行政院國家科學委員會專題研究計畫 成果報告
立體阻障雙芽配基金屬化物之催化聚合應用(3/3)
計畫類別: 個別型計畫 計畫編號: NSC93-2113-M-002-004- 執行期間: 93 年 08 月 01 日至 94 年 10 月 31 日 執行單位: 國立臺灣大學化學系暨研究所 計畫主持人: 劉緒宗 報告類型: 完整報告 報告附件: 出席國際會議研究心得報告及發表論文 處理方式: 本計畫可公開查詢中 華 民 國 95 年 2 月 13 日
行政院國家科學委員會補助專題研究計畫
X
成 果 報 告
□期中進度報告
立體阻障雙芽配基金屬化物之催化聚合應用
計畫類別:X 個別型計畫 □ 整合型計畫
計畫編號:NSC 94-2113-M-002-004
執行期間: 93 年 8 月 1 日至 94 年 10 月 31 日
計畫主持人:劉緒宗
成果報告類型(依經費核定清單規定繳交):□精簡報告 x 完整報告
本成果報告包括以下應繳交之附件:
□赴國外出差或研習心得報告一份
□赴大陸地區出差或研習心得報告一份
x 出席國際學術會議心得報告及發表之論文各一份
處理方式:除產學合作研究計畫、提升產業技術及人才培育研究計畫、列
管計畫及下列情形者外,得立即公開查詢
□涉及專利或其他智慧財產權,□一年□二年後可公開查詢
執行單位:台灣大學化學系
中 華 民 國 95 年 1 月 26 日
中英文摘要 中文摘要 配位體在聚合與催化反應的應用是計畫的主要內容,此計畫為三年期計畫,設計一系列 具有立體阻障的雙芽供體,並合成其過渡金屬化合物作為催化劑應用於不飽和化物如烯類等 的聚合反應與催化反應。此報告的研究主體中計合成多芽的配體,並由其與金屬的錯合反 應,其中探討了結構的特性。利用了這些金屬化合物作了 C-C 鍵形成與氧化催化反應測試, 而發現金屬錯合物中可以很有效率的將苯甲醇轉換成苯甲醛與衍生物,並且對 Heck ,Suzuki coupling 反應有很好的效應;另外多芽配體合成與金屬化物的純化與鑑定。Part I. Palladium complexes in catalytic polymerization; Part II Copper complex in Oxidation; Part III Palladium nanoparticles in C-C bond formation.
關鍵詞: 多芽配位體,催化,膦,亞胺,銅,鈀,氧化,C-C 鍵
Abstract:
Ligand effect on catalysis and mediated polymerization was the major issue of this three-year research project. Design of a series of multiple dentates particularly with steric hindreance for transition metal complexes was the initial step for the study. Preparation and characterization of the metal complexes with the designed ligand were carried out via various approaches. Finally, the use of the prepared complexes for catalytic study was to search the best activity of catalyst for a certain reaction. This report includes three part. Part I. Palladium complexes in catalytic
polymerization; Part II Copper complex in Oxidation; Part III Palladium nanoparticles in C-C bond formation.
Keywords: Multiple dentate, Catalysis, Phosphine, Imine, Copper, Palladium, Oxidation, C-C-bond.
Part I. Palladium complexes in catalytic polymerization.
Late transition metal catalyzed polymerization and/or copolymerization of unsaturated substrates via migratory insertion manner is of great current interest. Many research groups are actively engaged in developing efficient catalysts with late transition metal ions coordinated by various donors. These investigation illustrate that both electronic and steric environment of the ligands are crucial in stabilizing the metal ion as well as in the selectivity/activity of the polymerization.
Due to the different donor/acceptor properties of the phosphorus and nitrogen donor centers, study of phosphine-imine (P~N) bidetnates on either coordination or metal catalyzed organic transformation has received much attention.1-28
Preparations of L1 and its palladium complex [(L1)PdMeCl] 1 are reported in our earlier work, whereas L2 was prepared according to the previously published procedures. The ligands L3 and L4 were prepared by simple condensation of 2-diphenylphos-phinobenzaldehyde with slight excess of aniline or 2,6-di- isopropyl- aniline respectively in methanol solution. By stirring at room temperature overnight, the corresponding imine product was isolated quantitatively, which was further char- acterized by spectroscopic methods. Some selected spectral data of L1-L4 are given in the Table 1. 31P NMR shows a single peak at -13.6 and -14.9 ppm for L3 and L4 respectively, being 2 - 3 ppm upfield than those of the starting 2-diphenylphosphino- benzaldehyde (-11.7 ppm in CDCl3). In the 1H NMR spectra the imine proton appears as a doublet at 9.06 and 8.94 ppm due to JP-H = 5.1 Hz for L3 and JP-H = 5.7 Hz for L4 respectively. Such long-range coupling is comparable with other known P~N ligands.
Reactions of equal molar amount of ligands (L2 – L3) with Pd(COD)MeCl [COD = 1,5-cyclooctadiene] in THF solution afforded the complexes [Pd(P~N)MeCl] (P~N = L2, 2; L3, 3) in quantitative yield. Cationic Pd(II) complexes with acetonitrile coordination were prepared by treating the related neutral [PdMeCl(P~N)] with one equiv. of AgBF4 in a mixture of dichloromethane and acetonitrile solution.
