A hypersensitive response was induced by virulent bacteria in transgenic
tobacco plants overexpressing a plant ferredoxin-like protein (PFLP)
Hsiang-En Huang
a,b, Mang-Jye Ger
c, Mei-Kuen Yip
a, Chao-Ying Chen
b,
Ajay-Kumar Pandey
a, Teng-Yung Feng
a,*
a
Institute of Botany, Academia Sinica, Nankang, Taipei 115, Taiwan, ROC
b
Department of Pathology and Microbiology, National Taiwan University Taipei 106, Taiwan, ROC
c
Department of Life Science, National University of Kaohsiung, Kaohsiung 811, Taiwan, ROC Accepted 28 May 2004
Abstract
The hypersensitive response (HR) displayed by resistance plants against invading pathogens is a prominent feature of an incompatible plant pathogen interaction. It has been shown that tobacco cell cultures transgenic for a plant ferredoxin-like protein (PFLP) that functions as an electron acceptor of Photosystem I increased harpin-mediate HR. In this work we report increased bacterial disease resistance of pflp transgenic tobacco. Compared to the controls, four distinctive characteristics were found in the pflp-transgenics after inoculation with virulent bacterial cells Erwinia carotovora subsp. carotovora and Pseudomonas syringae pv. tabaci: (i) instead of typical disease symptoms, an HR-like necrosis was observed; (ii) the proliferation of the virulent pathogen was highly retarded; (iii) the expression of hsr203j, an HR marker gene, was apparently induced; (iv)H2O2 accumulation was induced immediately. Together, those results
demonstrate that enhanced production of PFLP in the transgenic plant conditions the induction of a hypersensitive response during compatible pathogen attack.
q2004 Elsevier Ltd. All rights reserved.
Keywords: Ferredoxin-like protein; Erwinia carotovora subsp. carotovora; Pseudomonas syringae pv. tabaci; hsr203j
1. Introduction
Avirulent pathogens elicit a rapid collapse of the challenged host cells in the so-called hypersensitive response (HR) that results in a restricted necrotic lesion from surrounding healthy tissue. Although some host tissue is damaged during the HR process, the localized host cell death contributes to the limitation of pathogen spread
[22,24,26]. A battery of inducible defense-related responses often accompanies an HR, including the generation of antibiotics [5], an oxidative burst [37] and enzymes involved in the general phenylpropanoid pathway
[9]. Many defense-related signal molecules, such as
salicylic acid, ethylene and jasmonic acid was also induced
under HR condition [27]. These molecules have emerged
as a key signal in the establishment of disease resistance and are able to protect the plant against further pathogen infection [8,11].
Harpin is one group of glycine-rich, cysteine-lacking, heat-stable proteins that can elicit HR in the absence of bacteria [41]. Three genera of plant bacterial pathogens, Erwinia, Pseudomonas, and Ralstonia spp. export harpins via the type III protein secretion system [3,12]. Genetic evidence indicates that harpins may play a minor role in bacterial elicitation of the HR, but it may assist the delivery of other pathogenesis proteins across the plant cell wall[12]. HR induced by harpin from Erwinia amylovora (HrpNEa) or
Pseudomonas syringae pv. syringae (HrpZPss) was
pre-vented by inhibition of calcium influx and ATPase activity in tobacco cell suspensions [16]. Harpin has a pronounced
effect on the plasmalemma, affecting HC
-ATPase, ion
channels or membrane carriers [30,33]. It also causes
0885-5765/$ - see front matter q 2004 Elsevier Ltd. All rights reserved. doi:10.1016/j.pmpp.2004.05.005
www.elsevier.com/locate/pmpp
* Corresponding author. Tel.: C886-2-26521867; fax: C886-2-2782-7954.
KC
efflux and extracellular alkalization in cell suspension cultures but not in protoplasts[19]. A secret able form of harpin from P. syringae pv. phaseolicola can elicit HR when expressed endogenously in plants[39]. These results indicate that the site of harpin action is the plant cell membrane/cell wall.
Previously, we reported that PFLP (plant ferredoxin like protein) was able to increase the generation of harpinpss
-mediated AOS and HR in tobacco suspension cells [7].
However, it is not know whether higher AOS generation in transgenic plant would assist in bacterial pathogen defense. In this paper we generated pflp transgenic tobacco to study the effect on defense against plant pathogenic bacteria. The pflp transgenic tobacco showed higher sensitivity to harpin than the wild type. When inoculated with the virulent pathogens Erwinia carotovora subsp. carotovora and Pseudomonas syringae pv. tabaci, the interaction was incompatible and the pflp transgenic tobacco showed disease resistance. Moreover, the accumulation of H2O2
and the expression level of HR marker gene hsr203j were highly induced in pflp-transgenic lines. These results imply that an enhanced amount of PFLP in the transgenic plants condition the induction of a hypersensitive response during virulent pathogen attack.
