Paul Kwok-Keung Ho,' Kung-Kai Cheung," Shie-Ming Peng and Chi-Ming Che *?" Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong Department of Chemistry, National Taiwan University, Taipei, Taiwan
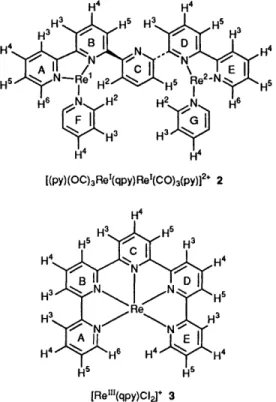
Three 2,2' : 6',2" : 6",2"' : 6"',2"-quinquepyridine (qpy) complexes of rhenium, [Cl(OC)3Re'(qpy)Re'(C0)3Cl] 1, [(py)(OC)3Re1(qpy)Re'(CO)3(py)][C104]2 2 (py = pyridine) and [Re"'(qpy)C1,][C1O4] 3, have been prepared and their physicochemical properties studied. The crystal structures of 2 and 3 have been determined: 2 is a monohelical bimetallic complex with two fac-tricarbonyl rhenium(1) moieties linked by the qpy ligand and the five pyridyl rings of qpy divide in the fashion bipy-py-bipy (bipy = bipyridine fragment and py = pyridine spacer); 3 is a seven-co-ordinated monohelical rhenium(Ir1) complex with pentagonal-bipyramidal geometry in which the two chloride ligands are trans to each other. Complexes 1 and 2 display photoluminescence in the spectral range 560-600 nm. The electrochemical properties of 2 and 3 are discussed.
Rhenium complexes containing pyridine-type ligands have received considerable attention in recent years. Rhenium(1) tricarbonyl complexes containing a-diimine ligands have been extensively used for photochemical studies. Their high stability, long excited-state lifetime, high emission quantum yield and the ease of tuning of the physicochemical properties through the variation of peripheral moieties accounts for their versatility. '3' Recent studies by Harman and co-workers also highlighted the potential applications of rhenium polypyridine complexes in organometallic chemistry. However, there are few examples of rhenium complexes containing long-chain oligopyridines and the recent examples are some seven-co-ordinated 0x0- and nitrido-rhenium(v) complexes of 2,2' : 6',2" : 6",2'"-quaterpyrid- showed that polydentate ligands based on oligopyridines spontaneously assemble mono- and bi-nuclear helical complexes upon reactions with Cu', Cu", Ag', Co", Ni" and Pd". Many of these examples were obtained with 2,2' : 6',2" : 6",2"' : 6"',2"- quinquepyridine (qpy). Herein we describe the preparation of monohelical bimetallic rhenium(1) and monohelical rhenium(iI1) complexes of qpy, their crystal structures, electrochemical and photophysical properties.
Studies by Constable and co-workers
Experimental
MaterialsPentacarbonylrhenium(r) chloride and silver perchlorate monohydrate were obtained from Strem Chemicals, lithium perchlorate from Aldrich. 2,2' : 6',2" : 6",2"' : 6"',2"-Quinque- pyridine and [ReVOC13(PPh3),]
*
were prepared according to the literature procedures. Tetrabutylammonium hexafluoro- phosphate was obtained from Southern Analytical Chemicals. Acetonitrile (Ajax, AR) was purified by treatment with KMnO, and then distilled over CaH,. Dichloromethane (Ajax, AR) was purified by washing with concentrated H2S04 followed by 5% aqueous Na,CO, solution and then distilled over CaH,. Methanol (Merck, GR) and all the other chemicals and solvents were used as received. All solvents for syntheses were analytical grade.Measurements
Proton NMR spectra were obtained on a JEOL 270 Fourier- transform spectrometer with tetramethylsilane as internal reference. Elemental analyses were conducted by Butterworth Laboratories Ltd. Electron impact (EI) and FAB mass spectra were collected on a Finnigan MAT 95 high-resolution
spectrometer, as were electrospray mass spectra using acetone as mobile phase. Cyclic voltammetry was performed with a Princeton Applied Research (PAR) model 175 Universal Programmer and model 173 potentiostat-galvanostat. The working electrode was glassy carbon. All measurements were made against Ag-AgNO, (0.1 mol dm-, in MeCN). The UV/VIS spectra were recorded on a Perkin-Elmer Lambda 19 UV/VIS/NIR spectrophotometer, infrared spectra as Nujol mulls on a Nicolet 20-FXC FT-IR spectrophotometer and steady-state emission spectra on a Spex Fluorolog-2 spec- trofluorometer. Emission lifetime measurements were per- formed with a Quanta Ray DCR-3 Nd-YAG laser system (pulsed output 355 nm, 8 ns). Solutions for photochemical experiments were degassed by at least four freeze-pumpthaw cycles.
Syntheses
[Cl(0C),Re1(qpy)Re1(CO),CI] 1. A mixture of [Re(CO),Cl] (100 mg, 0.24 mmol) and qpy (47 g, 0.12 mmol) in degassed methanol (20 cm3) was heated to reflux under an argon atmosphere for 12 h. The resulting bright yellow suspension was filtered off and washed with cold methanol. The yield was quantitative. The product was recrystallized by diffusion of diethyl ether into dichloromethane solution (Found: C, 37.20; H, 1.65; N, 7.10. Calc. for C,,H,7C12N,0,Re,: C, 37.30, H, 1.70; N, 7.00%). Mass spectrum (FAB): m/z 999 (1) and 964 (1 - Cl). IR (Cco/cm-', Nujol mull): 1902 and 2015.
