Abstract
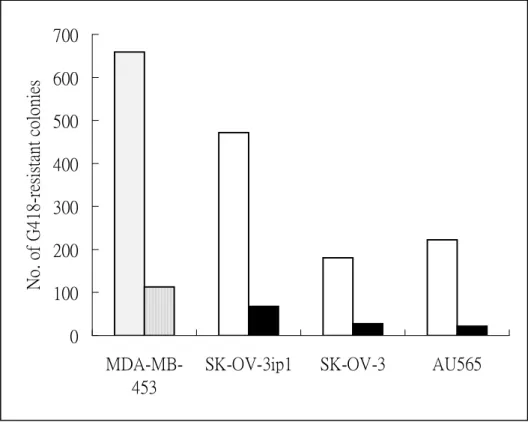
We showed previously that simian virus 40 (SV40) T/t-common polypeptide, which contains the N-terminal common domain of SV40 large T and small t antigens, can repress HER-2/neu expression in HER-2/neu-overexpressing ovarian carcinoma SK-OV-3 cells and consequently suppressed the tumorigenic potential of these cells (Oncogene 19:2704-2713, 2000). To further investigate the tumor-suppressing activity of T/t-common, we asked whether T/t-common could induce apoptosis of tumor cells. When the T/t-common-encoding plasmid was cotransfected with a plasmid encoding the neomycin resistance gene into HER-2/neu-overexpressing cancer cells, the number of G418-resistant colonies obtained was much lower than that obtained with vector cotransfection. This data suggests that T/t-common may induce cell death in these HER-2/neu-overexpressing cancer cells. Similar experiment was also performed with cancer cells that do not overexpress HER-2/neu and non-transformed cells. The killing effect of T/t-common was not observed, suggesting that T/t-common may specifically induce cell death in HER-2/neu-overexpressing cancer cells.
To further investigate the death-inducing activity of T/t-common, we asked whether T/t-common could induce apoptosis in HER-2/neu-overexpressing cancer cells. We first tested whether T/t-common-expressing SK-OV-3 cells underwent apoptosis under low-serum condition. Both TUNEL and FACS analyses indicate that this is indeed the case. We then used T/t-common-carrying adenovirus or vector adenovirus to infect HER-2/neu-overexpressing cancer cells, low
HER-2/neu-expressing cancer cells, and non-transformed cells. Both TUNEL and FACS analyses showed that T/t-common adenovirus infection could induce apoptosis in HER-2-overexpressing cancer cells but not in low HER-2/neu-expressing cancer cells and non-transformed cells. We next tested whether T/t-common induced
apoptosis through repressing HER-2/neu expression. We found that this is indeed the case, because re-expression of a large amount of HER-2/neu in the
T/t-common-expressing cells blocked apoptosis induced by T/t-common. Taken together, these data suggest that T/t-common could specifically induce apoptosis in HER-2-overexpressing cancer cells and this ability of T/t-common may contribute to its tumor-suppressing activity.
