Purifi'cation
and Characterization of Sucrose Synthetase
from the
Shoot of Bamboo
Leleba
oldhami1
2
Received forpublication May 21, 1976 and in revised form February 24,1977
JONG-CHING SU
Department ofAgricultural Chemistry, National Taiwan University, andInstituteof
Biological Chemistry,
Academia Sinica
JEN-LEIH Wu
Institute
of Zoology, Academia Sinica
CHAo-LUAN YANGInstitute
of Biological Chemistry, Academia
Sinica, Taipei, Taiwan,Republic of China
ABSTRACT
A105-fold purificationofthesucrosesynthetasefrom theextractof
the shoot of bamboo Lelaba oldkami was achieved by ammonium
suffatefsctionation, calium
pbosphate
gel adsorption,andcbromato-grapuic
separatiomouSephadex
G-100aaddietbylamiaoetbyl-ceulosee_sluns. Some properties of this enzyme, namely thermal and pH taiities, stabilization by aqueous glycerol, pH optimum, substrate
spedilcities,effects ofmetallic ios,effects ofsufydrylreagents,
mo-bllr
weight,
sedimentationconstants,isoelectricpoint, and sbdstrate sturationkinetics had been investigated.Thesubstratesturationkinetics indicated thattheenzymecould be an aflostercenzyme withthe saccharide substrtes (sucrose and fuc-tose) serving as the
homotropic
alosterc effectors inreglating
thebiosynthesis
anddegradationofsucrose.These results imply that thesynthesis of different polysacchar-ides at the different stages of plant growth is regulated by the availability ofsomesugarnucleotidesnotonlyasthe substrates but also as the modulators. It therefore becomes necessary to investigate into the sugarnucleotide-synthesizingsystems ofthe plant.
UDPG is doubtless the precursor of all sugar nucleotides mentioned above. Fromthephysiological propertiesofbamboo shoot and the resultsof apreliminary survey, wesuspectedthat UDPG could be supplied by the reaction catalyzed by sucrose synthetase in the direction ofsucrosecleavage. Sucrose synthe-tase from bamboo shoot may be aregulatory enzyme which is moreimportantforsynthesis ofUDPG rather than forsynthesis
ofsucrose.Here,we report thepropertiesofsucrosesynthetase
purified from the shoot of bamboo L. oldhami, especiallythose relevant to thepostulatedphysiologicalfunctions of the enzyme explained above.
EXPERIMENTAL PROCEDURES
Aseries of studies aimedattheelucidation of the biochemical mechanism of cell wall maturation in higher plants have been conducted in ourlaboratory. The shoot of bambooLeleba old-hami has been chosen as the main research material. Previous investigations revealed the types of glycosidic linkages presentin bamboo cell wall polysaccharides (14),the distribution of sugar nucleotidesinthe tissue(12), andthechanges ofpolysaccharide constituentsaccompaniedwiththegrowthand maturation of the plant(13). Unlike many other plants, bamboo shoot is incapable ofutilizing GDP-D-glucose, which could not be detected in the plant,asthe precursor for synthesizing cell wallpolysaccharides.
UDPG,themost abundant soluble nucleotidein bamboo shoot
(12), is agood precursor for synthesizing a 83-1,3-glucan (15). Thissynthetic ability is strongly inhibited by UDP-D-xylose(and moderately by xylose and xylobiose), which has been demon-stratedtobeagood precursor ofbambooshoot pentosans(16). Thepentosansynthesisfrom theprecursor UDP->-xylose, which israpidly isomerized toamixture of UDP->-xylose and UDP-L-arabinoseby the pentosan synthetase preparation,isinhibitedby UDP-D-galactose, theprecursor ofbamboo shootgalactan.
1This work wassupported bya grant to J. C. S. from the National ScienceCouncil, Republicof China.
2Part II in the
series
"SucroseSynthetase." ForPartI,seeYeh DB,JC Su 1972 J Chinese Biochem Soc 1: 37.
MATERIALS
The shoots of bamboo L. oldhami grown in the vicinity of Taipei were sampled. Only the edible part was used as the enzyme source. All of the commercially available chemicals except UDPwereused withoutpurification. The UDP obtained from the Sigma Co. was contaminated with UDPG and UMP, theformer of which interfered with the assay of sucrose synthe-taseby the UDPG dehydrogenase coupled method. These con-taminants were removed by paper chromatography according to the method ofPaladini andLeloir(10). The filter paper used for thispurification procedure was prewashed in sequence with 1 M oxalic acid anddistilled H20 and dried. Calcium phosphate gel wasprepared according to Keilin and Hartree (4).