[(PY)(oC),Re'(qPY)Re'(cO)~(PY)l [C10,1, 2 (PY = pyridine). A suspension of complex 1 (1 00 mg, 0.092 mmol) and AgCIO, (38 mg, 0.184 mmol) in degassed pyridine ( 5 cm3) was heated to reflux under an argon atmosphere in the dark for 8 h. The resulting greenish yellow solution was filtered through Celite and the volume of the solution was reduced under vacuum. The yellow slurry obtained was purified by chromatography on a 10 x 300 mm neutral alumina column and was eluted with a gradient of dichloromethane-acetonitrile. Two kinds of crystal, greenish yellow needles and prisms, were obtained by slow diffusion of diethyl ether into acetonitrile solution. They were found to have the same elemental compositions and absorption and emission properties, but their 'H NMR spectra were different. As discussed, these two crystals are suggested to be two stereoisomers (Found: C, 38.20; H, 2.00; N, 7.65. Calc. for C,,H,7CI,N70,4Re,: C, 38.30; H, 2.10; N, 7.65). 'H NMR (270 MHz, CD,CN, -35 OC, numbering of hydrogens as in Scheme 2): needle form, 6 9.20 (d, 1 H, HE3), 8.95 (d, 1 H, HA3),
8.63 (d, 1 H, HD3/D5), 8.57 (d, 1 H, HA6), 8.49 (d, 1 H, HD31D5), 8.43 (d, 2 H, HB31R5 and HE6), 8.35 (m, 4 H, HB4, Hc3/c5, HD4 and HE5), 8.30 (t, 1 H, HA4), 8.10 (d, 1 H, HR31B5), 8.06 (d, 1 H, Hc3ic5), 8.01 (dd, 1 H, HC4), 7.94 (d, 2 H, HG2), 7.85 (t, 1 H, HE4), 7.80 (t, 1 H, HF4), 7.67 (m, 3 H, HF2 and HA’), 7.23 (t, 2 H, HF3), 7.10 (t, 1 H, HG4), and 6.69 (t, 2 H, HG3); prism form, S 9.1 5 (d, 2 H, HA3 and HE3), 8.54 (s, 3 H, Hc3, Hc4 and HC5), 8.43 (t, 2 H, HB4 and HD4), 8.39 (d, 2 H, HB31B5 and ), 8.34 (d, 2 H, HA6 and HE6), 8.24 (t, 2 H, HA4 and HE4), 8.23 (t, 2 H, HF4 and HG4), 7.80 (d, 2 H, HB3iB5 and HD3’D5), 7.78 (d, 4 H, HF2 and HG2), 7.75 (t, 2 H, HA5 and HE5) and 7.25 (t, 4 H, HF3 and HG3). Mass spectrum (FAB): m / z 1087 (2) and 1008 (2 - py). IR (~co/cm-l, Nujol mull): 1917 and 2029.
H D ~ / D S
[Re1”(qpy)C1,] [ClO,] 3. A suspension of [ReOCl,(PPh,),] ( I 00 mg, 0.12 mmol) and qpy (46 mg, 0.12 mmol) in degassed ethanol (30 cm3) was heated to reflux for 24 h. The resulting deep blue solution was filtered and the filtrate treated with a saturated methanolic solution of LiCIO,. Deep blue microcrys- tals precipitated upon cooling and were filtered off and recrystallized by diffusion of diethyl ether into an acetonitrile solution. Crystals suitable for X-ray analysis were obtained by slow evaporation of an acetonitrile solution of the [Re”’(qpy)- C12][CI04] complex (Found for MeCN solvate: C, 41.55; H, 2.50; N, 10.70. Calc. for C27H,oC13N604Re: C, 41.35; H, 2.55; N, 10.70%). ‘H NMR 1270 MHz, (CD,),SO, numbering scheme as in Scheme 21: 6 8.99 (d, 2 H, HA3 and HE3), 8.89 (d, 2 H, HB3/B5 and HD31D5), 8.75 (d, 2 H, HB31B5 and HD3/D5), 8.63 (d, 2 H, Hc3 and HC5), 8.18 (t, 2 H, HA4 and H“), 7.83 (t, 2 H, HR4 and HD4), 7.51 (t, 2 H, HAS and HE5), 7.44 (d, 2 H, HA6 and HE6) and 7.41 (t, 1 H, Hc4). Mass spectrum (FAB): m / z 644 (3) and 609 (3 - Cl).
Crystal structure determinations
Complex 2. Crystal data. C41H27C12N701,Re2, M , =
1285.02, triclinic, space group P i (no. 2), a = 8.739(2), b =
83.77(1)”, U = 2161.4(8)A3,Z = 2, D, = 1 . 9 7 4 g ~ m - ~ , p ( M o - KK) = 57.98 cm-’, F(OO0) = 1236, T = 298 K.
