Hydrodictyon reticulatum (Hydrodictyaceae, Chlorophyta), A New
Recorded Genus and Species of Freshwater Macroalga in Taiwan
Jui-Yu Chou
1, Jui-Sheng Chang
2, Wei-Lung Wang
1,2*
1Department of Biology, National Changhua University of Education Changhua, Taiwan
2
Center of General Education, Yu-Da University Miaoli, Taiwan
(Received: 21 November 2005, accepted: 20 February 2006)
ABSTRACT
Hydrodictyon reticulatum (water net), a colonial, unattached, filamentous green algae, was found in rice paddies in northern Taiwan. This species is a new addition to the algal flora of Taiwan. It is distinctive from other green algae in having a cylindrical colony with six-edged mesh structure (that is visible to the naked eyes). In this study, we describe morphological characters in detail, and also use partial atpB sequences to examine its phylogenetic relationships to the sisters.
Key words: atpB gene, Hydrodictyon, Taiwan, Water net
Introduction
Hydrodictyon reticulatum L. Lagerheim
(Hydrodictyaceae, Chlorophyta) is a free-floating filamentous alga that forms a macroscopic, closed, cylindrical net with pentagons and hexagonal meshes. This distinctive form can be easily seen by the naked eyes, and is commonly known as a “water net.” It is widespread all over the world, while seldom form a persistent nuisance growth that exists otherwise in filamentous, such as Cladophora Kützing, Oedogonium Link, and Spirogyra Link (Pocock 1960; Wells et al., 1999). Its prominent bloom is related to the degree of watermass eutrophication and usually has ecological impacts (Wells et al. 1999; Wells and Clayton, 2001). The plants rapidly grow, when the environment is suitable, and produce a cylindrical daughter coenobia within it, which enlarges to produce minature nets with parent cells that are released when the cenobium cells disintegrate at maturity. Sexual reproduction, resulting in the formation of resistant spores, has been well documented (Marchant and Pickett-Heaps, 1971, 1972a, 1972b, 1972c, 1972d).
This species is widely distributed in the Northern Hemisphere (Pocock, 1960), while is
seldom recorded from the Southern Hemisphere (Pocock, 1960), except in South Africa, Australia, and New Zealand (Pocock, 1960; Coffey and Miller, 1988). This alga has never been recorded from Taiwan (Huang and Chiang, 1986; Wang et al., 1999; Wu, 1999, 2001; Liu and Wang, 2004). Recently, the species was collected from rice paddy in northern Taiwan as a weed. In this study, its morphological characters and the physical-chemical parameters of its habitat are described in detail. We also use atpB gene sequence to examine its phylogenetic relationships.
Materials and Methods
Morphological observations
Thalli were collected from a rice paddy, with clear water, at a depth of 15-30 cm in Hsinchu, northern Taiwan on 11 June 2005. The pH, dissolved oxygen (mg/L) and conductivity (µS/cm) of water at the localities were measured by pH meter, DO (dissolved oxygen) meter, and conducometer in situ, respectively. A roughly 1000 mL water sample was collected, stored at 0-4°C in a refrigerator immediately, and transported to the laboratory. Dissolved inorganic nitrogen (DIN, including NO2 + NO3 + NH3) and dissolved
2 inorganic phosphate (DIP, PO4) were measured with a SMART spectro photometer (LaMotte, Maryland, USA). Specimens were preserved in 95% alcohol for molecular analyses, while materials for morphological observations were preserved in 4-5% formalin solution or dried as herbarium specimens. The liquid specimens were examined. In LM observations, the vegetative and reproductive structures were examined using a light microscope at 100x and 200x (Zeiss Axioskop 2) and a dissecting microscope (Zeiss Stemi SV11). Voucher specimens were deposited at the Department of Biology, National Changhua University of Education, Taiwan.