METHODS
The protein content was estimated with Lowry's phenol method (6) using crystalline BSA as the standard.
Indensity gradientcentrifugationanalyses, a linear gradient of sucrosefrom 5 to20% and ofglycerol from 8 to33% (specific
gravities from 1.0173 to 1.0806) were employed. The density gradientcolumns werecentrifuged inaswingingbucket rotor at 112,000gand 4Cfor 10 hr. Yeastalcoholdehydrogenase
(S20,
a = 6.72) and fibrinogen (s20 , = 7.63, mol wt 341,000) were used as the marker proteins and the results were calculated 17SU, WU, AND YANG Plant
Physiol.
Vol.60,
1977 according to Martin and Ames (7). Molecular weight of theenzyme was estimated by gel filtration through a Sephadex G-200 column according to Andrews (1) and also from the sedi-mentation data (7). Bovine y-globulin, bovine hemoglobin, horse heart Cyt c, yeast alcohol dehydrogenase, and apoferritin were used in calibrating the column.
Isoelectric focusing of the enzyme was carried out in a density gradient electrophoresis apparatus from the Instrumentation Specialties Co., Lincoln, Neb., according to the procedure de-scribed in the brochure supplied by the manufacturer (8). The
carrierAmpholine with a pH range of 3.5 to 10 was purchased from LKB, Sweden. Total focusing time was 24 hr during which period the column was maintained at 10 C by circulating chilled waterthrough the jacket of the column. The enzyme obtained from the gel filtration step was used in this experiment.
The activity of sucrose synthetase was assayed by one of the
followingmethodsaccording to the situation.
Method1. DeterminationofUDPGby UDPG
Dehydrogen-ase-CatalyzedReaction. The method reported by Avigad(2)for the assay of sucrose synthetase from sugar beet was found not suitable for the assay of bamboo shoot enzyme because the bamboo shoot enzyme hadapHoptimum much lower than that ofbovine liver UDPG dehydrogenase. The following two-step method was developed.
The reaction mixture (0.80 ml) contained 50 ,umol sucrose, 0.1
itmol
UDP, 1 ,umol MgCI2, 0.1itmol
EDTA, 1 j,mol 2-mercaptoethanol, and 25;Lmol
sodium phosphate (pH6). After temperature equilibration at 37 C, the reaction was started byadding 0.1 ml of properly diluted enzyme solution. A blank without the addition of UDP was run simultaneously. After 10 min ofincubation, 0.1 ml of 1 M tris-HCl (pH 8.8) was added and themixture heated ina waterbathat68C for 10 mintostop thereaction. Eight-tenths ml of the reaction mixturewas
trans-ferredintoacuvette,towhich50 ,ul of 20mmNAD+and 140,ul ofH20wereadded. After addition of 10 ,ul (33units) of UDPG
dehydrogenase,the absorbance readings at340 nm of the solu-tionwererecorded untilnofurtherincrease could be noted. The net increase in the reading corrected for the blank value was
taken for calculating the amount of UDPG produced. This methodwasthe one most used in thisinvestigation.
Method 2.
Determination
of Fructose by a Reducing Sugar Method.Thereducing sugar formed in method 1 wasestimatedby Nelson's modification of Somogyi's method (9). The assay method was used when various nucleotides were tested as the substrates.
Method 3.Determinationof Sucrose FormedintheDirection ofSucrose Synthesis.The methoddescribedbyLeloir and Car-dini(5)wasfollowed. This methodwasemployedforanalyzing
thekinetics ofsucrose synthesizing reaction.
One unit of enzyme is definedasthatamountofenzyme which produces 1 ,umol ofUDPG, offructose, or sucrose/min under the specified assay condition, and the specific activity is the number of the enzyme unit exhibitedby 1 mg ofprotein.
The enzyme was extractedand purified according tothe fol-lowing procedures. One hundred g of the edible portion of bamboo shoot, prechilledat 4C for 2 to 3 hr andchoppedinto about 1-cm cubes, was homogenized with 1 ml of 1 M K-phosphate buffer (pH 7) in a Waring Blendor for 1 min. The homogenatewassqueezedthroughtwolayersof cheesecloth and the milky filtrate centrifuged at 10,000g for 30 min. From the clear supernatant, the bulk of the enzyme was precipitated
between 0.35 to 0.50 saturation of ammonium sulfate. The precipitate wasdissolved in a small amount of 50 mm
K-phos-phate(pH 7) containing 1 mmEDTA and 5 mm
2-mercaptoeth-anol and dialyzed overnight against 1 mm K-phosphate
(pH
7) containing 0.1 mM EDTAand 1 mm2-mercaptoethanol.