Keyword: HER-2/neu, SV40, T/t-common, Apoptosis
中文摘要
我們先前的報告證明了猴病毒 40 型大 T 抗原和小 t 抗原胺基端共同片段 ( T/t-commom polypeptide ),在大量表現 HER-2/neu 基因的人類卵巢癌細胞株 SK-OV-3 , 可 以 特 異 性 地 抑 制 HER-2/neu 表 現 和 腫 瘤 形 成 能 力 (Oncogene 19:2704-2713,2000)。為了了解 T/t-commom 的抑癌能力,我們探討其在癌細胞中
是否會誘發細胞凋亡。我們將帶有 T/t-commom 的質體和帶有抗 G418 基因的質 體共同轉染到會大量表現 HER-2/neu 基因的癌細胞中,發現其抗 G418 的細胞聚 落比載體轉染的少很多,這表示 T/t-commom 可能會誘導大量表現 HER-2/neu 基 因的癌細胞死亡。同樣地,我們也對少量表現 HER-2/neu 基因的癌細胞和正常細 胞進行相同的實驗,發現 T/t-commom 無法造成這些細胞死亡,因此證明了 T/t-commom 可能專一地誘導大量表現 HER-2/neu 基因的癌細胞死亡。 為了證明 T/t-commom 使得大量表現 HER-2/neu 基因的癌細胞死亡是經由誘 發細胞凋亡所導致。首先探討 SK-OV-3 在低血清的培養條件下,T/t-commom 是 否會導致細胞凋亡,TUNEL 和 FACS 的結果皆證明了此推論。再者將帶有 T/t-commom 的腺病毒感染到大量表現、少量表現 HER-2/neu 基因的癌細胞和正 常細胞,並利用 TUNEL 和 FACS 分析,發現 T/t-commom 只會誘導大量表現 HER-2/neu 基因的癌細胞凋亡。 為了進一步證明 T/t-commom 誘導大量表現 HER-2/neu 基因的癌細胞凋亡是 透過抑制 HER-2/neu 基因的表現,於是在表現 T/t-commom 的癌細胞中,重新大 量表現 HER-2/neu,發現會抑制 T/t-commom 誘發細胞凋亡。此結果證實以上推 論。綜合以上結果我們發現 T/t-commom 可專一地誘導大量表現 HER-2/neu 基因 的癌細胞進行細胞凋亡,這個能力可能和 T/t-common 抑制腫瘤形成有關。 關鍵詞:HER-2/neu, 猴病毒 40 型, T/t-common, 細胞凋亡 Introduction
The HER-2/neu proto-oncogene encodes a molecular weight of 185-kDa epidermal growth factor receptor-related transmembrane protein with intrinsic tyrosine kinase activity (Coussens et al., 1985; Hung et al., 1986; Yamamoto et al., 1986). The HER-2/neu receptor contains an extracellular domain, a transmembrane domain, and an intracellular tyrosine kinase domain. The ligand for the HER-2/neu receptor has not been well defined. Identification of the oncogenic HER-2/neu oncogene and the oncogenic property of the HER-2/neu were originally
demonstrated in the rat neu oncogene that was isolated from rat neuroblastoma (Bargamann et al. 1986; Hung et al. 1986). The activated rat neu oncogene contains a point mutation in the transmembrane domain of its protein, which results in a constitutively active tyrosine kinase (Bargmann et al. 1986; Hung et al. 1989). This increase in HER-2/neu tyrosine kinase activity can also be found in many human cancers that overexpress HER-2/neu. In human, the normal HER-2/neu gene is frequently overexpressed or amplified without mutation in many types of cancer, including cancers of the breast, ovary, lung, kidney, colon, bladder, esophagus,
stomach, mouth and salivary gland (reviewed by Hynes and Stern, 1994). Enhanced expression of the HER-2/neu gene is correlated with chemotherapeutic drug
resistance in some cancer cells (Tsai et al., 1993; Tsai et al. 1994; Ueno et al. 1997; Yu et al. 1998; Hynes and Stern, 1994), enhanced metastatic potential in human breast, ovarian, and non-small-cell lung carcinoma cells (Slamon et al., 1987; Tan et al. 1997; Yu et al. 1993; Hynes and Stern, 1994) and shortened survival in breast, ovarian, and prostate cancer patients (Slamon et al., 1987; Slamon et al., 1989; Berchuck et al., 1990; Sadasivan et al., 1993; Hynes and Stern, 1994). Transgenic mice carrying mutation-activated HER-2/neu or overexpressing normal HER-2/neu can induce mammary adenocarcinoma with a high frequency of metastasis (Muller et al., 1988; Guy et al., 1992; Hynes and Stern, 1994). Enhanced expression of the HER-2/neu gene can also increase experimental metastasis in human lung cancer cells (Yu et al., 1994). Taken together, these results indicate that HER-2/neu is a potent oncogene, and its overexpression plays a crucial role in the development of human malignancies. These results also suggest that suppression of HER-2/neu expression may lead to the treatment of the HER-2/neu-overexpressing human cancers. In fact, inhibition of HER-2/neu activity by various means, such as by anti-HER-2/neu antibodies, have been demonstrated to be an effective way to treat HER-2/neu-overexpressing cancers (Yu and Hung, 2000).