A yellow, long thin needle crystal of dimensions 0.08 x 0.05 x 0.45 mm was used for data collection at 25 “C on an Enraf-Nonius CAD4 diffractometer at The University of Hong Kong with graphite-monochromatized Mo-Ka radiation ( h = 0.710 73 A) using 0-28 scans with a-scan angle (0.75
+
0.35 tan 8)’ at a scan speed of 1.73-5.49” min-’. Intensity data (28,,, = 42”; h 0-8, k - 14 to 14, 1 - 18 to 18; three standard reflections measured every 2 h showed no decay) were corrected for Lorentz and polarization effects, and empirical absorption corrections were based on the y~ scan of four strong reflections (minimum and maximum transmission factors 0.927 and 1 .OOO). Upon averaging the 5035 reflections, 4638 of which were uniquely measured (Rint = 0.019), 3476 with I > 30(I) were observed and used in the structural analysis. The centric space group P i was confirmed in the successful refinement of the structure which was solved by heavy-atom Patterson methods and expanded using Fourier techniques and refined by full- matrix least squares using the TEXSANga package on a Silicon Graphics Indy computer. All non-H atoms were refined anisotropically. Hydrogen atoms were placed at calculated positions with thermal parameters equal to 1.3 times that of the attached atoms but not refined. Convergence for 595 variable parameters by least-squares refinement of F with w = 4FO2/o2(Fo2), where 0 2 ( F o 2 ) = [ 0 2 ( I )+
(0.001 FO2)’] for 3476 reflections with I > 30(I), was reached atR = 0.028 and R‘ = 0.026 with a goodness of fit of 1.67; (A/O),,~ = 0.01. The final Fourier-difference map was
featureless, with maximum positive and negative peaks of 0.83 and 0.61 e
A-3
respectively.14.414(2), c = 18.717(3) A, K = 68.92(2),
p
= 79.64(2), y =Complex 3eMeCN. Crystal data. C2,H20C13N604Re, M , = 785.05, triclinic, space group P i , a = 9.023(12), b = 12.220(6),
L‘ = 13.729(6)
A,
K = 69.19(3),p
= 78.02(7), y = 83.69(8)”, U = 1383(2)A3,
2 = 2, D, = 1.885 g cm 3 , ~(Mo-Kcr) = 47.83 cm’,
crystal dimensions 0.20 x 0.20 x 0.20 mm, F(OO0) = 764.Intensity data were collected as for complex 2, at National Taiwan University, using the 0-28 scan mode with 28,,, =
45.0”. The crystal quality was not good. The peak width for the 8-28 scan was 1.1” in 0 and 2.2” in 28. An empirical uy-scan absorption correction was applied. Three sets of reflections with
x
values close to 90” and 10” intervals inw
were collected. A total of 1 1 I reflections were collected and the absorption curve as a function of uy was obtained and applied for absorption correction.’
All data reduction and structure refinement were performed using the NRCC-SDP-VAX p a ~ k a g e . ~ ’ The struc- ture was solved by the Patterson method and refined by least squares. The weighting scheme was w-’ = 0 2 ( F ) . The last least-squares cycle was calculated with 61 atoms, 371 para- meters and 3022 reflections (IIol > 2.O0lI0l) of 3606 unique reflections, giving R = 0.058, R’ = 0.057, goodness of fit =3.82. The final Fourier-difference map showed residual extrema in the range of -2.850 to 2.370 e 8,
’.
The atomic coordinates of the complexes are listed in Table 2 and selected bond distances and angles in Table 1.Complete atomic coordinates, thermal parameters and bond lengths and angles have been deposited at the Cambridge Crystallographic Data Centre. See Instructions for Authors,
J. Chem. SOC., Dalton Trans., 1996, Issue 1.
Results
and Discussion
The synthesis of 2,2‘ : 6‘,2” : 6”,2“‘ : 6”’,2””-quinquepyridine by the Krohnke method resulted in a high yield. The co-ordination versatility of qpy and related compounds has been demonstrated by the formation of many mono- and bi-metallic, single- and double-helical transition-metal complexes. Examples include [Fe,(bm~qpy),(O,CMe)]~ + [bmsqpy = 4‘,4”’-bis(methylsul- fanyl)-2,2’ : 6‘,2” : 6”,2“‘ : 6”’,2’’’’-quinquepyridine], [Co,(qpy),- (MeOH)], + 5 f . g [bcpqpy = 4’,4”’-bis(p-chlorophenyl)-2,2’ : 6’,
2“ : 6”,2”’ : 6”’,2”’’-quinquepyridine), [Ni,(qpy),(O,CMe)] + , 5 d [Pd2(qpy),14 + 5 h and [Ag(qpy)] +
.