Phylogenetic analysis
Extraction of total DNA, PCR amplification of DNA fragments, and direct sequencing of the PCR products followed the methods of Sakayama et al. (2004). For phylogenetic analysis, a partial atpB (617bp) gene sequence from 13 operational taxonomic units (OTUs) and two related species as outgroups (Tab. 1) was subjected to neighbor- joining (NJ) analysis based on Kimura 2-parameter genetic distance (Kimura, 1980) using PAUP* 4.0b10 (Swofford, 2002). Support for nodes of the MP tree was assessed by calculating 1000 bootstrap resamplings of the heuristic searches based on random stepwise additions, MULTREES and TBR (Felsenstein, 1985). Support for the nodes of the NJ tree was determined by calculating bootstrap proportion values based on 1000 resamplings of
neighbor-joining searches by applying the Kimura 2-parameter method (Kimura, 1980)
Results
Morphological observations
Hydrodictyon reticulatum (L.) Lagerheim cf.
Pocock 1937, 24: 263-281. (Figures 1A-1D)
Basionym:
Conferva reticulatum Linnaeus, 1753, Spec. pl. ed.
1., 1165. Synonyms:
Hydrodictyon utriculatum Roth, 1800, Tent. F1.
Germ. III, 531.
Hydrodictyon pentagonum Vaucher, 1803, Hist.
Conf., 88.
Specimen examined.
The specimens were collected from Hsinchu, Taiwan (E121°08’024’’, N24°50’565’’), on 11 June 2005, No: NCUE-JYC- 940611.
Diagnosis.
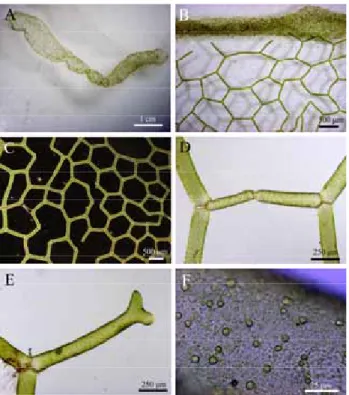
Plants are net-like, free-floating, cylindrical coenobium, closed at both end, and 5 to 20 cm in length (Fig. 1A). Young daughter nets are 125 µm long and 30 µm wide, displaying several pyrenoids. Mature cells (coenocytes) are up to 500 µm long and 125 µm wide, with 5-6-sided end-to-end meshes (Fig. 1B). Y-shaped cells occurring at the terminals of the net are not connected with other Table 1. List of species used in atpB gene analysis and accession numbers in GenBank.
Taxon References Accession number
Ingroup
Astrephomene gubernaculifera Nozaki et al, 2000 AB044181 Chlamydomonas moewusii Nozaki et al, 1999 AB014035 Chlamydomonas noctigama Nozaki et al, 2003 AB101502 Eudorina elegans Nozaki et al, 1999 AB014011 Eudorina minodii Nozaki and Krienitz, 2001 AB047068 Hydrodictyon reticulatum This study DQ176419
Pandorina morum Nozaki et al, 2000 AB044179
Pediastrum duplex Nozaki et al, 2002a AB084306 Pteromonas angulosa Nozaki et al, 1999 AB014038 Scenedesmus quadricauda Nozaki et al, 2002a AB084305 Vitreochlamys ordinata Nozaki et al, 1999 AB014036
Volvox barberi Nozaki et al, 1999 AB014001
Volvox gigas Nozaki et al, 2002b AB076112
Outgroup
Coleochaete sp. 18b3 Cimino and Delwiche, 2002 AY082322 Coleochaete sp. 528a3 Cimino and Delwiche, 2002 AY082327
Figure 1. Specimens of Hydrodictyon reticulatum L. Lagerheim. (A) Habit. (B) Mature cells arranged end to end to form 5-6 sided meshes. (C) Y-shaped cells occurring at the terminals of the net are not in contact with other cells. (D) Mature cells with numerous pyrenoids.
cells (Fig. 1C). Mature cells are multinucleate with numerous pyrenoids (Fig. 1D).
Habitats.