The dialyzedenzyme solution was diluted with 5 volumes of 10 mm K-phosphate (pH 6.8), and calcium phosphate gel
sus-pensionwasaddedtoittomakeafinalgeltoprotein ratio(w/w)
of 5.Afterstirring for 10min, the gel was collected by
centrifu-gationand washedstepwise with 6-ml aliquots of16,20, 24, and 28 mm of K-phosphate (pH 8). The last two buffer solutions contained 5% ammonium sulfate. The desorbed enzyme solu-tions showednearlyidenticalspecific activities. Thus, theywere
combinedfor furtherpurification. Solid ammonium sulfatewas addedtothegel eluate and the precipitate formed between 0.30 to 0.60 saturation of the salt was collected. The bulk of the precipitate was dissolved in a minimum amount of 10 mm Na-phosphate (pH 7) and the insoluble matter was removed by
centrifugation. One ml of the enzyme solution from the second ammonium sulfate fractionation step was applied to aSephadex
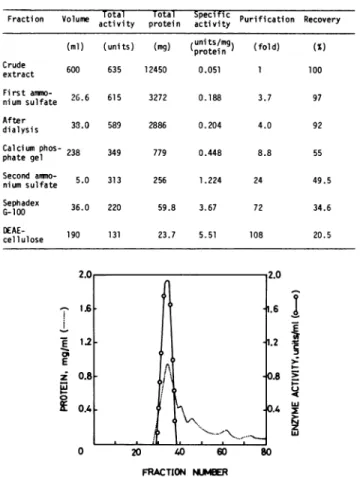
G-100 column(2.5 x 38cm) whichwaspreequilibrated with 2 mm Na-phosphate (pH 7.6). Before it wasintroduced into the column, 50 mg of sorbitol was added/ml of the enzyme solution. The flow rate was adjusted to 0.5 ml/min and the eluate col-lected as 2.5-ml fractions(Fig. 1). Sucrose synthetase obtained from thispurification step was free from invertase. The eluate was concentrated by ultrafiltration and could be used for the studies ofkinetics andgeneral properties.
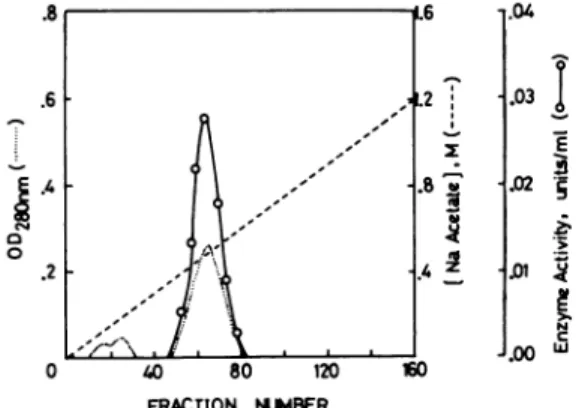
For further purification, a DEAE-cellulose column (2 x 28 cm) preequilibrated with 2 mm Na-phosphate buffer (pH 7.6) wasused. Theenzyme eluate fromtheSephadex G-100 column wasdirectly introduced into the ion exchangecolumn. Afterthe adsorption was complete, the column was eluted by a linear gradient with 2 mmNa-phosphate (pH 7.6) as the starting buffer and 50mmNa-phosphate (pH 6.4), which is 1 M with respect to Na-acetate, as the supplementary buffer to the mixing chamber.
Table I. Sunmnary of purification steps.
Results are based on 1 kg of edible tissue.
Fraction Volume activityTotal Total Specific Purification Recovery protein activity
(ml) (units) (mg) (units/mg)proteinI (fold) (X)
Crude 600 635 12450 0.051 1 100 First
ammno-niunrsulfate
26.6 615 3272 0.188 3.7 97 nium sulfate After 38.0 589 2886 0.204 4.0 92 dialysis Calcium phos- 238 349 779 0.448 8.8 55 phate gel Second anmmo- 5.0 313 256 1.224 24 49.5 nium sulfate Sephadex 36.0 220 59.8 3.67 72 34.6 G-100 DEAE- 190 131 23.7 5.51 108 20.5 cellulose 1.61 E £ 1-a 12 0.81. O.4 0 20 40 1.6 i.2 c 0.8 ' 0.4 W 60 80 FRACTION NUMBERFIG. 1. Gelfiltration pattern of the enzyme from the second ammo-nium sulfatestepon aSephadex G-100 column.