At 25 oC, consecutive bubbling of CO and ethylene into the dichloromethane solution of 1a uniquely resulted in the formation of 2, 3, 4 and 5 (Scheme 1).16 Intermediates 3 and 5 isolated in solid state are stable. By contrast acyl complexes 2 and 4 decompose slowly in solution as well as in solid state. Appearance of single 31P signal for 2, 3, 4 and 5 at 18.4, 36.7, 19.9 and 36.8 ppm respectively suggests the quantitative formation of only one product in each step. Infrared
spectrum of the compound 5 show two C=O stretching bands at 1712 and 1629 cm-1
corresponding to free and coordinated carbonyl groups respectively. Coordination of the C=O moiety to the palladium is well documented via the formation of 5- membered chelation.10 Possibility of six- membered chelation in compound 4 is ruled out based on 13C spectrum, which show two peaks at 223.1 and 206.6 ppm corresponding to metal bound acyl and free carbonyl
group respectively. No shift in later peak is observed upon ethylene insertion leading to the formation of 5. Chelated carbonyl carbon in 5, however, is down field shifted and appeared at 231.3 ppm.
Copolymerization of CO and olefins. Using the new imine-phosphine complexes
[Pd(P-N)(CH3)(CH3CN)](BF4) (P-N = L1) as catalyst, copolymerization of E-CO can be carried out under mild conditions. In one of the typical reactions, 1a (22.0 mg, 0.03 mmol) with CO and ethylene (40 psi for each in a 200 mL autoclave) in 75ml of CH2Cl2 at 75-80 oC produces 0.5 g of polyk etone after 48 hrs.14 Complex 1a also affords the copolymerization of norbornene /CO, but the fluorinated-benzaldimine-phos- phine derivative 1b (Ar = p-FC6H4-) provides a better yield. The resulting material is a white solid and soluble in most of orga nic solvents. Molecular weight was determined by GPC analysis (Mn = 2500, Mw/Mn = 1.26).
Dimerization and trimerization of ethylene. [Pd(P-N)(CH3)(CH3CN)](BF4)] has been found to be a good catalyst for dimerization and trimerization of ethylene. In constrast to the diimine-palladium complexes which are known to catalyze the polymerization of various olefins, the imine-phosphine complex developed in this study is suitable for oligomerization of ethylene. It indicates that either the coordination of ethylene is probably overwhelming over other α-olefins or insertion of high olefin is slow in such a system.
Insertion of alkynes . Neutral and Cationic
Pd(II)-alkyl and Pd(II)-aryl complexes with phosphine- imine (P~N) ligand show unusual reactivity toward alkynes. No insertion of alkyne has been observed into the neutral palladium
complexes or the cationic Pd-alkyl complex. On the other hand, smooth insertion of alkynes into the cationic complexes [(P~N)Pd(COMe)(MeCN)]+ and [(P~N)Pd(Ph)(MeCN)]+ was observed, resulting in the formation of singly and doubly insertion products respectively. All the inserted products were isolated and characterized by spectroscopic methods. Single crystal X-ray analysis for some alkyne insertion complexes indicate that the products are stabilized by intra- molecular coordination via either a carbonyl oxygen or a π-phenyl coordination with η2-mode. Higher order
insertions of ethylpropiolate in complexes [(P~N)Pd(Ph)(MeCN)]+ (5) or
[(P~N)Pd(C(Ph)=C(Ph)-C(Ph)=CPh2)(MeCN)]+ (13), leading to the oligomeric species is found to proceed smoothly, but the di-substituted alkynes such as diphenylacetylene does not undergo such insertion. The insetion intermediates leading to oligomers is characterized by both spectral and Mass analyses. It is clear that the mixed-donor ligand as well as the reacting substrates affect the migratory insertion path of alkynes. The complexes with unsymmetrical ligand which can differentiate between the migratory insertion of alkynes over the intramolecular cyclization, may be a promising candidates for polymerization of alkynes.
P h2 P N H P h P d M e C l + N a B A r4
P Pd N O COOEt OEt COOEt Ph Ph Ph Ph Ph n-2
Heck reaction. Pd(II) complexes of the phosphine- nitrogen (P-N) bidentate donors act as
efficient catalysts for the Heck reaction. In a typical example, reaction of phenyl iodide with methyl acrylate in N-methylpyrrolidinone (NMP) at ca 130 oC provides the Heck product with the turnover number up to 106. In addition, the coupling reaction of 4-bromoacetophenone with olefin in a quantitative yield was achieved by using the same catalyst in the presence of sodium iodide. [(P-N)PdPhI] O Br R O R Heck reaction
Polymerization of phenylacetylene. The phosphine-imine(P~N)rhodium(I) complexes (XX),
containing a P~N and a COD ligands is an effective catalyst for polyphenylacetylene formation. Aqueous medium serves a good reaction enviornment and provides a convenient way of separation for the polymers from the reaction mixture.