Two viral oncoproteins, adenovirus 5 E1A and simian virus 40 (SV40) large T, have been reported to repress transcription from the HER-2/neu promoter and consequently repress the transforming activity of HER-2/neu (Yu et al., 1990; Yu et al., 1991; Matin and Hung, 1993; Hung et al., 1995; Chen et al., 1997). When E1A is stably transfected into cells, it can repress HER-2/neu overexpression and
suppress the tumorigenic and metastatic potential of activated
HER-2/neu-transformed mouse 3T3 cells and dissemination of human ovarian cancer cells that overexpress HER-2/neu (Yu et al., 1991; Yu et al., 1992; Yu et al., 1993; Yu et al., 1995). Moreover, in mice bearing HER-2/neu-overexpressing ovarian, lung, or breast cancer cells, E1A delivered either by adenovirus or
liposome significantly inhibits tumor growth and prolongs mouse survival (Hung et al., 1995; Yu et al., 1995; Zhang et al., 1995; Chang et al., 1996; Chang et al., 1997). These results strongly suggest that by repressing HER-2/neu expression, it is
possible to achieve therapeutic effects on cancers that overexpress HER-2/neu. In fact, a clinical trial of E1A gene therapy in HER-2/neu-overexpressing breast and ovarian cancer has been conducted and promising results indicating the feasibility of this approach have been obtained (Hung et al., 2000; Hung, 2000).
Previously we have shown that SV40 small t and its derivative, T/t-common (which contains the N-terminal common domain of large T and small t), possess a
strong transcriptional repression activity (Wang et al., 1994). Subsequently, we showed that small t and T/t-common can repress the HER-2/neu promoter, with T/t-common has the strongest repressing activity between the two proteins (Lin et al., 2000). We then demonstrated that T/t-common can suppress the tumorigenic potential of the HER-2/neu-overexpressing ovarian carcinoma SK-OV-3 cells both in vitro and in vivo through repressing HER-2/neu expression (Lin et al., 2000). These results lead to a conclusion that T/t-common may be used as a therapeutic agent against the HER-2/neu-overexpressing cancers just like adenovirus E1A does. In this report, we demonstrate that SV40 T/t-common could specifically induce apoptosis in HER-2/neu-overexpressing cancer cells but not in
HER-2/neu-low-expressing tumor cells and non-transformed cells.
Materials and Methods Cell Lines and Culture
.The SK-Br-3, MDA-MB-453, SK-OV-3, and SK-OV-3 ip1 cells were grown in DMEM/F12 nutrient mixture (Gibco, Grand Island, NY) supplemented with 10% FBS in 5% CO2. The SK-OV-3 derivative cells (SK-T/t-1, SK-T/t-2, SK-T/t-3, SK-T/t-4,
SK-NEO-1, and SK-NEO-2) were grown under the same conditions, except that G418 (200 g/ml) was added to the culture media. The AU565, MCF-7, NPC-TW04, HEp2, HeLa, MRC-5, CV-1, and NIH3T3 cells were cultured in DMEM (Gibco, Grand Island, NY) supplemented with 10% FBS in 5% CO2.
Plasmids and Viruses.
Plasmids pRSV.3-BglII, pRSV-T/t-common, pCMVHER2, and pcDNA3 were described previously (Lin et al., 2000). The pRc/CMV plasmid, which contains the neomycin resistance gene, was purchased from Invitrogen. The recombinant
adenovirus rAdT/t which contains the T/t-common gene, and the vector adenovirus rAdV were constructed according to the protocol provided by B. Vogelstein (He et al., 1998).
Transfection and G418-resistant Colony Formation Assay.