5 b Scheme 1 illustrates the three possible bonding modes of qpy. It usually functions as ( 0 2 c ~ e ) 1 3 + , 5 e CCO,(~PY),I~+ , 5 e CCo(bcPqPY )(H 2 0)-CCu,(qPY),(O2Cwl3 + ,5a*c CZn,(bmsqpy),(O2CMe)I3 + 7
’
’
two discrete units (mode 1) but there are some cases where it acts as a pentadentate unit (mode 2) in which all the five pyridyl donors co-ordinate to one metal centre. In addition, there is a special bonding mode, in [Pd2(qpy)2]4+,5h in which each
Mode 2
Mode 1
Mode 3
Scheme 1
transition-metal cations
The three possible co-ordination modes of qpy with
H4 H4
H4
[Re'11(qpy)C12]+ 3
Scheme 2 Numbering scheme of hydrogens and pyridyl rings of qpy
rhenium complexes for assignment of 'H N M R spectra; for simplicity, bonded carbonyls of 2 and chlorides of 3 are omitted
palladium is in an irregular five-co-ordinate environment with four short contacts (1.941-2.085
A)
to a terpyridyl fragment of one ligand and a terminal pyridine from the other. The co- ordination sphere is completed by a long contact ( z 2.6A)
to the remaining pyridine of the second ligand.Scheme 2 shows the numbering scheme of the hydrogen atoms and pyridyl rings of qpy in complexes 2 and 3. In complexes 1 and 2 the bonding of the qpy ligand could be in the fashion bipy-py-bipy (mode 3, bipy = bipyridine fragment and py = pyridine spacer). This co-ordination mode could be rationalized by the fact that the Re'(CO), unit usually possesses a fuc geometry.'*2 Furthermore, owing to the higher steric hindrance encountered in forming the py-(Re-bipy)-(bipy-Re) bonding mode, the (Re-bipy)-py-(bipy-Re) mode is preferred. The solubility of complex 1 is very poor, but 2 has much higher solubility in MeCN and MeOH. The synthesis of [Re"'(qpy)CI,] + 3 is suggested to proceed through in situ reduction of [ReVOC13(PPh),] in ethanol. Rhenium(rr1) complexes containing pyridine-type ligands are not extensive. Some examples include [ReCl,(bipy)(PPh,)] (bipy = 2,2'-bipy- ridine) l 2 [ReC1(2Me-py)(en),12 + l 3 (2Me-py = 2-methylpyrid- ine, en = 1,2-diaminoethane), [ReO(terpy)(SC,H,Me-O,l+ and very recently [Re(terpy),C1I2+ (terpy = 2,2' : 6',2"-terpy- ridine). None of them was prepared from [ReOCI,(PPh,),] despite the fact that it is a useful starting material for the rhenium(II1) complexes of phosphines and alkyl isocyanides. It is interesting that the reaction between [ReOC13(PPh3),] and 2,2' : 6',2": 6",2"'-quaterpyridine (qtpy) under similar reaction conditions as in the preparation of 3 gives The crystal structures of complexes 2 and 3oMeCN have been determined. Figs. 1 and 2 show perspective views of the cations tively. The structures feature the first examples of rhenium- qpy complexes. Although two kinds of crystal were found for 2, only the structure of the needle-shaped crystal was determined. As shown in Fig. 1, the complex adopts a bimetallic monohelical structure with each rhenium atom in a distorted- octahedral geometry and the three carbonyls in a j u c [ReV(qtpy)O(OMe),l + .4
C(PY)(oC),Re(qPY)Re(~o~3(PY)12 + and CRe(qpy)CI,I + respec-
Fig. 1 Perspective view of complex 2
C( 13)
Fig. 2 Perspective view of complex 3
arrangement. The measured Re-N distances range from 2.154(8) to 2.225(8)
A,
which are comparable to the related values in [Re'(diimine)(CO),(py)] + complexes such as [Re(dmphen)(CO),(py)] '(dmphen = 4,7-dimethyl-l, 10-phen- anthroline.'
There is no n-n interaction within the monoheli- cal bimetallic complex. The two pyridines, the two bipyridine units and the central 1,3-pyridyl spacer are essentially planar. With reference to the numbering scheme given in Scheme 2, the dihedral angle between planes 1 (ring F) and 2 (A and B) is 120.50", that between 3 (G) and 4 (D and E) is 96.63". The dihedral angles between the pyridyl spacer plane 5 (ring C) and planes 1, 2, 3 and 4 are 55.00, 110.39, 53.53 and 112.01" respectively. The principal twists of the interpyridyl bonds of the qpy ligand in 2 occur a t C(21)-C(22) (between planes 2 and 5 ) and C(26)-C(27) (between 5 and 4) with dihedral anglesTable 1 Selected bond distances (A) and angles (") of [(py)(OC),Re'- (4PY )Re'(CO) 3(PY )I CC104lZ 2 and CRe"'(qpy )C121 CC1O,I*MeCN 3 Complex 2 Re(1)-N( 1) 2.225(8) N(1)-Re(l)-C(l) 176.1(4) Re( 1 )-N(2) 2.1 54(8) N(2)-Re( 1)-C(3) 172.8(4) Re(1)-N(3) 2.216(7) N(3)-Re(l)-C(2) 173.5(4) Re(2)-N(5) 2.223(7) N(5)-Re(2)-C(6) 171.0(4) Re(2)-N(6) 2.161 (8) N(6)-Re(2)<(5) 174.9(4) Re(2)-N(7) 2.169(8) N(7)-Re(2)-C(4) 177.1(4) Complex 3 Re-CI 2.430(5) Cl-Re-Cl 178.5(2) Re-N( 1 ) 2.073( 14) N( 1 )-Re-N(2) 70.8(5) Re-N(2) 2.104( 13) N(2)-Re-N( 3) 77.3(5) Re-N( 3) 2.07 1 ( 1 3) N( 3)-Re-N(4) 70.7(5) Re-N(4) 2.050( 14) N(4)-Re-N( 5 ) 75.1(5) Re-N( 5 ) 2.164( 13) N(5)-Re-N( 1) 75.3(5)
being 110.39 and 112.01" respectively. There are minor twists at the interpyridyl bonds of the two bipyridine units (3.98 and 7.55" respectively). The Re Re separation is 7.723 A, which precludes any direct interaction.
