys. 92, 1056 (2002). 22. C.M. Palanduz and D.M. Smyth: The effect of cation place
ex-change on the electrical conductivity of SrBi2M2O9(M⳱ Ta, Nb). J. Eur. Ceram. Soc. 19, 731 (1999).
23. W.C. Shin and S.G. Yoon: Improvement in ferroelectric properties of SrBi2Ta2O9 thin films with Bi2O3 buffer layers by liquid-delivery metalorganic chemical-vapor deposition. Appl. Phys. Lett. 79, 1519 (2001).
24. S.Y. Chen, B.C. Lan, and C.S. Taso: Film structure and ferroelec-tric properties of vanadium-doped Sr0.8Bi2.3Ta2O9 thin films. J. Appl. Phys. 91, 10032 (2002).
25. S.H. Kim, D.J. Kim, J.P. Maria, A.I. Kingon, S.K. Streiffer, J. Im, O. Auciello, and A.R. Krauss: Influence of Pt heterostructure bottom electrodes on SrBi2Ta2O9thin film properties. Appl. Phys. Lett. 76, 496 (2000).
26. R. Bungener, W. Pamler, and U. Gosele: Diffusion of Sr, Bi, and Ta in amorphous SiO2. Mater. Sci. Semicond. Process. 6, 43 (2003).
27. Q. Tan, J. Li, and D. Viehland: Role of lower valent substituent-oxygen vacancy complexes in polarization pinning in potassium-modified lead zirconate titanate. Appl. Phys. Lett. 75, 418 (1999). 28. D. Viehland and Y.H. Chen: Random-field model for ferroelectric domain dynamics and polarization reversal. J. Appl. Phys. 88, 6696 (2000).
29. B.H. Park, S.J. Hyun, S.D. Bu, T.W. Noh, J. Lee, H-D. Kim, T.H. Kim, and W. Jo: Differences in nature of defects between SrBi2Ta2O9and Bi4Ti3O12. Appl. Phys. Lett. 74, 1907 (1999).
30. D.A. Shirley: High-resolution x-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 5, 4709 (1972). 31. J.H. Thomas III and L.H. Hammer: A photoelectron spectroscopy
study of carbon tetrafluoride/hydrogen reactive ion etching resi-due on tantalum disilicide. J. Electrochem. Soc. 136, 2004 (1989). 32. S. Hofman and J.M. Sanz: Quantitative XPS analysis of the sur-face layer of anodic oxides obtained during depth profiling by sputtering with 3 keV Ar+ions. J. Trace Microprobe Technol. 1, 213 (1982).
33. G.L. Yuan, J-M. Liu, Y.P. Wang, D. Wu, S.T. Zhang, Q.Y. Shao, and Z.G. Liu: Fatigue study of metalorganic-decomposition-derived SrBi2Ta2O9thin films: The effect of partial switching. Appl. Phys. Lett. 76, 2208 (2000).
34. D. Wu, A. Li, H. Ling, T. Yu, Z. Liu, and N. Ming: Temperature-dependent fatigue behaviors of ferroelectric ABO3-type and lay-ered perovskite oxide thin films. Appl. Phys. Lett. 84, 3352 (2004). 35. J. Robertson, C.W. Chen, W.L. Warren, and C.D. Gutleben: Elec-tronic structure of the ferroelectric layered perovskite SrBi2Ta2O9. Appl. Phys. Lett. 69, 1704 (1996).
![FIG. 3. (a) Intensity ratios {I F(111) /[I B(115) + I F(111) ]} of the fluorite phase and (b) texturing ratios {I (200) /[I (115) + I (200) ]} of SBT films containing different Ta contents as a function of annealing time.](https://thumb-ap.123doks.com/thumbv2/9libinfo/7652658.139469/5.877.55.416.118.702/intensity-fluorite-texturing-containing-different-contents-function-annealing.webp)