2. Materials and methods
2.1. Construction of the transformation vector
The coding sequence of pflp gene was amplified from the sweet pepper clone[7]by PCR with the following primers:
B5-SPF: 50-CGG GAT CCC GAT GGC TAG TGT CTC
AGC TAC CA-30, and S3-PF: CGA GCT CGT TAG CCC
XCG AGT TCT GCT TCT-03. The PCR product was
digested with BamH I and SacI. The full-length pflp fragment replaced the GUS protein coding sequence from pBI121 vector with CaMV 35S promoter (Clontech, Palo Alto, CA, USA.), and the insert was verified by DNA sequencing. The resultant plasmid, pBISPFLP, was then transformed into Escherichia coli DH5a.
2.2. Generation of transgenic tobacco lines
Agrobacterium tumefaciens C58C1 was transformed with pBISPFLP vectors as described by Holsters et al.
[17]. Transformation of tobacco (Nicotiana tabacum cv.
Xanthi) was performed by the standard leaf disc
transformation method using kanamycin selection
(100 mg/ml) [18]. PCR analysis and DNA gel blotting
confirmed six independent transformant lines. All trans-genic plants were grown in a growth chamber (16 h light/ 8 h dark at 30 8C). The irradiances of growth chamber are 48 mmol mK2sK1. Two transgenic lines were self-ferti-lized and the seeds were collected for seeding and PCR analysis.
2.3. Extraction and gel blot analysis of DNA, RNA, and protein
Genomic DNA was extracted from young leaf tissue by the Qiagen genomic kit protocol (Qiagen). Digestion with restriction enzymes, electrophoretic separation on agarose gels, and transfer to nylon membranes (Boehringer Mannheim) were performed by using standard procedures
[35]. Membranes were hybridized at 65 8C with the full
length NPT II marker probe PCR-label with digoxigenin-11-dUTP (Boehringer Mannheim) according to the manu-facturer’s protocols. After hybridization, membranes were washed under high stringency conditions (2!SSC, 0.1% SDS) and detected using the DIG luminescent detection kit (Boehringer Mannheim).
Total RNA was isolated from tobacco leaves using the Qiagen Plant RNA Kit (Qiagen) and quantified by
spectrophotometry, assuming A260Z40 mg/ml [35]. Total
RNA (15 mg) was electrophoresed through 1% agarose/ formaldehyde gels and then transferred onto nylon mem-branes. Membranes were hybridized at 55 8C overnight with hsr203j probes PCR-label with digoxigenin-11-dUTP (Boehringer Mannheim) according to the manufacturer’s protocols. After hybridization, membranes were washed under high stringency conditions and detected using the DIG Luminescent detection kit (Boehringer Mannheim).
Proteins were extracted by homogenizing 0.2 g offresh leaf tissue in 0.5 ml Tris buffer (150 mM NaCl, 50 mM Tris pH 7.5) using a plastic pestle fitted to a 1.5 ml centrifuge tube. The protein concentrations of the samples were determined with Coomassie brilliant blue dye using a microassay method as recommended by the manufacturer (BioRad). Two micro-grams of each protein sample were electrophoresed through gels containing 12.5% polyacrylamide plus SDS (SDS-PAGE). These gels were then either stained with Coomassie blue or electro-transferred onto nitrocellulose membranes using the BioRad blue tank method. PFLP proteins were detected on Western blots using anti-PFLP antibodies followed with mouse anti-rabbit IgG-peroxidase conjugate. 2.4. HarpinPsspreparation and plant hypersensitive
response assay
The harpinPssclone was provided by Dr H.-C. Huang at
the Agricultural Biotechnology Laboratories, National
Chung-Hsien University, Taiwan. HarpinPss protein was
extracted according to He et al.[16]. Escherichia coli DH5a (pSY10) which harbors the harpinPssgene (hrpZ) was grown
in Luria Broth containing ampicillin (50 mg/ml) at 37 8C in the dark with shaking overnight in the presence of isopropylthio-b-D-galactoside. To obtain harpinPss, the bacteria were first washed and sonicated for 30 s in 10 mM phosphate buffer pH 6.5 and boiled for 10 min. After boiling, the extracts were centrifuged at 10,000!g for 10 min. Supernatants were desalted by a Microconcentrator (Amicon) and stored at 4 8C.