DNA transfection was performed as described previously (Wang et al., 1994). Cells were seeded in 35-mm-diameter plates 24 h before transfection. Cells were transfected at 70% confluency by the calcium phosphate coprecipitation technique (Sambrook et al., 1989); 1 g of pEGFP-C1, a plasmid which contains the CMV promoter-driven GFP gene, was also cotransfected as an internal control for
transfection efficiency. The cells were exposed to the transfection precipitate for 8 h, washed twice with fresh medium, and fed with fresh DMEM containing 0.5% FBS and appropriate concentration of G418. After 5 days, the media were removed and cells were refed with DMEM containing 10% FBS and appropriate concentration of
G418. The G418-resistant colonies were counted two weeks latter. Flow Cytometry Analysis.
For flow cytometry analysis of apoptosis by propidium iodide staining, floating and attached cells were collected, combined, and washed three times in PBS, and fixed in 80% ethanol at -20oC. Fixed cells were washed twice in PBS/1% BSA/0.1% Tween, and stained in 1ml of PBS containing 50 g of propidium iodide and 10 g of boiled RNase, at 4 oC for 1 h or longer. Stained cells were then analyzed on a flow cytometer (Becton Dickinson). Results were obtained as the percentage of subdiploid nuclei, which represent the apoptotic cells.
TUNEL Assay.
Cells were fixed in 4% paraformaldehyde for 45 min at room temperature. After rinsing with PBS, cells were permeabilized in a solution of Triton X-100 (0.1%) in sodium citrate (0.1%) for 2 min on ice. Samples, washed with PBS, were then
incubated in the TUNEL reaction mix (Roche) for 1 h at 37 oC in the dark. The stained samples were washed three times in PBS containing 0.2% BSA and 0.1% Triton X-100, and then visualized by a fluorescence microscope.
Results and Discussion
I. T/t-common could specifically induce cell death in HER-2/neu-overexpressing cancer cells but not in non-transformed cells and HER-2/neu low-expressing cancer cells.
Previously we generated a recombinant adenovirus, rAdNT/t, carrying the T/t-common gene. We found that rAdNT/t infection could permanently suppress the soft-agarose colony-forming ability of the HER-2/neu-overexpressing cancer cells SK-OV-3 and AU565. Since the expression of T/t-common is transient in
rAdNT/t-infected cells, the above finding implies that T/t-common may induce cell death in rAdNT/t-infected cells. To test whether T/t-common could indeed induce cell death in HER-2/neu-overexpressing cancer cells, we cotansfected either a
T/t-common-expressing plasmid (pRSV-T/t-common) or its vector control plasmid (pRSV.3-BglII) together with a neomycin resistance gene-encoding plasmid
(pRc/CMV) into HER-2/neu-overexpressing cancer cell lines SK-OV-3 (ovarian cancer cell line), SK-OV-3 ip1(ovarian cancer cell line, MDA-MB-453 (breast cancer cell line) and AU565 (breast cancer cell line), or HER-2/neu low-expressing cancer cell lines MCF-7 (breast cancer cell line), NPC-TW04 (nasopharyngeal cancer cell line) , HeLa (cervical cancer cell line) and HEp-2 (laryngeal cancer cell line), or non-transformed cell lines CV-1 and NIH3T3. We found that T/t-common cotransfection led to much less neomycin-resistant colonies than control
contrast, in non-transformed cell lines and HER-2/neu low-expressing cancer cell lines, cotransfection with T/t-common or vector plasmid led to similar number of neomycin-resistant colonies (Fig. 2). These data suggest that T/t-common could specifically induce cell death in HER-2/neu-overexpressing cancer cell lines but not in non-transformed cells and HER-2/neu low-expressing cancer cells.