18
i
CM 0 I w .5 z .04 .03 -g .02 5 .5 ._1 Di-W .nn W FRACTION NUMBERFIG. 2. Ion exchange chromatographic pattern of the enzyme from the gelfiltration stepon aDEAE-cellulose column.
140 - 120
9 100 80 60~
MOLECULAR WEIGHT (daltons)
FIG. 3. Estimation of the molwtofbamboo shootsucrosesynthetase by gel filtration chromatography on a Sephadex G-200 column.
Al-though thetruemolwtof y-globulin is160,000, the protein exhibitsan
apparent molwtof205,000 by gelfiltration (1).
Thevolume ofthe bufferwas 150 ml in each chamber. The flow
rate of the buffer through the column was 1 ml/min and the
eluate was cut into 2-ml fractions. The enzyme was eluted
between 0.36 and 0.61 M Na-acetate (Fig. 2). The fractions
containing the bulk of the enzyme activity were combined,
dialyzed, andlyophilized.
RESULTS AND DISCUSSION
Through six stepsoftreatment, a 108-foldpurification ofthe
enzyme was achieved (Table I). The chromatographic patterns
on aSephadexG-100column andaDEAE-cellulosecolumnare
shown in Figures 1 and 2, respectively. After the gel filtration step, the preparation wascompletely free from invertase
activ-ity. The finalenzymepreparation showedanearly homogeneous
disc electrophoretic pattern. From these results, it has been estimated that the enzyme comprises nearly 1% of the soluble proteinsinthebamboo shootextract.Thefollowingstudieswere
doneunderconditionswhere the initial velocities of theenzyme
reaction werelinear with respectto reaction time.
Heat Stability. The crude enzyme after the first ammonium
sulfate step was quite stable; storage at -20 C for a month
resulted in no loss ofactivity and 52% of the activity was lost
after 6 months' storage. The enzyme afterthe DEAE-cellulose
stepbecame unstable and could not be stored fora prolonged
timeevenatthe freezingtemperature.Theenzymeshowedgood stability between pH6.4 and8.5. Heatingattemperaturebelow 50C for 10minresulted innoloss of theenzymeactivity. Above
50C,theenzymeactivitywaslost rapidly. Theenzymefrom the
gelfiltration steplost90% of itsactivity afterstorage at4 C for 15 days. When the enzyme was stored in 25, 20, 15, or 10% glycerol at4C, there remained 50, 50, 30,or20% of the initial activity after 68days. It is thus evident that glycerol is capable of
stabilizing theenzyme in solution.
pH Optimum. The optimum pH range ofsucrose synthetase
assayed in the direction ofsucrosecleavagewasbetween 5.8and 6.8 with pH 6 showing the highest reaction rate. As can be predicted, the optimum pHfor the reaction in the direction of sucrose synthesis, which involves the release of 1
proton/mole-cule ofUDPG utilized, is about 1.5 pH unitshigher.
Substrate Specificity. UDP, ADP, IDP, and TDP were the nucleotide substrates of sucrose synthetase. At 0.1 mm, when the activity of UDPasthe substrate wastaken as 100,those of ADP, IDP, and TDPwere21, 9.5, and 25, respectively. Other nucleoside diphosphates were completely inactive as the sub-stratefor the enzyme.
Fructose is the onlymonosaccharide thatcan be the acceptor ofglucosylgroupfromUPDG in the enzyme-catalyzed reaction. Among sucrose, maltose, lactose, cellobiose, trehalose, gentio-biose, raffinose, meligentio-biose, and turanose, only sucrose, turan-ose, gentiobiose, and melibiose at 50 mm showed activities as glucosyl donors in the ratio 100:11.5:7.0:1.5.
Effectors. When the concentration was lower than 0.8 mm, Mg2+ was an activator; at 0.5 mm,it could enhance the activity by 18%. The rest of thedivalent cations tested, namely Hg2+, Cu2+, Mn2+, Fe2+,andCa2+wereinhibitors.Among them,Hg2+ was the most effective; at the concentration of0.2
iM,
itcould inhibit the enzymeactivity by 73%.Cyanide, azide, and fluoride were inhibitors in decreasing order of effectiveness. At 5 /.M, cyanide inhibits 80% of the enzyme activity.