The HR assay was performed according to Huang et al.
[20]. Fully expanded tobacco leaves were wounded to form tiny pricks on the lower surface of the leaves by a 25-gauge
needle. HarpinPss was prepared in 50 mM Tris buffer
(pH 7.5) and infiltrated by pressing a 1 ml syringe without a needle to the prick. The infiltrated plant was incubated in a growth chamber (16 h light/8 h dark at 25 8C).
2.5. Transgenic tobacco resistance assays
All transgenic tobacco resistance assays were carried out with T2 progenies of the transgenic plants in independent experiments. Fully expanded upper leaves of plants, approximately 80-days old, were used for infection with pathogen. Inoculations with Pseudomonas syringae pv. tabaci and Erwinia carotovora subsp. carotovorawere performed. Each inoculation was repeated four times in individual plants of each transgenic line. Bacteria inside the leaf discs were released by grinding the infiltration area in sterile water in a microfuge tube and then plated on Nutrient Broth agar plates. The plates were cultured at 30 8C overnight, and the colonies were counted the following day. 2.6. Determination of H2O2
H2O2was determind according to method of Jana and
Choudhuri[21]. Tobacco leaves (100 mg) were
homogen-ized with 0.6 ml of 50 mM phosphate buffer (pH 6.5) with 10 mM 3-amino-1, 2, 4-triazole. The homogenates were centrifuged at 6000g for 25 min. Titanium sulphate (0.2 ml of 0.1%) in 20% (v/v) H2SO4was added to the supernatant
and centrifuged at 6000g for 15 min. The intensity of the yellow colour of the supernatant was measured at 410 nm to determine the level of H2O2.
3. Results
3.1. Screening and expression analysis of pflp transgenic tobacco plants
Tobacco plants expressing heterologous pflp gene isolated from sweet pepper were generated by Agrobacter-ium-mediated transformation. pBI based plasmid construct containing sweet pepper pflp cDNA was prepared for tobacco transformation. (Fig. 1). Viable transformation
lines were selected and designated as T-SPFLP. In order to identify the distribution of pflp transgene in transgenic tobacco genomes, genomic DNA was extracted from the transgenic lines and subjected to Southern analysis. Given that wild type tobacco contains the ferredoxin gene [7], neomycin phosphotransgeraseII (NPT II) cDNA was used as a probe to identify the transgenic loci. Genomic DNA from T-SPFLP10-1 and T-SPFLP18-1 were digested with EcoRI restriction enzyme.Fig. 2showed that, after probing with the NPTII cDNA probe, DNA of the transgenic tobacco lines T-SPFLP10-1 and T-SPFLP18-1 exhibit one band, respectively. The patterns of Southern blotting suggest that T-SPFLP10-1 and T-SPFLP18-1 result from independent transformation events and incorporate the pflp transgene at different chromosomal locations. Western blot analysis showed that PFLP protein levels in T-SPFLP transformants was two to three fold increased compared to the controls (Table 1).
Fig. 2. Gel blots analysis of genomic DNA, DNA gel blot analysis of T-SPFLP transformed tobacco using NPT II cDNA as probe. Genomic DNA (15 mg) was digested with EcoR I for T-SPFLP10-1 and T-SPFLP18-1 as indicated and subjected to Southern blot analysis.
Fig. 1. Map of the relevant portions of the transformation plasmids. pBR-spflp, NOS-proZthe promoter region of A. tumefaciens nopaline synthase, NOS-terZthe terminator region of the same gene, NPT IIZthe coding sequence of neomycin phosphotransferase II gene, CaMV 35S-proZthe CaMV 35S promoter sequence, pflpZthe coding sequence for the sweet pepper PFLP and SPZsignal peptide of sweet pepper PFLP.
Table 1
Quantitative PFLP expression levels in transgenic tobacco
Transgenic lines Protein (fold)a
T-SPFLP10-1 3.13G0.21 T-SPFLP18-1 2.80G0.58
Wild type 1.00G0.21
a
Protein levels were determined with western blot. The PFLP protein level of wild type tobacco was defined as 1.
3.2. pflp transgenic plants showed a high sensitivity to harpinPss
In order to investigate the relationship between the PFLP and harpinPss-mediated HR in vivo, different dosages of
harpinPss were infiltrated into the intercellular spaces of
transgenic and wild type tobacco leaves and HR necroses were subsequently examined. After infiltration with 10 and 1 mg harpinPssover 24 h, in the transgenic line leaves showed
HR necroses almost to the full extent of the infiltration area, but wild type tobacco leaves exhibited much less HR necrosis. Even infiltration using lower concentrations of harpinPss
(0.1 mg) over 24 h in the transgenic line leaves showed a slight HR necrosis but not in wild type leaves (Fig. 3A).