In a typical experiment, a round-bottom flask (25 mL) charged with catalyst (0.01 mmol) was evacuated and flushed with nitrogen for several times, then phenylacetylene along with solvent was added. The reaction was carried out at 30 oC for 12 h and a polymer was isolated as an orange solid by filtration. The selectivity of cis-stereochemistry inclined to the polymers is observed in all instances. It seems that there is no obvious correlation of the stereo-regularity of polyphenylacetylene with the solvent systems. Molecular weight of the polymer can reach up to 30000 with PDI ~ 1.99. Ph2 P N Ph Rh(COD) XX BF4
influence of the chelation as well as the ancillary ligand on the insertion process with palladium center. Clearly, the phosphine-imine linked through o-phenylene backbone provides a unique ligand system to stabilize the metal-acyl species such as 14, which also reflects in the coplymerization of CO/ethylene. By examining the angle of N-Pd-P, the smaller bite angle appears in 14 might have effect on catalysis of copolymerization.
Part II Copper complex in Oxidation
In order to understand and mimic functions of various families of copper proteins, there has been much research interests in developing model copper(II) complexes in both structure and reactivity aspects. Among various coordination mode for copper(II), complexes with five-coordinate adopting in a square pyramidal geometry were found in natural occurring metalloprotein such as hemocyanin, bleomyc in and galactose oxidase. Thus a great number of copper(II) complexes containing chelating nitrogen donors including pyridinyl or imidazoyl ligands in model study were reported.1-8 In such pursuit, we synthesized a designed tetradentate 1, which includes two pyridine nitrogens, one secondary amine group and phosphine-oxide in its ligand set. The preparation and structural characterization of five-coordinate Cu(II) with this ligand are reported.
P NH O Ph Ph N N 1
Ligand preparation. The desired ligand was prepared through the reaction of
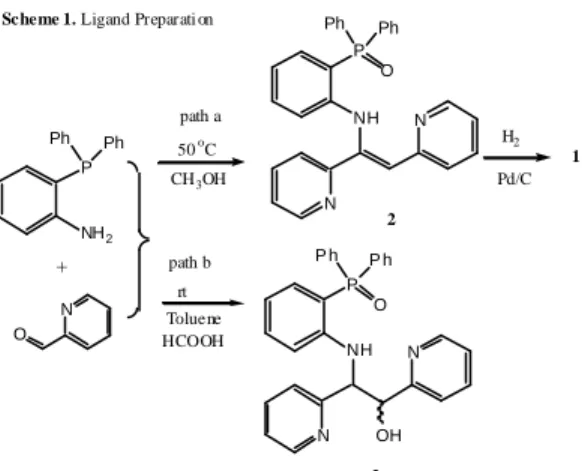
2-diphenylphosphinoaniline with 2-pyrdinecarbaldehyde followed by the hydrogenation (scheme 1, path a). Without any acid catalysis, the condensation reaction took place immediately in methanol at 50 oC and quantitatively provided the formation of enamine product 2. Upon the recrystallization, this compound appears to be a clear, colorless single crystal. This reaction has also been investigated by Doherty (scheme 1, path b).9 In the presence of formic acid, the adduct 3 from imine and aldehyde was obtained at ambient temperature in toluene. Presumably, the reaction at higher temperature in the polar solvent readily facilitates the dehydration to form 2. Metal catalyzed hydrogenation of 2 in the presence of atmospheric hydrogen gave the desired ligand 1.
P NH O Ph Ph N N P NH2 Ph Ph O N CH3OH H2 Pd/C 1 P NH O P h P h N N OH Toluene + path a path b HCOOH 50 oC rt 2 3 Scheme 1. Ligand Preparati on
Compound 2 was characterized by both spectroscopic and x-ray crystallographic methods. A shift at 30.7 ppm in the 31P NMR spectrum of 2 shows the existence of tertiary-phosphine oxide in the molecule. In addition to the aromatic protons, the appearance of one singlet absorption at 6.23 ppm in 1H nmr, which is in the typical range for olefinic protons, suggests the formation of carbon-carbon double bond functionality. Although the spectral data of 2 are consistent with the proposed structure, the confirmation comes from the single crystal analysis. Figure 1 shows its ORTEP plot with 30% ellipsoid. All bond lengths and angles are in agreement with the reported values. The C(19)-C(20) bond length of 1.356(4) Å is typical distance for C=C, whereas the distance of 1.379(3) Å for C(19)-N(4) clearly illustrates the single bond nature between two atoms. These observations also confirm the enamine moiety in the presence of the molecule. As for the hydrogenated product 1 is easily characterized by both nmr spectroscopy and elemental analysis.
O(1) P(1) C(7) C(1) C(13) C(18) N(1) C(19) C(20) C(21) N(2) N(3) C(26)
Figure 1. ORTEP Plot of 1. Selected bond distances and angles: P(1)-C(1) 1.806(3) Å; P(1) -O(1) 1.483(2) Å;
P(1)-C(13) 1.811(3) Å; C(18)-N(1) 1.412(3) Å; N(1) -C(19) 1.379(3) Å; C(19) -C(20) 1.356(4) Å; C(18) -N(1)-C(19) 125.3(2)o; N(1)-C(19)-C(20) 122.8(3)o; N(1)-C(19)-C(20) 118.2(2)o.