II. T/t-common-expressing SK-OV-3 cells underwent apoptosis under low serum condition.
To investigate whether T/t-common induced apoptosis in
HER-2/neu-overexpressing cancer cells, we grew previously established
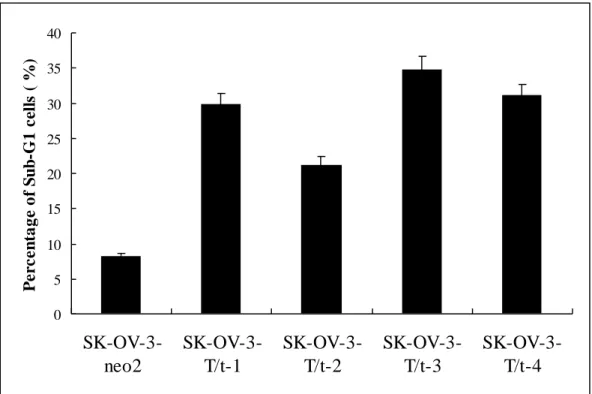
T/t-common-expressing SK-OV-3 clones or vector-transfected SK-OV-3 clone in low-serum media. The cells were harvested 4 days later, fixed, stained with propidium iodide, and subjected to FACS analysis. As shown in Fig. 3, the
T/t-common-expressing clones (SK-OV-3-T/t-1, SK-OV-3-T/t-2, SK-OV-3-T/t-3, and SK-OV-3-T/t-4) contained more cells in sub-G1 phase than did the vector-transfected clone (SK-OV-3-neo2). These data indicate that T/t-common-expressing SK-OV-3 cells underwent apoptosis under low serum condition. To further confirm that these T/t-common-expressing clones underwent apoptosis, TUNEL assays were performed. The T/t-common-expressing SK-OV-3 clones or the vector-transfected clone were incubated in low-serum media. Four days later, the cells were fixed and subjected to TUNEL assay. As shown in Fig. 4, all of the T/t-common-expressing clones
(SK-OV-3-T/t-1, SK-OV-3-T/t-2, SK-OV-3-T/t-3, and SK-OV-3-T/t-4) were TUNEL-positive while the vector-transfected clone (SK-OV-3-neo2) was
TUNEL-negative. The nuclei of the cells were also stained with DAPI. While the nuclei of the T/t-common-expressing cells displayed apoptotic feature, that is they were condensed and stained brightly with DAPI, the nuclei of vector-transfected cells were evenly stained and not condensed (data not shown). Together these data strongly indicate that the T/t-common-expressing SK-OV-3 cells underwent apoptosis under low serum condition.
III. T/t-common could induce apoptosis in HER-2/neu-overexpressing cancer cells but not in HER-2/neu low-expressing cancer cells and non-transformed cells.
The above data indicate that T/t-common could induce apoptosis in
HER-2/neu-overexpressing ovarian carcinoma SK-OV-3 cells. To investigate whether T/t-common could induce apoptosis in other cells, recombinant adenovirus carrying T/t-common (rAdT/t) and vector adenovirus (rAdV) were used to infect
HER-2/neu-overexpressing breast cancer cells (SK-Br-3 and AU565), HER-2/neu-overexpressing ovarian cancer cells (SK-OV-3), HER-2/neu
low-expressing breast cancer cells (MCF-7), and non-transformed cells (CV-1 and MRC-5). After infection, the cells were incubated in low-serum media. Various times
after infection, the cells were harvested, fixed, stained with propidium iodide, and subjected to FACS analysis. As shown in Fig. 5, compared to rAdV infection, rAdT/t infection dramatically increase the number of apoptotic cells (i.e., sub-G1 cells) in HER-2/neu-overexpressing cancer cells (SK-Br-3, AU565 and SK-OV-3). In contrast, in HER-2/neu low-expressing cancer cells (MCF-7) and non-transformed cells (CV-1 and MRC-5), rAdT/t infection caused similar number of apoptotic cells as rAdV infection. These data indicate that T/t-common could specifically induce apoptosis in HER-2/neu-overexpressing cancer cells but not in HER-2/neu low-expressing cancer cells and non-transformed cells. The TUNEL assays also confirmed the above
conclusion (data not shown).