The HR molecular marker gene hsr203j [31,32] that
accumulates specifically in tissues undergoing HR was examined. After infiltration with 10 and 1 mg of harpinPss
over 12 h, the hsr203j messenger accumulation was three fold increase in transgenic lines when compared to the wild type. When infiltrated with 0.1 mg harpinPss, the activation of
hsr203j could be detected in transgenic tobacco but not in wild type (Fig. 3B). These results implied that the pflp transgenic tobacco have higher sensitivity to the HR elicitor. 3.3. pflp transgenic tobacco exhibited resistance to virulent bacterial pathogens
To determine the effects of the pflp gene on plant disease resistance, two individual transgenic lines were challenged
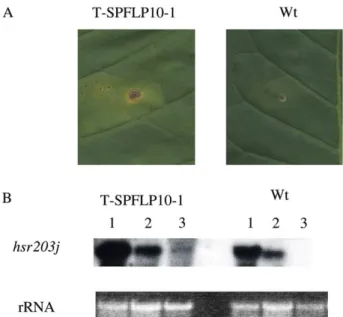
Fig. 3. The harpinPss-mediated hypersensitive response (HR) in transgenic
and wild type tobacco. (A) Low concentration of harpinPssinduced slight
HR in transgenic tobacco. HarpinPss(0.1 mg) was infiltrated into transgenic
(T-SPFLP10-1) and wild type (Wt) tobacco leaves. Photograph was taken 2 days post infiltration. (B) Induction of hsr203j gene expression by harpinPss.
The transgenic (T-SPFLP10-1) and wild type (Wt) tobacco were infiltrated with 10 mg (lane 1), 1 mg (lane 2) and 0.1 mg (lane 3) of harpinPss. Total
RNA in the infiltration areas of tobacco leaves were extracted after 12 h. RNA blots (15 mg per lane) were probed with the hsr203j cDNA probe. Ethidium bromide staining of rRNA was used to verify the loaded amount of total RNA.
Fig. 4. Resistance of pflp transgenic tobacco to virulent pathogens. Fully expanded upper leaves of transgenic (T-SPFLP18-1, TSPFLP10-1) and control tobacco (Wt) were infiltrated with 100 ml of bacterial suspension P. syringae pv. tabaci (1.0!108cfu/ml)(upper panel) or E. carotovora subsp. carotovora
with the virulent bacterial plant pathogens P. syringae pv. tabaci or E. carotovorasubsp. carotovora. The wild fire symptom occurred in wild type 3-days post P. syringae
pv. tabaci(1!108cfu/ml) inoculation and the symptoms
expanded continuously. However, the leaves of transgenic lines exhibited HR-like symptoms after 1-day post inocu-lation and this necrosis was dehydrated and limited in the infection site, even on 7-days post inoculation (Fig. 4A–C). Transgenic plants were also evaluated for resistance to bacterial soft rot disease caused by E. carotovora subsp. carotovora. E. carotovora subsp. carotovora (1!108cfu/ml) cause tissue maceration in wild type tobacco leaves (Fig. 4D). As shown inFig. 4E and F, pflp transgenic tobacco leaves exhibited HR-like necrosis and the necrosis was limited even after 2-days post inoculation. Bacterial populations in the infiltrated areas were calculated post inoculation at different times. A ttest demonstrated that differences in pathogen number between transgenic and wild type tobacco 2-days post infections were significant (P!0.05). Postinoculation (2-days), the population of P. syringae pv. tabaci and E. carotovora subsp. carotovora in the wild-type tobacco was approximately 10 times higher than that found in the transgenic lines (Fig. 5). These results indicated that overexpression of pflp in transgenic tobacco could enhances resistance against both kinds of virulent pathogens, E. carotovora subsp. carotovora and P. syringae pv. tabaci.