Copper Complexes. Under refluxing conditions, reaction of CuCl2 with equimolar amount of 1
in absolute ethanol for 3 h provided the green complex [(1)CuCl2] 4, whereas complexation of 1 with Cu(ClO4)2.6H2O gave the ionic complex [(1)Cu(H2O)](ClO4)2 5 in green solids. By the recrystallization, x-ray suitable crystals for both complexes can be accomplished. ORTEP plots of both complexes 4 and 5 are deposited in Figures 2-3, respectively. The copper(II) center in both complexes has the distorted square pyramidal geometry with one nitrogen donor [N(2)] from the ligand seated in the apical position. The basal plane in complex 4 was formed by two chloride
donors and two nitrogen atoms [N(1) and N(3)] from the ligand. The formation of a stable five- member chelate ring by the secondary amine N(3) and pyridine nitrogen N(1) is presumably an exp lanation for these two donors in the equatorial direction. The coordination mode of nitrogen donors in complex 5 is similar to those in 4, but the oxygen atom [O(1)] of phosphine-oxide moiety of the ligand is bounded to metal center. Thus donor atoms of N(1), N(3), and O(1) as well as a water molecule formed the basal plane in 5.
O(1) P(1) C(7) C(13)C(18) N(1) N(2) N(3) C(6) Cl(1) Cl(2) O(1) P (1) C(7) N(1) N(2) N(3) C(6) O(2) Cu
Figure 2. Molecular Structure of Copper Complex 4. Figure 3. ORTEP Drawing of the Cationic Part of 5.
The bond distances and bond angles of basal plane of both complexes are summarized in the Figure 4. A significant difference in the structure of 4 compared to that of 5 is that all Cu-N lengths in 4 are longer those in 5 particularly the apical one.[Cu-N(2) = 2.334(3) Å in 4; 2.254(8) Å in 5] Also, bond lengths of Cu-N(1) are somewhat shorter than those of Cu-N(3), which is attributed to the different hybridization of nitrogen donors; the N(1) is a pyridinyl nitrogen, whereas N(3) is a secondary amine group. In both complexes, the distance of the apical nitrogen to copper center is longer by ca. 0.3 Å than those of equatorial ones, indicating a weak interaction in the apical orientation. All bond distance and bond angles in both complexes lie within normal ranges except N(2)-Cu-N(3) [80.8(1)o]. However, it is noticed that the angles Cl(1)-Cu-Cl(2) in 4 [96.36(4)o] much derived from the 90o as compared to the smaller derivation of O(1)-Cu-O(2) [87.9(2)o]. Cu N(1) N(3) Cl(2) Cl(1) N(2) 2.257(1) 2.258(1) 2.034(3) 2.074(3) 2.334(3) Cu N(1) N(3) O(1) O(2) N(2) 1.955(6) 1.921(5) 1.975(7) 2.040(7) 2.254(8)
Figure 4. Comparsion of bond distances and angles around the metal centers between 4 and 5
N( 1)- Cu-N( 3) 81.1(1) o N( 3)- Cu-Cl(2) 89.30( 9)o Cl(2) -Cu- Cl( 1) 96.36(4) o Cl(1) -Cu- N(1) 92.80( 9) o N( 3)- Cu-Cl(1) 173.80( 9) o N( 1)- Cu-Cl(2) 161.74( 9) o N( 2)- Cu-N( 1) 91.6(1) o N( 2)- Cu-N( 3) 80.8(1) o N( 2)- Cu-Cl(1) 100.36( 9)o N( 2)- Cu-Cl(2) 102.22( 9) o N(1) -Cu-N (3) 81.8( 3)o N(3) -Cu-O(2) 93.7(3)o O( 2)-C u-O( 1) 87.9(2) o O( 2)-C u-N(1) 94.3(3) o N(3) -Cu-O(2) 169.3(3) o N(1) -Cu-O(1) 166.3(3) o N(2) -Cu-N (1) 90.6( 3) o N(2) -Cu-N (3) 88.6( 3) o N(2) -Cu-O(1) 102.2(2)o N(2) -Cu-O(2) 101.4(3) o
In addition to crystallography, both EPR spectroscopic data and electronic absorption are in agreement with the structure. The X-band EPR spectra of 4 and 5 in methanol glass (80K) were determined and their data were collected in table 3. It appears that both complexes with the
parameters g// > g- are typical monomeric square pyramidal copper(II) complexes with dx2-y2 ground state.10 The spectra of 4 and 5 recorded as mulls show a broad band centered at 540 and 567 nm, respectively, which are in agreement of the d-d transitions for a square pyramidal copper(II) complex with pyridine donors.11 Table 1 also includes the electronic absorptions in methanol of both complexes. The absorptions in UV region are assigned as the transition from the ligand itself, whereas the λmax around 370 nm is believed due to the LMCT band. In addition, complex 4 in methanol exhibits a d-d band around 740 nm with a shoulder to lower wave-numbers, a characteristic profile for the copper(II) species in square pyramidal geometry. This outcome also indicates that the complexes remain the same structural feature in solution.
Table 1. EPR spectroscopic dataa and electronic absorptions of 1, 4 and 5.b
g// g- A//(G) λmaxb
1 210(41.93) 260(13.06) 326(5.05)
4 2.284 2.071 144 211(21.68) 261(15.01) 321(1.91) 371(0.85) 421(0.62) 743 (0.11)
5 2.311 2.078 173 211(31.78) 261(10.79) 324(2.98) 369(2.13) 516(0.18) 700 (0.07)
a. in methanol glass (80K). b. in methanol, ε is given in the parenthesis. (x 103 M-1cm-1)
This new polydentate ligand gave the formation of square- pyramidal copper(II) complexes with all three nitrogen donors in facial arrangement, providing a possible model for the enzymatic reactions. The catalytic oxidation of alcohols and alkanes using these copper complexes are currently under investigation.