Incubation withp-mercuribenzoate or iodoacetate at 4 Cand concentration ofeither 0.8 or 0.4mm resulted in thecomplete
FRACTION NUMBER
FIG. 4. Isoelectricfocusingpatternof bamboo shootsucrose
synthe-taseinacarrierampholytewithapHrangeof 3.5 to10.
4.0 3.2.-gC g 2.4 (wbMgC%) E1.6 1 o 200 S(SUCROSE]. (mM)
FIG. 5. Effect ofsucroseconcentration on therateof
enzyme-cata-lyzed reaction. The maximum velocity was obtained from the double
reciprocal plot (not shown) and used in drawing the Hill plot. Inset shows the Hillplotfromwhich theS0.5wasevaluated.
Cytfvre C Acohol OHase (-GIobLdin BlueooEnzm
murixtian
19
Plant
Physiol.
Vol.60,
1977Plant Physiol. Vol. 60, 1977
0 50 100 150 200
S(UDP),(jM)
FIG. 6. Effect of UDP concentrationontherateofenzyme-catalyzed reaction. The maximum velocity and Kmwereobtained from the double
reciprocal plot (not shown) and the formerwasemployed in drawing the Hillplotasshown in the inset.
9.0 6.0 l 2 n=1.8
~3.0.
> /1* logS 0 5 10 15 20 S(FRUCTOSE).(mN)FIG. 7. Effect offructose concentrationon therateof
enzyme-cata-lyzed reaction. The maximum velocity wasevaluatedfrom thedouble reciprocal plot (not shown)andusedindrawing theHillplot(inset).
loss of the enzyme activity. When reduced glutathione was
added to thep-mercuribenzoate-inhibited enzyme to the final concentration of 9mmand themixture incubatedat 4C for 20 min, theenzymerecovered52% ofitsactivity.Fromthistest,it
maybe concluded thattheenzyme isan -SHenzyme.
MolecularWeightandSedimentation Properties. By
compar-ing the elution volume (Ve) of the enzyme with the standard
curveoflog(MW)versusVeobtained with standardproteinson a
Sephadex G-200 column, the mol wt of bamboo shootsucrose
synthetase wasestimatedas280,000 (Fig. 3).
The sedimentation constant ofthe enzyme wascalculated to
be 7 by asucrose density gradient ultracentrifugation method. However,inaglyceroldensity gradient, the sedimentation
con-stantof theenzymewasestimatedtobe 7.3.Thedecrease in the sedimentation constant of the enzyme by about 4% under the
presence of its substrate sucrose was in agreement with the properties ofaspartate transcarbamylase from Escherichia coli (3); the sedimentation coefficient of ATCase was reduced by
3.6% when succinate and carbamyl phosphate were present. This experimental result can be taken as evidence to show the bindingbetweensucroseandtheenzyme.Themolwtcalculated from the sedimentation constantof the enzyme in sucrose and that of the standard protein fibrinogen was 295,000, in good
agreementwith that obtainedby thegelfiltration technique. pi.The isoelectricpointof theenzymewasfoundtobe 5.2by the isoelectric focusing technique (Fig. 4). Among the four
protein peaks recorded automatically by a double beam UV analyzer,thelargestonthe farright side ofthefigurewasdueto the protein precipitate formed during the course of isoelectric focusing.
Kinetics. The saturation curves for the four substrates and
their respective Hill plots are shown in Figures 5 to 8. The kinetic constantsestimated from these plotsare summarized in
Table II.
In determining the saturation curve of one substrate, the
concentration ofthe othersubstratewasfixedat alevelatleast two times higher than the Km (or
S0.5)
value (fixed substrate concentrations: sucrose, 100 mM; UDP, 2 mM; fructose, 5 mM; and UDPG, 3.4 mm). For the substrate with a very low Kmvalue, adequate amount was provided soas to ensure that the
kinetic behavior of the reaction does not change during the
course ofactivitymeasurement.
The saturation curves for the two carbohydrate substrates
were both sigmoidal in shape withnvaluesof 1.8(sucrose with
magnesium chloride and fructose)or 1.7 (sucrose without mag-nesium chloride), while those of the nucleotide substrates were
bothhyperbolic in shape with n values closeto unity.
It has been revealed in our previous investigation that the
bamboo shoot isveryrich inUDPG and that the sugar nucleo-tide can also be formed by the sucrose-cleaving reaction
cata-lyzed byasucrosesynthetase, although UDPG
pyrophosphoryl-ase,which is knowntobetheenzymeresponsible for
synthesiz-ing UDPG from UTP and G-1-P inmany livingsystems, isalso
presentin the bamboo shoot (11). Our data have revealed that the sucrose-cleaving reaction catalyzed by the bamboo shoot
sucrose synthetase is modulated by the availability ofsucrose.