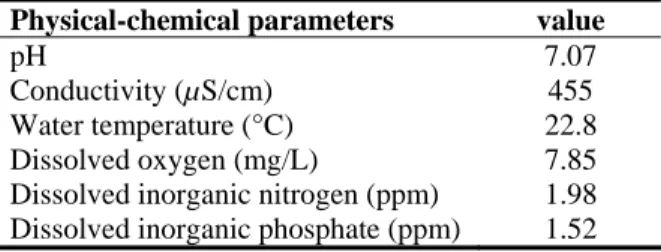
The rice paddy in Hsinchu had an area of 225 square meters with an average depth of 15 cm and a maximum depth of 30 cm. The physical parameters of the rice paddy (1400 hrs, 11 June 2005) were as follows: the water temperature was 22.8°C, pH was 7.07, conductivity was 455 µS/cm, and dissolved oxygen was 7.85 mg/L. The chemical parameters were as follows: the dissolved inorganic nitrogen was 1.98 ppm, and dissolved inorganic phosphate was 1.52 ppm. Hydrodictyon reticulatum was distributed around the margins of the rice paddy and mixed with aquatic plants, Nuphar shimadae Hayata, Egeria densa Planchon., Euryale ferox Salisbury and a Charalean, Chara braunii Gmelin.
Remarks.
Hydrodictyon reticulatum and H. indicum
Iyengar are typically a closed, cylindrical sac of thousands of cells. Hydrodictyon patenaeforme Pocock and H. africanum Yamanouchi can be distinguished from the former two species in having
flattened nets, single layered with several hundred cells. H. indicum will separate at maturity, we can use this character to distinguish it from H.
reticulatum.
Phylogenetic analysis
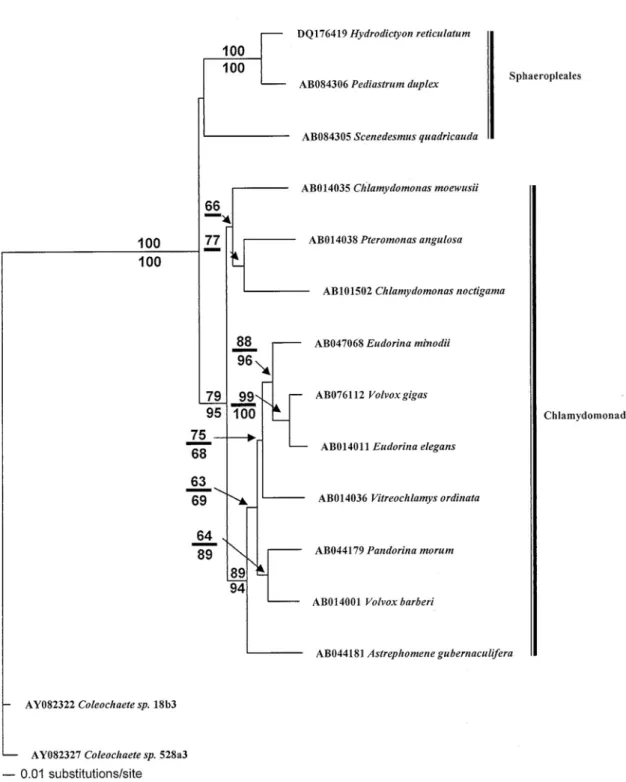
A NJ tree was recovered (Fig. 2), in which only the branches supported with bootstrap values ≧ 50% in the MP (above the branch) and NJ (below the branch) analyses were indicated. The minimum evolution score is 0.54. In the tree, H.
reticulatum was clustered with another coenobial
organism, Pediastrum duplex Meyen, based on the
atpB gene sequence, a result consistent with the
phylogenies based on 18S rDNA and 26S rDNA gene sequences (Wicox et al., 1992; Buchheim et
al., 2001). One species belonging to Sphaeropleales, Scenedesmus quadricauda (Turp.) Breb., is sister to H. reticulatum and P. duplex. Monophyly of
Sphaeropleales was suggested, although without a significant bootstrap value. In contrast, monophyly of the Chlamydomonadales was significant supported.