Seven-co-ordinated rhenium(rI1) complexes have been reported " 9 ' and the ones containing pyridine-type ligands are [Re(terpy),XI2+ (X = CI-, OH- or NCS-)." The rhenium atom in the monohelical [Re(qpy)Cl,]+ complex 3 is in a distorted pentagonal-bipyramid geometry with the two C1 atoms trans to each other [Cl(l)-Re-C1(2) 178.53"]. The two measured Re-Cl distances [2.430(5)
A]
are much longer than that of the other related trans-dichlororhenium(rrr) complexes such as 2.337(1) 8, in [Re(dmpe),C12]+,'9 2.322(3) and 2.331(3) 8, in [ReCl,(CNPr'),(PMePh,),]+ 18' and 2.349(1) 8, in[ReCl,(ampy),] +
'
where ampy = 2-(aminomethy1)pyridine and dmpe = 1,2-bis(dimethyiphosphino)ethane. This could be rationalized by the rhenium atom in the seven-co-ordinated complex 3 being more electron rich than that in six-co- ordinated complexes.The 'H NMR spectra of the two forms of complex 2 in CD,CN at -35 "C are shown in Fig. 3. Owing to the restriction imposed by the facial geometry of the two tricarbonylrhenium(1) units, only three stereoisomers (each has its enantiomer) are feasible, the structures of which are illustrated in Scheme 3. As described in the Experimental section, only two kinds of crystals of 2, the yellow needles and prisms, have been obtained. From the X-ray analysis the needle crystal corresponds to isomer 2a. As there exists no mirror plane nor rotational symmetry in 2a, its 'H NMR spectrum is more complex than that of 2b and 2c. The spectrum of the yellow prism crystals reveals a centre of symmetry in the molecule. As a result, it could be isomer 2b or 2c, both of which have a two-fold rotational axis. These two isomers could interconvert through rotation of the interannu- lar bonds between rings C and D and D and E (Scheme 3). The infrared spectra for 1 and 2 display two carbonyl- stretching bands, consistent with a facial tricarbonyl structure. The absorption, emission and photophysical data of 1 and 2 are listed in Table 3. The absorption spectra show broad and structureless lowest-energy absorptions at 350-385 nm, which are very similar to the metal-to-ligand charge- transfer (m.1.c.t.) transitions offac-[Re(bipy)(CO),Y]"+ (Y = C1 or py),ly2 and hence they are tentatively assigned as 7c*
(qpy) c- d, (Re') charge transfer in nature. Complexes 1 and
2 show photoluminescence in the spectral range 560-600 nm. The emission spectra of 2 measured in MeOH at room temperature and at 77 K are shown in Fig. 4. The excitation spectra closely match the corresponding absorption spectra. In both cases the emission bands are broad and featureless. For 2 the emission maximum measured in methanol glass at 77 K shows a substantial blue shift of 1320 cm-' when compared
(a 1 9.0 8.6 8.2 7.8 7.4 7.0 6.6 HWm5 a n d HD3/O5 HA6, and HB4 a n d ~ € 6 - HA3 a n d HE3 ( b 1 H", Hc4 and Hcs
7
I HA' a n d HE4 HF' a n d Ha HF2 a n d HG2ii
9.2 8.8 8.4 8.0 7.6 7.2 6.8 6 Fig. 3crystal, ( b ) prism-form crystal
Proton NMR spectra of complex 2 in CD3CN: ( a ) needle-form
co
\ co 2a co P Y J R d J p R f P Y \co
co 2b 2cScheme 3 The three stereoisomers of complex 2
with that recorded in the same solvent at room temperature. Changing the solvent from CH,Cl, to MeCN also causes a blue shift of the emission maximum and shortening of the luminescence lifetime. These observations are reminiscent of the m.1.c.t. excited state of rhenium(1)-a,a'-diimine com- plexes. ' 9 ,
The 'H NMR spectrum of complex 3 in (CD,),SO with assignments is given in Fig. 5. It can be rationalized on the basis