Thus,sucroseis regardedasthehomotropic allosteric effector of
theenzymeandtheenzymeis turnedononly when the
concen-tration of sucrose in the shoot tissue reaches a certain level
through translocation from the mother plant, and the turningon
of the enzyme makes available to the tissue UDPG, the key
sugar nucleotide from which various precursors of cell wall
polysaccharides arederived.
9.L C~~~~~~ .0 -0 E 0 2 4 6 8 10 S(UDPG).(mM)
FIG. 8. Effect of UDPG concentrationonthe rate of enzyme-cata-lyzed reaction. Themaximum velocityand Km valueswere estimated from the doublereciprocal plot (not shown)and the formerwas
em-ployedindrawingthe Hillplot(inset).
Table II. Kinetic constantsof bamboo shoot sucrosesynthetase
catalyzed reaction
Substrate Sucrose UDP Fructose UDPG
..t2+ 4g2+
SO5( orKu* 59 56 0.044 2.50 1.36
Will coef.
(n) 1.7 1.8 1.1 1.8 1.2
SO.5 foraucrose and fructoseandgm forUDSP and UDPG.
LiTERATURE CrFED
1.ANDsaw P1965ThbeSefiltrationbehavior of proteins relatedtotheir molecular weights
over awiderange.Biochem J 96:595-606
2. AVIGwADG 1964Sucrose-uridinediphosphate glucosyltransferasefrom Jerusalem artichoke tubers. J Biol Chem 239:3613-3618
3. GastA JC,HKScHAcwL4m 1968Allostericinteractions inaspartatetranscaramylase.
II.Evidencefordiffcrent conformationalstatesof theprotein in thepresenceandabsence ofspecificlgands. Biochemistry7:538-552
4. Km1 D, EF HAsza 1938 On themecbanism of the decomposition of hydrogen peroxidebycatalse.Proc R Soc Lond B 124:399
5. Lzwsa LF,CE CAsuNI 1962 UDPG-fructosetransglucosyls from wheatgerm.Methods Enzymol 5: 167-171
6. Lawny OH, NJ RosaoucW ,LAFA=,RJ RANDALL 1951Proteinmeasurementwith the Foliopbenolreagent.J Biol Chem193: 265-275
7. MARI RG,BNAsFs 1961 Amethodofdeterminingthesedimentation behaviorof
enzymes:applcation toproteinmixtures.JBiol Chem 236: 1372-1379
8. NssLsoNJW, WB ALUNGroN, CG ARoN 1974Isoelectric focusing with ISCO density
gradient eekrphoresisequipoent.ISCOApplicationReerch Bulletin No. 15. Instru-mentationSpecalies Co,Lincoln Neb
9. NLssoN N 1944 A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153: 379-380
10. PAADINI AC, LFLenoa 1952 Studies on uridine-diphosphate-glucose. Biochem J 51: 426-430
11.SinU S-M 1976 Purification and properties of uridinediphosphate glucose pyrophospho-rylase from bambooshoots. Master thesis. Inst Agric Chem, National Taiwan Univ, Taiwan, Rep China
12. Su JC 1965 Carbohydrate metabolism in the shoots of bamboo Lekba oldhami. III. Separation and identification of nucleotides. J Chin Agric Chem Soc Sp Iss: 45-54 13. SuJC, IN CHOU,MJ TsM. 1969 Carbohydrate metabolism in the shoots of bamboo Lekba
oldhami. VII.Changes of polysaccharide constituents accompanied with the growth of the plant. J ChinAgricChem SocSp Iss: 16-20
14. Su JC, DS Tsou, HHTu 1967Carbohydrate metabolisminthe shootsof bambooLekba oldhami. IV.Astructuralstudy of cell wall polysaccharides. Bot Bull Acad Sinica 8: 339-352
15. Su JC, HFYuAN, HY SUNG 1968 Carbohydrate metabolism in the shoots of bamboo Lekba oldhami.V.Thecallosesynthesizing system. JChin Agric Chem Soc Sp Iss: 1-11 16. SUNGHY, HF YuAN, JC Su 1971 Carbohydrate metabolism in the shoots of bamboo L*Ieba oldhami. IX. Some properties of the pentosan synthesizing system. J Chin Agric Cbem SocSp Iss: 62-73