Discussion
The genera Hydrodictyon A. Roth was derived from within Pediastrum, and indicated the pattern of colony-form evolution within the Hydrodictyaceae from two-dimensionality to three-dimensionality based on 26S rDNA sequences (McManus and Lewis, 2005). Prongs structure also indicates a close phylogenetic relationship between
Pediastrum and Hydrodictyon (Pickett-Heaps,
1975). The hypothesis is supported by recent molecular analysis (Wicox et al., 1992; Buchheim
et al., 2001; McManus and Lewis, 2005). We can
found that Hydrodictyon was clustered with
Pediastrum in the NJ tree (Fig. 2) based on partial atpB gene sequences in this study, consistent with
the result. With hopes of constructing a more robust estimate of the phylogeny of this family and the validity of aforementioned hypothesis, additional taxa and molecular data from plastid-encoded atpB gene should be added in the future study.
There are four species in Hydrodictyon: H.
africanum, H. patenaeforme, H. indicum, and H. reticulatum (Pocock, 1937). In H. africanum, the
ultimately spheroid coenocytes, and final regular disjunction into separate spheres, are unique. Pocock (1937) pointed out that the net-like form of
4
Figure 2. The NJ tree is in which we show the branches supported by ≧ 50% bootstrap values in the MP (above the branch) and NJ (below the branch) analyses. The minimum evolution score = 0.54.
H. patenaeforme is determined by environmental
conditions. Iyengar (1925) described H. indicum as “resembling those of H. reticulatum,” except in certain minor details, but no complete nets were seen in his study. The differences of aforementioned species are in size, the breaking up of the net by the separation of the coenocytes, and the thick wall with numerous peg-like ingrowths. Pocock (1937)
suggested that when these features are examined critically, their diagnostic values are found to be limited. He pointed out that the size of
Hydrodictyon was dependent on the external
environment. Pocock also observed that the breaking up of nets also depends on the external environment or possibly on different reproductive stages. Besides, the formations of peg-like
ingrowths on the inner side occur frequently in old coenocytes of H. reticulatum. Except H. indicum, other three species are distinct based on 26S rDNA sequences in recent study (McManus and Lewis, 2005). Based on these characteristics, we identified the specimen collected from northern Taiwan as H.
reticulatum.
According to our own observations, protrusions were formed at one or both ends at an angle of about 120˚ to the long axis of the cylindrical cells, which corresponds to the angle between two adjacent cells in the hexagonal net. This connection inevitably results in a hexagonal geometrical arrangement of the cells. Y-shaped cells have seldom been observed in normal colonies (McReynolds, 1961; Tiwari et al., 1980). In our study, we observed that the Y-shaped cells occurring at the terminals of the net do not contact with other cells. The angle between the two arms and the main axis of the Y-shaped cell was also 120˚. These facts show that the bifurcation of the cell end at an angle of 120˚ to the long axis of the cell is an innate characteristic of H. reticulatum, when the end of the zoospore fails to contact with other zoospores (Hatano and Maruyama, 1995).
Pocock (1960) suggested that the typical habitat of H. reticulatum is a warmer region with alternating periods of inundation and drought such as the Nile delta of Egypt and the paddy fields of China. In Taiwan, crop rotation is usually conducted, rice paddies are therefore dry in winter but flooded in late spring or early summer, explaining why H. reticulatum grows in rice paddies. It provides a suitable habitat for H.
reticulatum. Prescott (1970) used H. reticulatum as
an indicator of high pH and hard water. But this is not substantiated by its occurrence in water of pH 7.07 in this study (Tab. 2). On the basis of this conflicting information on habitat requirements for the plant, we cannot assume that H. reticulatum is unsuited to the conditions in Taiwan. This plant may occur as a bloom as a result of its getting full sunlight. Hall and Payne (1997) also pointed out that this seasonal pattern was not linked to DIN or DIP, but was likely due to seasonal irradiance levels.
Hydrodictyon reticulatum has been considered a
nuisance where it occurs (Coffey and Miller, 1988), as resistant to herbicides such as copper sulphate (Fitzgerald, 1981), and regrows rapidly after re-watering (Pocock, 1960). Its spread within Taiwan could cause heavy weed problems.
Table 2. Some physical-chemical parameters recorded at the collecting locality.
Physical-chemical parameters value
pH 7.07
Conductivity (µS/cm) 455 Water temperature (°C) 22.8 Dissolved oxygen (mg/L) 7.85 Dissolved inorganic nitrogen (ppm) 1.98 Dissolved inorganic phosphate (ppm) 1.52
Acknowledgements
The authors were grateful to Mr. Sheng-Yu Wu and Mr. Shi-Qiang Liu for their helps with field collection. This study was supported by Academic Sinica Grant APEC II (Asia Paleo-Environment Changes II) and National Science Council Grant NSC 92-2611-M-018-001.