Table 2 Atomic coordinates of non-hydrogen atoms for complexes 2 and 3 Atom X Complex 2 Re( 1) 0.255 96(5) Re(2) 0.1 88 70(5) C(1) 0.1 17 3(4) C(2) 0.505 8(5) O(1) -0.012(1) 0.121 (1) o(2) (43) 0.034 7(9) O(4) 0.508 4(10) O(5) 0.102( 1) O(6) 0.320(1) O(7) 0.394(1) O(8) 0.624( 1) O(9) 0.438(2) O(10) 0.537(2) O( 1 1) 0.265( 1) O(12) 0.1 13(1) O(13) 0.00 1 ( 1 ) O(14) 0.086(1) N(1) 0.458 9( 10) N(2) 0.434 6(9) N(3) 0.366 4(9) N(4) 0.171 4(9) N(5) 0.104 8(9) N(6) 0.230 4(9) C(1) 0.093( 1) N(7) -0.040 5(9) (22) 0.180( 1) (23) 0.121(1) C(4) 0.388( 1) C(5) 0.133( 1) C(6) 0.269( 1) C(7) 0.555(1) C(8) 0.688(2) Complex 3 Re 0.01 I 9(1) CK2) 0.164 7(5) N(I) 0.045 9( 16) Cl(1) -0.146 7(5) N(2) -0.114 4(18) N(3) -0.146 6(17) N(4) 0.05 1 4( 16) N(5) 0.241 4(14) C(1) 0.097 2(22) C(2) 0.131 6(23) C(3) 0.1 10 3(23) (24) 0.040 7(25) C(5) 0.011 8(22) C(6) -0.084 l(21) C(7) -0.146 9(2l) C(8) -0.250 O(24) C(9) - 0.289 4(22) C( 10) - 0.227 8(20) C( 1 1) - 0.253 7(22) C(12) -0.362 4(21) Y 0.246 92(3) 0.206 16(3) 0.172 7(2) 0.502 O(4) 0.395 2(7) 0.370 2(6) 0.202 2(6) 0.035 4(6) 0.039 4(6) 0.464( 1) 0.528(2) 0.593( 1) 0.436 7(9) 0.204 7(7) 0.144 2( 9) 0.245 7(7) 0.090 O(6) 0.332 7(6) 0.172 2(6) 0. I34 7(5) 0.225 2(6) 0.344 2(6) 0.326 3(6) 0.21 1 4(6) 0.166 5 ( 8 ) 0.340 7(9) 0.31 8 5(9) 0.207 O(7) 0.103 6(8) 0.100 5(8) 0.371 6(8) 0.417 8(9) 0.1 18 5(7) 0.208 90(7) 0.382 O(4) 0.033 2(4) 0.305 5( 12) 0.138 8(13) 0.1128(12) 0.217 7(12) 0.267 4( 12) 0.414 5(16) 0.472 O( 17) 0.41 9 2( 18) 0.310 l(19) 0.257 8( 16) 0.156 6(16) 0.093 2( 17) 0.008 6( 18) 0.054 2( 15) 0.050 2( 17) -0.01 19(16) -0.013 2(17) Z 0.032 6 l(2) 0.459 85(2) 0.785 6(2) 0.274 7(2) 0.046 6( 5) 0.1 12 8(4) 0.360 l(5) 0.41 6 O(4) 0.591 2(5) 0.249 2(8) 0.220 4(8) 0.290( 1) 0.344 9(7) 0.749 9(6) 0.864 7(6) 0.759 6(7) 0.771 4(7) 0.024 9(5) 0.127 4(4) 0.261 8(4) 0.372 6(4) 0.496 l(5) 0.526 3(5) 0.041 2(6) 0.086 2(6) 0.396 9(6) 0.430 O ( 5 ) 0.543 l(6) -0.107 2(5) - 0.025 4(4) -0.057 3(7) -0.042 4(7) - 0.047 8( 10) 0.150 66(6) 0.081 O(4) 0.223 3(4) 0.240 9( 1 1) 0.304 6(10) 0.129 7(10) -0.0044(11) 0.088 2( 12) 0.206 6( 14) 0.266 O( 15) 0.375 5( 17) 0.419 4(14) 0.352 5( 15) 0.383 5( 13) 0.490 7( 14) 0.506 7( 15) 0.422 2( 15) 0.310 2(16) 0.214 3( 13) 0.205 l(15) X 0.724(2) 0.628(2) 0.495( 1) 0.460( 1) 0.592(2) 0.704( 1) 0.678( 1) 0.544(1) 0.507(1) 0.605( 1) 0.564(1) 0.421( 1) 0.321( 1 ) 0.168( 1) 0.032( 1) - 0.107( 1) - 0.107( 1) 0.033( 1) 0.039( 1) - 0.020( 1) 0.054( 1) 0.115(1) 0.191(1) 0.222( 1) 0.288( 1) 0.324( 1 ) 0.295( 1) -0.028(1) - 0.061( 1) -0.203(1) -0.337(1) - 0.3 18( 1) - 0.170(2) - 0.370 6(24) -0.267 9(22) -0.160 3(21) -0.044 5(21) - 0.036 O(22) 0.086( 3) 0.184 O(21) 0.165 7(21) 0.278 5(22) 0.423 3(21) 0.520 6(21) 0.485 4(22) 0.343 3(20) 0.350 8(6) 0.198 5(17) 0.41 I 6(22) 0.368 3( 19) 0.422 9(23) 0.461 8(24) 0.537(3) 0.637(3) Y 0.424( 1) 0.388 2(10) 0.342 7(8) 0. I88 8(8) 0.153( 1) 0.097 7(9) 0.078 2(8) 0.1 14 4(7) 0.095 8(7) 0.041 0(8) 0.021 5(8) 0.058 0(8) 0.115 2(7) 0.154 l(7) 0.1 12 9(8) 0.150 3(9) 0.226 9(8) 0.260 5(7) 0.348 4(7) 0.438 9(9) 0.523 7(9) 0.520 3(7) 0.428 O(7) 0.419 2(8) 0.500 8(8) 0.488 3(9) 0.393 5( 10) 0.314 5(8) 0.200 9(9) 0.201(1) 0.21 O( 1) 0.220( 1) 0.219 3(9) 0.012 4(18) 0.047 5( 18) 0.109 8(16) 0.175 7(15) 0.193 O(17) 0.257 8( 18) 0.303 O(16) 0.278 3(16) 0.308 O( 15) 0.351 8(17) 0.352 O(18) 0.305 7(18) 0.266 4( 16) 0.710 8(5) 0.706 4( 15) 0.768 7( 17) 0.772 3( 15) 0.603 5( 15) 0.233 3(17) 0.303 8(21) 0.387 4(20) 7 0.018(1) 0.087 6( 10) 0.088 l(7) -0.102 2(6) -0.137 5(6) - 0.095 4(8) 0.017 O(7) 0.01 7 6(6) 0.101 5(6) 0.153 l(7) 0.230 8(7) 0.256 7(6) 0.204 6(5) 0.232 9(5) 0.232 8(6) 0.260 6(6) 0.287 5(6) 0.287 6(5) 0.311 3(5) 0.266 5(6) 0.281 2(7) 0.341 l(6) 0.387 2(5) 0.453 3(5) 0.471 2(6) 0.534 O(7) 0.579 6(7) 0.560 5(7) 0.601 l(6) 0.646 9(6) 0.61 5 9(7) 0.539 l(7) 0.498 9(6) 0.108 2(16) 0.022 2( 15) 0.037 9(14) - 0.044 8( 1 3) -0.149 5(14) - 0.220 7( 14) -0.183 4(14) - 0.077 O( 13) - 0.028 6( 12) -0.075 2( 14) - 0.009 O( 16) 0.098 7( 14) 0.140 7( 12) 0.331 l(4) 0.368 6( 12) 0.381 9(12) 0.216 6(12) 0.344 5( 15) 0.363 6(14) 0.364 2( 17) 0.359 2( 19)
Table 3 Absorption, emission and photophysical data for complexes 1 and 2