IV. The ability of T/t-common to induce apoptosis of HER-2/neu-overexpressing cancer cells was derived from its ability to repress HER-2/neu expression.
As described in our previous paper (Lin et al., 2000), T/t-common could
suppress the tumorigenic potential of HER-2/neu-overexpressing cancer cells through repressing HER-2/neu expression. Previous studies (Roh et al., 2000; Cuello et al., 2001) showed that repression of HER-2/neu expression in HER-2/neu-overexpressing cancer cells can lead to apoptosis. Taken together, these observations suggest that T/t-common may induce apoptosis through repressing HER-2/neu expression in
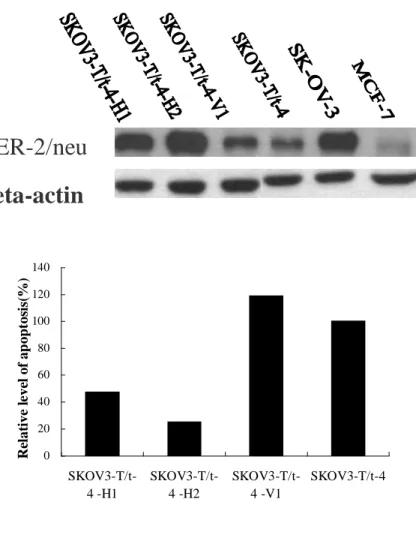
HER-2/neu-overexpressing cancer cells. To investigate whether this hypothesis is true, we reintroduced the HER-2/neu gene under the control of CMV IE promoter (which is not repressed by T/t-common) into the T/t-common-expressing SK-OV-3 clone (SK-OV-3-T/t-4). As shown in Fig. 6 upper panel, both clones SK-OV-3-T/t-4-H1 and SK-OV-3-T/t-4-H2 (lanes 1and 2, respectively) produced a large amount of
HER-2/neu, while the vector-transfected clone (SK-OV-3-T/t-4-V1; lane 3) produced similar amount of HER-2/neu as the parental SK-OV-3-T/t-4 clone (lane 4). The parental SK-OV-3-T/t-4 clone, the vector-transfected clone (SK-OV-3-T/t-4-V1), and the HER-2/neu-reexpressed clone (SK-OV-3-T/t-4-H1 and SK-OV-3-T/t-4-H2) were incubated in low serum media and then subjected to TUNEL assays. As shown in Fig. 6 lower panel, while the parental cells and the vector-transfected cells contained a significant amount of apoptotic cells, the HER-2/neu-reexpressed cells contained much less apoptotic cells. These data indicate that blockage of T/t-common’s ability to inhibit HER-2/neu in HER-2/neu-overexpressing cancer cells could lead to abolishment of its ability to induce apoptosis in these cells. To further verify this conclusion, we also reintroduced the HER-2/neu gene under the control of CMV IE promoter into another T/t-common-expressing SK-OV-3 clone (SK-OV-3-T/t-3). As shown in Fig. 7, similar results as those shown in Fig. 6 were obtained. Therefore T/t-common’s ability to induce apoptosis of HER-2/neu-overexpressing cancer cells was derived from its ability to repress HER-2/neu expression.
References
Bargmann CI, Hung M-C, and Weinberg RA. (1986) Cell, 45, 649-657.
Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, McKenzie S, Yin S and Bast JRC. (1990). Cancer Res., 50, 4087-4091
Chang JY, Xia W, Shao R and Hung M-C. (1996). Oncogene, 13, 1405-1412.
Chang JY, Xia W, Shao R, Sorgi F, Hortobagyi GN, Huang L and Hung M-C.
(1997). Oncogene, 14, 561-568.
Chen H, Yu D, Chinnadurai G, Karunagaran D and Hung M-C. (1997). Oncogene,
14, 1965-1971.
Chen H and Hung M-C. (1997). J. Biol. Chem., 272, 6101-6104.
Coussens L, Yang-Feng TL, Liao Y-C, Chen E, Gray A, McGrath J, Seeburg PH,
Libermann TA, Schlessinger J, Francke U, Levinson A and Ullrich A. (1985).