References
Buchheim MA, Michalopulos EA and Buchheim JA. 2001. Phylogeny of the chlorophyceae with special reference to the Sphaeropleales: a study of 18S and 26SrDNA data. J. Phycol. 37: 819-835.
Cimino MT and Delwiche CF. 2002. Molecular and morphological data identify a cryptic species complex in endophytic members of the genus
Coleochaete Breb (Chlorophyta:
Coleochaetaceae) J. Phycol. 38: 1213-1221. Felsenstein J. 1985. Confidence limits on
phylogenies, an approach using the bootstrap. Evolution 39: 783-791.
Fitzgerald GP. 1981. Selective algicides. In: Environment and Water Quality Operational Studies, Technical Report E-81-7 of the US Environmental Protection Agency. Proceedings of Workshop on algal management and control. Office, Chief of Engineers, US Army,Washington, D.C. 20314. pp. 15-32.
Hall J and Payne G. 1997. Factors controlling the growth of field populations of Hydrodictyon
reticulatum in New Zealand. J. Appl. Phycol. 9:
229-236.
Hatano K and Maruyama K. 1995. Growth pattern of isolated zoospores in Hydrodictyon
reticulatum (Chlorococcales, Chlorophyceae).
6 Huang S and Chiang YM. 1986. Notes on
freshwater algae of Taiwan (I). Biol. Bull., NTNU. 21: 41-46.
Kimura M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16: 111-120.
Liu SL and Wang WL. 2004. Two new members of freshwater red algae in Taiwan: Compsopogon
tenellus Ling et Xie and C. chalybeus Kutzing
(Compsopogonaceae, Rhodophyta). Taiwania 49: 32-38.
MacReynold JS. 1961. Growth determination in
Hydrodictyon. Bull. Torrey Bot. Club. 88: 397-
403.
McManus HA and Lewis LA. 2005. Molecular phylogenetics, morphological variation and colony-form evolution in the family Hydrodictyaceae (Sphaeropleales, Chloro- phyta). Phycologica 44: 582-595.
Marchant HJ and Pickett-Heaps JP. 1971. Ultrastructure and differentiation of
Hydrodictyon reticulatum. Part II Formation of
zooids within the coenobium. Aust. J. Biol. Sci. 26: 471-486.
Marchant HJ and Pickett-Heaps JP. 1972a. Ultrastructure and differentiation of
Hydrodictyon reticulatum. Part III. Formation
of the vegetative daughter net. Aust. J. Biol. Sci. 25: 265-278.
Marchant HJ and Pickett-Heaps JP. 1972b. Ultrastructure and differentiation of
Hydrodictyon reticulatum. Part IV.
Conjugation of gametes and the development of zygospores and azygospores. Aust. J. Biol. Sci. 25: 279-291.
Marchant HJ and Pickett-Heaps JP. 1972c. Ultrastructure and differentiation of
Hydrodictyon reticulatum. Part V. Develop-
ment of Polyhedra. Aust. J. Biol. Sci.25: 1187-1197.
Marchant HJ and Pickett-Heaps JP. 1972d. Ultrastructure and differentiation of
Hydrodictyon reticulatum. Part VI. Formation
of the germ net. Aust. J. Biol. Sci. 25: 1199-1213.
Nozaki H, Ohta N, Takano H and Watanabe MM. 1999. Re-examination of phylogenetic relationships within the colonial Volvocales (Chlorophyta): an analysis of atpB and rbcL gene sequences J. Phycol. 35, 104-112.
Nozaki H, Misawa K, Kajita T, Kato M, Nohara S and Watanabe MM. 2000. Origin and evolution of the colonial volvocales (Chlorophyceae) as inferred from multiple, chloroplast gene sequences. Mol. Phylogenet. Evol. 17: 256-268.