Absorption," h/
Complex nm (&/dm3 mol-' cm-I) Emission," h/nm TOb/ns 44
1' 385 ( 5 670) 605 50 8.5 x 10-4
2' 360 (1 2 600) 565 400 0.038
2 d 350 ( 13 600) 570 250 0.01 7
2' 350 ( 1 3 000) 570 240 0.017
2 1 530
" Absorptions and emissions of the lowest-energy maxima. Lifetimes and luminescence yields were obtained by monitoring the corresponding lowest-energy emission maxima at 298 K and the luminescence yield data are referenced to [R~(bipy)~]'+ in degassed water. In CH'Cl, solution at 298 K. In MeCN solution at 298 K . ' In MeOH solution at 297 K . In MeOH glass matrix at 77 K .
of first-order coupling and shows five doublets and four triplets indicating a centre of symmetry on the qpy ligand. The protons
are all displaced downfieid compared with those in other double-helical transition-metal complexes of qpy. 5 , 6 Presum-
35 30 .E 25
*
e
202
15 10 5 0 h v) 3 c2
c .- v)-
400 500 600 700 800 UnmFig. 4 Emission spectra of complex 2 measured in MeOH at 298
HB4 and HM
I HA6 and HE6
1
HA5andHE5!I
-
->, '7 7 I " 'I " " I 1 , " , " ' " I " " " '.- ' " " I " ' ' ' ' ' ' ~ B ' . '9.0 8.5 8.6 8.4 8.2 8.0 7.8 7.6 7.4
6
Fig. 5 Proton NMR spectrum of complex 3 in (CD,),SO
ably this is due to lack of n stacking in 3. This is good evidence that [Re(qpy)CI,] + remains monomeric in solution. The molecular structure of 3 in solution has also been verified by its electrospray mass spectrum. This shows one group of peaks centred at mjz = 644, with a virtually identical isotopic mass distribution to that calculated for the parent mass peak of [Re(qpy)CI,]+. This implies that 3 remains as a monomeric cation in solution. The UVjVIS spectrum exhibits two intense absorptions at 453 (E = 4590) and 570 nm ( I = 10 010 dm3 mol- cm-'> in acetonitrile solution at room temperature. Since intense visible absorptions are also found for other rhenium(m-- polypyridine complexes such as [Re(en),(py)CI] +, l 3 [Re(bi-
py)(PPh,)CI,] l 2 and [Re(terpy),C1]2+,'S but are absent for
[Re111(PR3)3X3],20 we tentatively assign the absorption bands of 3 to the d, (Re"') __t n* (qpy) m.1.c.t. transition.
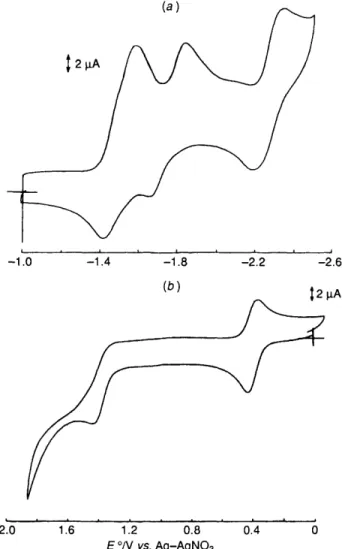
The cyclic voltammograms of complexes 2 and 3 in MeCN
are shown in Fig. 6. Complex 2 displays three quasi-reversible reduction waves and a broad irreversible oxidation wave. The latter is tentatively assigned to the oxidation of Re' since the qpy ligand is electroinactive at this potential. The two reduction waves at - 1.48 and - 1.73 V are absent for 3, and hence they are assigned to sequential reduction of the two Re' in the bimetallic complex 2. If 2 behaves as two discrete
[Re(diimine)(CO),(py)] + there should be only one two- electron metal-centred reduction wave. Thus a certain extent of electronic coupling, represented by the 250 mV difference in potential between the two reduction waves, exists between the two Re' upon reduction. In fact, the broadness of the irreversible oxidation wave could also be rationalized by
-1
.o
-1.4 -1.8 -2.2 -2.6I 1
2.0 1.6 1.2 0.8 0.4 0
EON vS. Ag-AgNOB
Fig. 6 Cyclic voltammograms of complexes 2(a) and 3(b)
the potentials required for the oxidation of the two rhenium(1) centres, to be close to each other. Similar electrochemical behaviour has been observed for other bimetallic double-helical metal complexes. The remaining reduction wave at - 2.28 V,
which is also present for 3, is tentatively assigned as a ligand- based reduction. For 3 two reduction waves are found at - 1.12 and - 1.25 V. With reference to previous electrochemical
studies on the related rhenium(rI1) complexes such as [Re(ampy),CI,] + , I 3 the reduction waves would involve the
couple Re"'-Re". There is a reversible oxidation wave with E ,
at +0.38 V, which is tentatively assigned to oxidation of Re" to Re". An additional irreversible oxidation wave was found at
+
1.4v.