Part III Palladium nanoparticles in C-C bond formation.
Suzuki-Miyaura coupling reaction provides a powerful method for preparation of
unsymmetrical biaryls,1and the palladium complexes are known to be the most efficient catalyst amo ng various metal systems. However, a high loading of catalyst and under inert atmosphere in most reactions are generally required for a better conversion, which does not meet the points of both economical and environmental senses.1-6 Thus searching of new palladium complexes as catalysts has received much attention particularly for the use of aryl chloride as substrates,2- 8 under aerobic conditions4 or even in aqueous solution.5 Accordingly, palladacycles were found to the most promising catalysts in this regard.1d,6-8 Recently, Bedford7 and Nájera8 have demonstrated that Suzuki coupling reaction of aryl chlorides with arylboronic acid in high conversion can be achieved by using the cyclopalladated imino-complexes 1 and 2, respectively. In a previous communication, we have found that palladium complex 3b could act as a catalyst for the coupling of aryl chloride with arylboronic acid in aqueous medium.9 Here, we would like to report the
detail studies of the preparation and characterization of a series of benzylic palladacyles as well as their catalytic activities toward Suzuki-Miyaura coupling reaction in ethanol under aerobic
R N Pd X PR3 N Pd Cl OH 2 R 1 2 N R R Pd Cl 2 3a R = Me 3b R = iPr
Preparation of Ligands and Complexes. Substituted trimethylbenzylideneamines were
prepared by the condensation of 2,4,6-trimethylbenzaldehyde with the substituted aniline
(scheme1). Both infrared absorptions near 1626 cm-1 and 13C nmr shifts around 162 ~ 163 ppm of imines 4a-b are characteristic for the functionality of C=N. Cyclopalladation reactions were carried out in a mixture of (CH3CN)2PdCl2, sodium acetate and the corresponding ligand in tetrahydrofuran at ambient temperature for 38 h. The desired palladium products 3a-b were isolated as air stable solids upon crystallization. In both instances, the cyclopalladation readily occurs at benzylic to form an endo six- membered chelating ring and the product is in a
chloro-bridged dipalladium structure, which is consistent with most of the related species.10The C-H activation at benzylic position is established by their 1H nmr and x-ray single-crystal determination. The appearance of signal 3.19 ppm for 3a and 3.24 for 3b with the integration of 2H in the 1H nmr spectra was assigned to the methylene protons of Pd-CH2- resulted from the C-H activation with the palladium complex.
N R R 4a R = Me 4b R = iPr NH2 R R
Scheme 1. Preparation of palladacycles.
O
3a-b
The detail structure of palladacyles 3a was proved by X-ray diffraction study on single crystals grown from a hexane/dichloromethane solution, whereas the structural characterization of
3b has published previously.9 Figure 1 displays the ORTEP plot of 3a and table 1 summarizes the selected bond distances, bond angles and torsion angles. In both instances, the palladium metal
displays a slightly distorted square-planar geometry with nitrogen and carbon donors in
cis-fashion. All bond distances and bond angles lie within normal ranges, which are essentially
similar to those for 3b except Pd(1)-N(1). The distance of Pd(1)-N(1) [2.024(3) Å ] for 3a is slightly shorter than that for 3b [2.043(3) Å], which is attributed to steric difference between methyl and isopropyl groups. The length of C(7)-N(1) [1.298(4)Å] is characteristic for C=N double bond. The small bite angle N(1)-Pd(1)-C(1) [86.0(1)o]is similar to those benzylic type palladacycles reported by Sales and coworkers.10e It is noticed that the angle of Pd(1)-C(1)-C(2) [115.8(2)o] deviating from the angle for a tetrahedral geometry, similar to that for 3b, is
presumably due to the strain forced by the chelating rings. Those dihedral angles along the chelating ring of Pd(1)-N(1)-C(8)-C(7)-C(2)-C(1) in 3a (table 1) indicates that this ring is adopted into a twist half-chair conformation, apparently resulting from the occurrence of two double bonds in the chelate ring.