Science, 230, 1132-1139.
Cuello et al., Cancer Res. 61:4892-4900, 2001
Gavrieli I, Sherman Y, Ben-Sasson SA. (1992). J. Cell Biol., 119, 493-501.
Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD and Muller WJ. (1992).
Proc. Natl. Acad. Sci. USA, 89, 10578-10582.
Hassan W, Sanford MA, Woo SLC, Chen S-H, and Hall SJ. (2000). World J. Urol.,
18, 130-135.
He T-C, Zhou S, DA Costa LT, Yu J, Kinzler KW and Vogelstein B. (1998). Proc.
Natl. Acad. Sci. USA, 95, 2509-2514.
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D and Levitsky H.
(1998b). J. Exp. Med., 188, 2357-2368.
Hung M-C. (2000). Abstract of International Symposium on DNA Vaccine and
Hung M-C, Hortobagyi GN, and Ueno NT. (2000). Adv. Exp. Med. Biol., 465,
171-180.
Hung M-C and Lau Y-K. (1999). Semin. Oncol., 26 (suppl. 12), 51-59.
Hung M-C, Chang JYJ and Xing XM. (1998). Adv. Drug Delivery Rev., 30,
219-227.
Hung M-C, Matin A, Zhang Y, Xing X, Sorgi F, Huang L and Yu D. (1995). Gene,
159, 65-71.
Hung M-C, Schechter AL, Chevray PY, Stern DF and Weinberg RA. (1986). Proc.
Natl. Acad. Sci. USA, 83, 261-264.
Hynes NE and Stern DF. (1994). Biochim. Biophys. Acta, 1198, 165-184.
Kao M-C, Liu G-Y, Chuang T-C, Lin Y-S, Wuu J-A and Law S-L. (1998).
Oncogene, 16, 547-554.
Lin Y-C, Peng J-M and Wang W-B. (2000). Oncogene, 19, 2704-2713.
Matin A and Hung M-C. (1993). Cell Growth Diff., 4, 1051-1056.
Muller WJ, Sinn E, Pattengale PK, Wallace R and Leder P. (1988). Cell, 54,
105-115.
Roh et al., Cancer Res. 60:560-565, 2000
Sadasivan R, Morgan R, Jennings S, Austenfeld M, van Veldhuizen P, Stephens R
and Noble M. (1993). J. Urol., 150, 126-131.
Shtrichman R and Kleinberger T. (1998). J. Virol., 72, 2975-2982.
Simons JW, Mikhak B, Chang JF et al. (1999). Cancer Res., 59, 5160-5168.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A and McGuire WL. (1987).
Science, 235, 177-182.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart
SG, Udove J, Ullrich A and Press MF. (1989). Science, 244, 707-712.
Tsai C-M, Chang K-T, Perng R-P, Mitsudomi T, Chen M-H, Kadoyama C and Gazdar AF. (1993). J. Natl. Cancer Inst., 85, 897-901.
Tsai C-M, Perng R-P, Chen M-H, Jan Y-H, Hung M-C, Ku T-Y, and Chang K-T.
(1994). J. Natl. Cancer Inst., 86, 1018-1020.
Ueno NT, Yu D, and Hung M-C. (1997). Oncogene, 15, 953-960.
Wang W-B, Bikel I, Marsilio E, Newsome D and Livingston DM. (1994). J. Virol., 68,
6180-6187.
Xing X, Martin A, Yu D et al. (1996). Cancer Gene Ther., 3, 168-174.
Yamamoto T, Ikawa S, Akiyama T, Semba K, Nomura N, Miyajima N, Saito T and Toyoshima K. (1986). Nature, 319, 230-234.
Yu D and Hung MC. (2000). Oncogene, 19, 6115-6121.
Yu D, Jing T, Liu B, Yao J, Tan M, McDonnell TJ, and Hung M-C. (1998). Mol. Cell,
2, 581-591.