Acknowledgements
We acknowledge support from The University of Hong Kong, the Hong Kong Research Grants Council and The Croucher Foundation.
References
1 M. Wrighton and D. L. Morse, J . Am. Chem. Soc., 1974, 96, 998; M . Wrighton, Chem. Rev., 1974,74,4801.
2 ( u ) J. V. Caspar and T. J. Meyer, J. Phys. Chem., 1983,87, 952; ( h )
L. A. Sacksteder, M . Lee, J. N. Demas and B. A. Degraff, J . Am. Chem. Soc., 1993,115, 8230.
3 L. E. Helberg, J . Barrera, M. Sabat and W. D. Harman, Znorg. Chem., 1995,34,2033.
4 C . M. Che, Y. P. Wang, K . S. Yeung, K . Y. Wong and S. M. Peng,
J . G e m . Soc., Dulton Trans., 1992, 2675.
5 (u) E. C. Constable, M. G. B. Drew and M. D. Ward, J. Chem. Soc.,
Chem. Commun., 1987, 1600; ( h ) E. C. Constable, M. G. B. Drew, G. Forsyth and M. D. Ward, J. Chem. Soc., Chem. Commun., 1988,
1450; (c) M . Barley, E. C. Constable, S. A. Corr, R. C. S. McQueen,
J. C. Nutkins, M. D. Ward and M. G . B. Drew, J. Chem. Soc., Dalton Trans., 1988, 2655: ( d ) E. C. Constable, M. D. Ward,
M. G. B. Drew and G. A. Forsyth, Polyhedron, 1989, 8, 2551; (e) E. C. Constable, S. M. Elder, P. R. Raithby and M. D. Ward, Polyhedron, 1991, 10, 1395; ( f ) E. C. Constable, J. V. Walker, D. A. Tocher and M. A. M. Daniels, J. Chem. Soc., Chem. Commun., 1992, 768; ( g ) E. C. Constable, M. A. M. Daniels, M. G. B. Drew,
D. A. Tocher, J. V. Walker and P. D. Wood, J. Chem. Soc., Dalton Trans., 1993, 1947; ( h ) E. C. Constable, S. M. Elder, J. Healy and M. D. Ward, J. Am. Chem. Soc., 1990,112,4590.
6 E. C. Constable and R. Chotalia, J. Chem. Sue., Chem. Commun., 1992, 64: E. C. Constable, M. J. Hannon, A. Martin and P. R. Raithby, Polyhedron, 1992, 11,2967.
7 F. Krohnke, Synthesis, 1976, I .
8 G. W. Parshall, lnorg. Synth., 1977, 17, 110.
9 ( a ) TEXSAN-TEXRAY Structure Analysis Package, Molecular Structure Corporation, Houston, TX, 1985: ( h ) NRCVAX, E. J. Gabe, Y. Le Page, J. P. Charland, F. L. Lee and P. S. White, J. Appl.
Crystallogr., 1989, 22, 384.
10 A. C. T. North, D. C. Philips and F. S. Mathews, Acta Crystallogr., Sect. A , 1968,24, 35 I .
1 1 K. T. Potts, M. Keshavarz-K, F. S. Tham, H. D. Abruna and
12 J. V. Caspar, B. P. Sullivan and T. J. Meyer, lnorg. Chem., 1984,23, 13 S. D. Orth, J. Barrera, M . Sabat and W. D. Harman, lnorg. Chern., 14 L. Chang, J. Rall, F. Tisato, E. Deutsch and M. J. Heeg, lnorg. Chim.
15 J. Rall, F. Weingart, D. M. Ho, M. J. Heeg, F. Tisato and 16 G. Rouschias and G. Wilkinson, J. Chem. Soc. A , 1967,993. 17 L. Wallace, C. Woods and D. P. Rillema, Znorg. Chem., 1995, 34,
2875.
18 ( a ) S. Warner and J. Lippard, Inorg. Chem., 1989, 28, 3008; ( h ) J.-M. Manoli, C. Potvin, J.-M. Bregeault and W. P. Griffith, J. Chem. Soc., Dalton Trans., 1979, 192.
19 J.-L. Vanderheyden, M. J. Heeg and and E. Deutsch, horg. Chem.,
1985,24, 1666.
20 H. P. Guntz and G. P. Leigh, J. Chem. Soc. A , 1971,2229. C. Arana, lnorg. Chem., 1993, 32, 4436.
2104. 1993, 32, 594. Acta, 1993, 205, 35.
E. Deutsch, Inorg. Chem., 1994,33, 3442.