Phosphine-substituted Complexes. Both palladacycles 3a and 3b readily underwent ligand
substitution reaction with phosphines (Eq. 1) to give the corresponding complex. Both complexes were in air-stable crystalline forms. Appearance of only one signal around 40 ppm in 31P NMR for both complexes 5a and 5b suggests the formation of single trialkylphosphine-substituted isomer out of the two possibilities. The benzylic methylene unit cis- to the phosphorus in 5a and 5b was established from the 1H NMR spectra, where methylene group bound to the palladium appears as a doublet with a coupling constant JP-H ≈ 2 ~ 5 Hz. This value is in the typical range reported for the cis-arrangement of the methylene group and phosphine in related species.10a,10e Even though both complexes could be easily characterized by means of spectral and elemental analysis, the crystal structure of 5b was determined to confirm the details. Figure 2 displays the ORTEP drawing of 5b and selected bond distances and bond angles are collected in Table 2. A method similar to that for the preparation of 5a-b was employed to produce the triphenylphosphine adduct
5c-d. Both spectroscopic and crystallographic analyses of 5c verify the structure of 5c-d (figure
3). N R R Pd Cl 2 N R R Pd Cl PR'3 PR'3 5a R = Me, R' = C6H11 5b R = iPr, R' = C6H11 5c R = Me, R' = Ph 5d R = iPr, R' = Ph ( 1)
Both 5b and 5c show the square planar arrangement around the metal center with the phosphine and imine donor trans to each other, presumably due to the trans influence of these donors. All bond distances and bond angles are lie in the normal range of the close related complexes such as [Pd(TFA)(?2N,C-C6H4CH2NMe2)(PCy3)] reported by Bedford and
coworkers.7a When two structures are compared, it is found that all bond distances and bond angles around the metal center are very close except the bond angle of P(1)-Pd(1)-C(1) and the bond length of Pd-P. The P(1)-Pd(1)-C(1) in 5b [93.1(1)o] is considerably larger than that of the analogous angle in 5c [88.57(9)o], reflecting a result of steric relief between the carbon donor and tricyclohexylphosphine. The Pd-P bond distance of 5b [2.2683(7) Å] is slightly longer than that of
5c [2.2388(7) Å], which is due to the contribution of palladium back-donation to the π-acidic nature of triphenylphosphine. For the conformation of chelating rings associated with metal center in 5b and 5c remains as a twist half-chair form as illustrated by the torsional angles along the ring consisted of Pd(1)-N(1)-C(8)-C(7)-C(2)-C(1). (table 2)
<<Insert Figures 2 and 3>> <<table 2>>
Another phosphine-substituted palladacycle 7 with five- member chelating was prepared via the substitution of 6 with tricyclohexylphosphine (Eq. 2). 31P nmr chemical shift of 7 appeared at 43.1 ppm, which is in the typical range of the trialkylphosphine palladium complexes.7a,7c Crystal structure of 7 was determined (Figure 4). As expected, the molecular structure 7 shows a square planar geometry around the metal center. Out of four coordination sites, two are occupied by N~C ligand in cis- fashion and the other two by phosphine and chloride with nitrogen and phosphorus donors in trans arrangement. The bond lengths between the Pd and C, N, P and chloride donor atoms for 7 (table 2) are within the range and comparable with complexes 5b. However, it is noticed that all bond angles around the metal center in 7 is much more derivated from the square-planar than in 5b,due to the five- member chelate ring.
N Pd Cl PCy3 7 N Pd Cl 2 6 PCy3 (2) <<Figure 4>>
Suzuki-Miyaura Coupling Reaction. All palladacyclic complexes prepared in this work were
subjected to evaluate their catalytic activities on the coupling reaction of phenylboronic acid with aryl halides. In a typical experiment for the reaction, aryl bromide, phe nylboric acid and K2CO3 in a ratio of 1:1.5:2 were placed in the flask, followed by the addition of the solvent and the catalyst. In all instances the solvent was used as obtained commercially without further purification, while the deionized water was used for aqueous systems. The organic product was isolated by extraction and then analyzed by the 1H nmr spectroscopy.
The initial screen on solvents were performed using 3b as the catalyst precursor (table 3, entries 5 - 8). It appeared that the protic solvents including water gave much better results than any other organic solvents.9 This coupling reaction running in DMF only provided moderate yield as compared with alcoholic solvents. This result is quite similar to that reported by Nolan and
coworkers for using palladacycle carbene complex in the coupling of aryl chloride with phenylboronic acid.3h It is noticed that the reactions can carried out under atmosphere of air. Therefore, the activities of various palladium complexes were screened using ethanol as the solvent under aerobic conditions. Results are summarized in table 3.
As the molar ratio of [substrate]/[Pd] remains ~ 20000, all palladacyclic catalysts show good catalytic activities in Suzuki coupling. Among them, complexes 3a and 3b ought to be the best ones, even better than those phosphine-substituted complexes 5a-d. For 3a and 3b appear that the steric hindrance of the ligand has less influence on the catalysis. However, the yield drops dramatically within a reasonable period of reaction time (~ 20 h) when the mol ratio of [substrate]/[Pd] increases up to 106 (entries 11,18), indicating a concentration limitation of these
Table 3. Results of coupling reactions catalyzed by palladacycles
entry cat.(Pd mmol ) ArBr [ArBr]/[Pd] atm.