Yu D, Hamada J-I, Zhang H, Nicolson GL and Hung M-C. (1992). Oncogene, 7,
2263-2270.
Yu D, Matin A, Xia W, Sorgi F, Huang L and Hung M-C. (1995). Oncogene, 11,
1383-1388.
Yu D, Scorsone K and Hung M-C. (1991). Mol. Cell. Biol., 11, 1745-1750.
Yu D, Suen T-C, Yan D-H, Chang L-S and Hung M-C. (1990). Proc. Natl. Acad. Sci.
USA, 87, 4499-4503.
Yu D, Wang S-S, Dulski KM, Tsai C-M, Nicolson, GL and Hung M-C. (1994).
Cancer Res., 54, 3260-3266.
Yu D, Wolf JK, Scanlon M, Price JE and Hung M-C. (1993). Cancer Res., 53,
891-898.
Evaluation of Accomplishment
The manuscript describing the finding reported here is under preparation. Overall
this finding indicates that SV40 T/t-common is a potential therapeutic agent
specifically targets HER-2/neu-overexpressing cancers.
Fig. 1. Inhibition of G418-resistant colony formation by T/t-common in HER-2/neu-overexpressing cancer cells.
0 100 200 300 400 500 600 700 MDA-MB-453
SK-OV-3ip1 SK-OV-3 AU565
No . o f G4 18 -r esis tan t co lo nies
Fig. 2. T/t-common did not inhibit G418-resistant colony formation in HER-2/neu low-expressing cancer cells and non-transformed cells.
Fig. 3. T/t-common-expressing SK-OV-3 clones underwent apoptosis under low serum condition. 0 20 40 60 80 100 120 140 160
NPC-TW04 Hep2 HeLa CV-1 NIH3T3 MCF-7
N O . o f G41 8 -r es is tan t co lon ie s
Vector
T/t-common
0 5 10 15 20 25 30 35 40 SK-OV-3-neo2 SK-OV-3-T/t-1 SK-OV-3-T/t-2 SK-OV-3-T/t-3 SK-OV-3-T/t-4 P e rc e n ta g e o f S u b -G 1 c ell s ( %)Fig. 4. TUNEL assay showed that T/t-common-expressing SK-OV-3 clones underwent apoptosis under low serum condition.
Fig. 5. T/t-common-containing adenovirus infection induced apoptosis in
HER-2/neu-overexpressing cancer cells but not in HER-2/neu low-expressing cancer cells and non-transformed cells.
SKOV3-neo2
SKOV3-T/t-1
SKOV3-T/t-2
SKOV3-T/t-3
SKOV3-T/t-4
0 5 10 15 20 25 30 SK-Br-3 AU565 SK-OV-3 MCF-7 CV-1 MRC-5
P
ercen
ta
ge
of
S
u
b
-G1
cel
ls(
%)
rAdV
rAdT/t
Fig. 6. Enhanced re-expression of HER-2/neu in SK-OV-3-T/t-4 cells blocked apoptosis induced by T/t-common. Upper panel, expression of HER-2/neu in various SK-OV-3 clones. Lower panel, relative apoptosis rate of various SK-OV-3 clones. 0 20 40 60 80 100 120 140 SKOV3-T/t-4 -H1 SKOV3-T/t-4 -H2 SKOV3-T/t-4 -V1 SKOV3-T/t-4 Re lat ive l e ve l of ap opt os is (%)
HER-2/neu
Beta-actin
Fig. 7. Enhanced re-expression of HER-2/neu in SK-OV-3-T/t-3 cells blocked apoptosis induced by T/t-common. Upper panel, expression of HER-2/neu in various SK-OV-3 clones. Lower panel, relative apoptosis rate of various SK-OV-3 clones. 0 20 40 60 80 100 120 140 160 180 SKOV3T/t3 -H1 SKOV3T/t3 -H2 SKOV3-T/t-3-V1 SKOV3-T/t-3 R e la ti v e lev el o f a p o p to si s (%) HER-2/neu Beta-actin