Nozaki H and Krienitz L. 2001. Morphology and phylogeny of Eudorina minodii (Chodat) Nozaki et Krienitz, comb. nov. (Volvocales, Chlorophyta) from Germany. Eur. J. Phycol. 36, 23-28.
Nozaki H, Onishi K and Morita E. 2002a. Differences in pyrenoid morphology are correlated with differences in the rbcL genes of members of the Chloromonas lineage (volvocales, chlorophyceae). J. Mol. Evol. 55: 414-430.
Nozaki H, Takahara M, Nakazawa A, Kita Y, Yamada T, Takano H, Kawano S and Kato M. 2002b. Evolution of rbcL group IA introns and intron open reading frames within the colonial Volvocales (Chlorophyceae). Mol. Phylogenet. Evol. 23: 326-338.
Nozaki H, Misumi O and Kuroiwa T. 2003. Phylogeny of the quadriflagellate Volvocales (Chlorophyceae) based on chloroplast multigene sequences. Mol. Phylogenet. Evol. 29: 58-66.
Pickett-Heaps JD. 1975. Green Algae: Structure, Reproduction and Evolution in Selected Genera. Sinnauer Associates, Inc. Massachussetts.
Pocock MA. 1937. Hydrodictyon in South Africa with notes on the known species of
Hydrodictyon. Trans. R. Soc. S. Afr. 24:263
-281.
Pocock MA. 1960: Hydrodictyon: a comparative biological study. J. S. Afr. Bot. 26: 167-319. Prescott GW. 1970. Algae of the Western Great
Lakes Area. Iowa, W. M. C. Brown Publishers.
Sakayama H, Hara Y and Nozaki H. 2004. Taxonomic re-examination of six species of
Nitella (Charales, Charophyceae) from Asia,
and phylogenetic relationships within the genus based on rbcL and atpB gene sequences. Phycologia 43: 91-104.
Swofford DL. 2002. PAUP*-Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0﹡Sinauer Associates, Sunderland MA.
Tiwari GL, Singh SP and Pandey DC. 1980. An interesting observation on Hydrodictyon
reticulatum (L.) Lagerheim. Phycologia 19:
80-84.
Wang CL, Wang WL, Chen PC. 1999. The Investigations of Freshwater Algae of Min-Sion Area(Chia-Yi Taiwan). J. of Chia-Yi Normal College 13: 203-224.
Wells RDS, Hall JA, Clayton JS, Champion PD, Payne GW and Hofstra DE. 1999. The rise and fall of water net (Hydrodictyon reticulatum) in New Zealand. J. Aquat. Plant Manage. 37: 49-55
Wells RDS and Clayton JS. 2001. Ecological impacts of water net (Hydrodictyon
reticulatum) in Lake Aniwhenua, New
Zealand. N. Z. J. Ecol. 25: 55-63.
Wicox LW, Lewis LA, Fuerst PA and Floyd GL. 1991. Assessing the relationships of autosporic and zoosporic chlorococcalean green algae with 18S rDNA sequence data. J. Phycol. 28: 381-386.
Wu JT. 1999. Occurrence of four freshwater rhodophytes in Taiwan. Taiwania 44: 145-153. Wu JT. 2001. Supplements to the freshwater
*
通信作者:王瑋龍(Wei-Lung Wang);FAX:886-4-7295019;E-mail:wlwang@cc.ncue.edu.tw 8
臺灣新紀錄大型淡水藻:網藻屬及水網藻(網藻科,綠藻門)
周睿鈺
1張睿昇
2王瑋龍
1* 1國立彰化師範大學生物學系 2育達商業技術學院通識中心 (收稿日期:2005.11.21,接受日期:2006.2.20) 摘 要水網藻(Hydrodictyon reticulatum L. Lagerheim)為群體、非附著型式的絲狀綠藻,首次在臺灣北 部稻田中發現,為台灣淡水藻相的新紀錄屬及種。與其他淡水綠藻不同處在於其用肉眼可見到六邊 形網狀構造,以此構造形成一圓柱形的個體。本文對此新紀錄種屬之形態作詳細描述外,同時以部 分葉綠體ATP合成酵素之B次單位(atpB)基因序列確定其親緣關係的分類地位。