t (h) Conv.b (%) 1 3a (1x10-4) p-MeOC 6H4Br 20000 air 3 100 2 3a (2x10-5) p-MeOC 6H4Br 100000 air 10 91 3 3a (2x10-6) p-MeOC 6H4Br 1000000 air 18 62 4 3a (2x10-3) p-MeOC 6H4Br 1000 air 20c 100 5 3b (1x10-4) p-MeOC 6H4Br 20000 air 3 100 6d 3b (1x10-4)e p-MeOC 6H4Br 20000 air 3 75 7e 3b (1x10-4)f p-MeOC 6H4Br 20000 air 3 28 8f 3b (1x10-4) p-MeOC 6H4Br 20000 air 3 100 9 3b (1x10-4) p-MeOC 6H4Br 20000 air 3 64 10 3b (2x10-5) p-MeOC 6H4Br 100000 air 10 86 11 3b (2x10-6) p-MeOC 6H4Br 1000000 air 20 57 12 3b (2x10-3) p-MeOC 6H4Br 1000 air 14c 100 13 5c (1x10-4) p-MeOC 6H4Br 20000 air 3 85 14 5d (1x10-4) p-MeOC 6H4Br 20000 air 3 95 15 5a (1x10-4) p-MeOC 6H4Br 20000 air 3 100 16 5b (1x10-4) p-MeOC 6H4Br 20000 air 3 92 17 6 (1x10-4) p-MeOC 6H4Br 20000 air 3 86 18 6 (2x10-6) p-MeOC 6H4Br 1000000 air 16 26 19 7 (1x10-4) p-MeOC 6H4Br 20000 air 3 83 20 3b (2x10-5) p-MeCOC 6H4Br 100000 air 5 77 21 3b (2x10-6) p-MeCOC 6H4Br 1000000 air 3 40 22 3b (2x10-6) p-MeCOC 6H4Br 1000000 air 20 100 23 3a (2x10-6) p-MeCOC 6H4Br 1000000 air 20 89 24 5a (2x10-6) p-MeCOC 6H4Br 1000000 air 20 100 25 5b(2x10-6) p-MeCOC 6H4Br 1000000 air 20 100 26f 3b (1x10-4) p-MeCOC 6H4Br 20000 air 3 100 27 3a (1x10-3) 2,4,6-tri-MeC 6H2Br 2000 air 3 76 28 3b (1x10-3) 2,4,6-tri-MeC 6H2Br 2000 air 3 69 29 5b(1x10-3) p-MeOC 6H4Cl 2000 air 17 72 30f 3a (2x10-4) p-MeCOC 6H4Cl 10000 air 1 96 31 5b (2x10-5) p-MeCOC 6H4Cl 10000 air 3 80 a
Reaction conditions: ArX (2 mmol), PhB(OH)2 (3 mmol), catalysts, K2CO3 (4 mmol), EtOH (5 mL), reflux. b Determined by 1H-NMR spetroscopy
catalysts. Among these studies, we found that the palladacycle with six- member chelate ring 5a-d were slightly better than those with five- member ones 6. This observation is also applied for the phosphine-substituted palladacycles, i. e. the catalytic activities of 5a-d are better than that of 7 (entries 13 –16 versus 19). The catalytic activities of 3a-b are generally as good as those for 5a-d, indicating that phosphine ligands are not necessary in this reaction. It is worthy to mention that the coupling reaction can be carried out at room temperature (entry 4). A quantitative conversion was observed for the coupling of p-bromoanisole with phenylboronic acid catalyzed by 3b in water with the presence of tetraalkylammonium salt (entries 8, 26).
As for the activated substrate (p-acetylphenyl bromide), the turnover number can reach up to 106 for 3b, 5a and 5b (entries 22-25). In addition, complexes 3a and 3b catalyzed the coupling of the steric bulky substrate such as 2,4,6-(Me)3C6H2Br with phenylboronic acid in good conversions (entry 27-28).
Under similar reaction conditions, the catalytic activities of these palladacycles toward aryl chloride appear to be lower than the bromo substrates (entry 29). However, the conversion can be improved by carrying the reaction in water medium and the presence of tetrabutylammonium salt (entries 30-31).9 Overall, these palladium complexes prepared in this work behave highly catalytic acitivity on the Suzuki-Miyaura coupling reaction.
Mechanistic Pathway of Catalysis. In order to probe the reaction pathway, the course of the
coupling reaction was monitored by taking samples and analyzed them by 1H nmr and TEM. First, an aliquot from the reaction of 3b with phenylboronic acid (2 eq.) in the presence of K2CO3 and ethanol was examined by transmission electron microscopy, showing the formation of palladium particles with the diameter in the range of 50 ~ 60 nm (figure 5). Upon the addition of
p-bromoanisole to the above solution, the coupling reaction underwent smoothly to yield p-methoxybiphenyl quantitatively, suggesting that the palladium nanoparticles might be the active
catalyst for the reaction.
Further studies, we found that complex 3b readily reacted with ethanol under basic conditions to generate acetaldehyde and the free ligand 4b accompanied with palladium nanoparticles, but aggregate to produce the palladium black without the presence of other substrates. The formation of acetaldehyde is presumably due to the substitution of chloride ligands by ethoxide under basic conditions followed by the β-elimination.3h Addition of phenylboronic acid to the above solution yielded benzene and biphenyl immediately (table 4). On
the other hand, p-acetophenyl bromide was converted into acetophenone and
4,4’-bisacetobiphenyl as evidenced by the 1H nmr spectroscopy. However, the reaction of
p-acetophenyl chloride was much slower than that of bromide (table 4, entry 2). The production of
biphenyl derivatives and the reduced compounds is presumably via the addition of phenylboronic acid or p-acetophenyl bromide to the palladium nanoparticles followed by the reductive elimination (scheme 2). We also found that the decomposition rate of aryl bromide is much slower than that of arylboronic acid, revealing that the oxidative addition of aryl halide to
the metal is the rate limiting step in the cross coupling reaction. This also explain that the use of excess of arylboronic acid in the Suzuki-Miyaura reactions is generally required for better conversions. It should be mentioned that the reduction of aryl halides or arylboronic acid proceeded superiorly than that of the homo-coupling reaction.
<<Table 4>> base + CH3CHO + Pd(0) 4b 3b + O O + p-MeCOC6H4Br p-MeCOC6H4Br O PhB(OH)2 PhB(OH)2 Scheme 2 EtOH nano-particle
The decomposition rate of palladcycles in basic ethanol solution follows the order: 6 > 3b >
5b > 5d (table 5). Complex 6 appeared to be slower than that of 3b, but aggregated into larger
nanoparticles (100 ~ 200 nm), which explained the activity difference between 6 and 3b due to the size effect.11 As for the phosphine substituted complex 5b and 5d, the decomposition was much slower than that of 3b presumably due to the stabilization of the coordinating phosphine. Another finding is that the surfactant stabilized palladium nanoparticles appears to be less active than the ones generated in situ. In the presence of tetrabutylammonium bromide, palladium nanoparticles was obtained from the decomposition of complex 3b in ethanol. The resulting palladium nanopartices was used as the catalyst for the coupling reaction of PhB(OH)2 and MeOC6H4Br to give the desired product but in a slower conversion rate by ca. 20 %.
It has been demonstrated that palladium nanoparticles can catalyze the C-C bond coupling reaction,12 particularly under Jeffery condition for Heck reaction.13 Unlike the palladium clusters stabilized by tetraalkylammonium salt, the nanoparticles generated from the reduction of palladacycles are presumably surrounded by the imine ligands, which tends to aggregate with the precipitation of palladium black. This reveals the weak stabilizing effect of these ligands toward nanoparticles. On the other hand, the loose protection gives these palladium particles in high activities toward the substartes.12
Furthermore, the low concentrations of palladium complexes may also avoid the rapid formation palladium black and the formation of larger nanoparticles, suggesting the extreme activity of metal clusters. However, the conversion decreases as the ratio [substrate]/[Pd] is higher than 106 (table3 entries 3 and 11), showing a limitation of these nanoparticles in catalysis.
We have synthesized and characterized a new series of air-stable palladacycles. These palladium complexes were successfully applied in the Suzuki-Miyaura coupling reaction and behaved good catalytic activities, for example the turnover frequency up to 107 mol/mol(Pd).h for 3b in the coupling of p-bromoanisole or p-bromoacetophenone with phenylboronic acid. Notable
were the reaction conditions employed, i. e. the moisture and air insentive. Several observations confirm that the Suzuki-Miyaura coupling catalyzed by palladcyclic complex are via the
palladium nanoparticles. Reactions of p-bromoacetophenone or arylboronic acid individually on the nanoparticles leading to the homo-coupled and reduced products was investigated, but the cross coupled ones were obtained when the aryl halide and arylboronic acid were presented. However, the pathway for the cross coupling and reduction process on these particles remains unclear. More work is necessary in order to work out the nature of these nanoparticles.
REFERENCES (published works)
Palladium(II) Complexes Containing P~N~O donors. Preparation and Reactivity
Ping-Yung Shi, Yi-Hong Liu, Shie-Ming Peng, Shiuh-Tzung Liu J. Chin. Chem. Soc. 2003, 50, 89.
New Bulky Phosphino-Pyridine Ligands. P~N~C Tridentate in Palladium Complexes. Hsin-Pei Chen, Yi-Hung Liu, Shie-Ming Peng, Shiuh-Tzung Liu Dalton Trans. 2003, 1419..
Hung-Ren Wu, Yi-Hung Liu, Shie-Ming Peng, Shiuh- Tzung Liu “Palladium(II) Complexes Containing a Pyridinyliminophosphorane Ligand” Eur. J. Inorg. Chem. 2003, 3152.
Hsin-Pei Chen, Yi-Hung Liu, Shie-Ming Peng, Shiuh-Tzung Liu “New Bulky Phosphino-Pyridine Ligands. Palladium and Nickel Complexes for the Catalytic Polymerization and Oligomerization of Ethylene” Organometallics. 2003, 22, 4893.
Chuan-Lin Chen, Yi- Hung Liu, Shie-Ming Peng, Shiuh- Tzung Liu “Substituent Effect on Cyclopalladation of Arylimines” J. Organomet. Chem. 2004, 689, 1806 –1815.
Chuan-Lin Chen, Yi- Hung Liu, Shie-Ming Peng, Shiuh- Tzung Liu “An efficient catalyst for Suzuki-Miyaura coupling reaction in aqueous medium under aerobic conditions.” Tetrahedron
Lett. 2005, 46, 521.
Weiwen Tsai, Yi- Hong Liu, Shie-Ming Peng, Shiuh- Tzung Liu “Structural Characterization and Catalytic Activities of Copper Complexes with Pyridine-amine-phosphine-oxide Ligand.” J.
Organomet. Chem. 2005, 690, 415.
Chuan-Lin Chen, Yi- Hung Liu, Shie-Ming Peng, Shiuh- Tzung Liu, “Air and Moisture Stable Cyclopalladated Complexes as Efficient Catalysts for Suzuki-Miyaura Coupling Reaction”
Organometallics 2005, 24,1075.
S. R. Korupoju, J.-Y. Lai, Y. -H. Liu, S.-M. Peng, S.-T. Liu“Synthesis and Characterization of Nickel and Palladium Complexes Containing Hetero-Multi- dentate PNO ligands” Inorg. Chim.