3.4. hsr203j gene expression was induced in transgenic tobacco by virulent pathogen infections
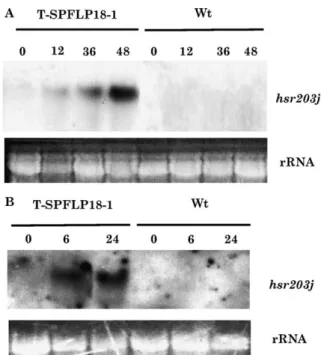
pflp transgenic tobacco plants showed HR-like necrosis when inoculated with compatible pathogens. To make sure those necroses was due to HR, an HR molecular marker gene hsrs203j was monitored after virulent pathogen inoculated in transgenic tobacco. The leaves of pflp transgenic (SPFLP18-1)
and wild type tobacco were infiltrated with 100 ml of E. carotovorasubsp. carotovora and P. syringae pv. tabaci, respectively (1.0!107cfu/ml). Total RNA in the infiltration areas of tobacco leaves were extracted and probed with the hsr203j. The HR marker gene hsr203j was highly induced at 6 h post E. carotovora subsp. carotovora inoculation and this induction was continued over 24 h in the pflp transgenic tobacco (Fig. 6A). Northern blot analysis was also performed when tobacco leaves were challenged with compatible
Fig. 5. The multiplication of bacterial pathogens were inhibited in pflp transgenic tobacco. Fully expanded upper leaves of two transgenic lines T-SPFLP10-1, T-SPFLP18-1 and control tobacco (wt) were infiltrated with 100 ml of bacterial suspension of P. syringae pv. tabaci (1.0!108cfu/ml) (A)or
E. carotovorasubsp. carotovora (1.0!108cfu/ml). (B) Bacterial populations were detected on successive times post inoculation. Data presented are mean and
standard deviation of four individual plants.
Fig. 6. Induction of hsr203j gene expression in transgenic tobacco by virulent pathogen infected. The transgenic (SPFLP18-1) and wild type (wt) tobacco were infiltrated with 100 ml of E. carotovora subsp. carotovora(A) and P. syringae pv. tabaci (B) individual (1.0!107cfu/ml). Total RNA in the infiltration areas
of tobacco leaves was extracted at the time points indicated. RNA blots (15 mg per lane) were probed with the hsr203j cDNA probe. Ethidium bromide staining of rRNA was use to verify the loaded amount of total RNA.
pathogen P. syringae pv. tabaci (Fig. 6B). The hsr203j was induced at 12 h post inoculation and this induction was continued over 48 h. It indicates that a virulent bacterial patho-gen indeed induce HR maker patho-gene in the pflp transpatho-genic plants. 3.5. H2O2was induced in transgenic tobacco by virulent
pathogen infections
The rapid production of peroxide (H2O2) by plant is one of
the most striking events during the early phase of the HR. To evaluate the production of H2O2in pflp transgic tobacco, two
different pflp transgenic tobacco lines (T-SPFLP18, T-SPFLP10) were infiltrated with 100 ml of E. carotovora subsp. carotovora (1.0!106cfu/ml) and the yield of H2O2
was measured as describe by Jana and Choudhuri[21]. The concentration of H2O2 was highly increased at 6 h post
E. carotovora subsp. carotovora inoculation and this induction was continued over 10 h in the pflp transgenic under light condition. This accumulation of H2O2was similar to avirulent
pathogen Pseudomonas syringae pv. syringae inoculated in the wild type tobacco (Fig. 7). The concentration of H2O2was
estimated to be 80–120 mM at the peak. However, no H2O2
accumulation was observed when inoculation with the avirulenct pathogen was followed by incubation in the dark.
4. Discussion
AOS accumulation has been shown to be required for plant pathogen defense[40]. Nevertheless, not all cases of AOS
accumulation increase in transgenic plant improve plant disease resistance. For example, AS1 transgenic tobacco that increases AOS accumulation was more sensitive to HR elicitor. However, the lesion cause by the virulent pathogen P. syringae pv. tabaci was more serious in AS1 transgenic tobacco than in the wild type [28]. Previously, we reported that PFLP was able to increase the generation of harpinpss
-mediated AOS and HR in tobacco suspension cells [7]. To
understand the effect of overexpression pflp in transgenic tobacco on disease resistance, pflp transgenic tobacco was generated and challenged with virulent bacterial pathogens.
The growth of P. syringae pv. tabaci in the pflp transgenic tobacco was significantly inhibited when compared with wild
type tobacco (Fig. 5). The visible necrosis induced by
P. syringae pv. tabaci was restrained significantly in the pflp transgenic tobacco (Fig. 4). To further confirm that this necrosis was due to HR, the HR marker gene hsr203j expression pattern was monitored. When inoculated with P. syringae pv. tabaci the hsr203j was activated in pflp
transgenic tobacco but not in wild type (Fig. 6B).
Additionally, Southern analysis showed that the pflp transgene incorporated at different loci in two independent transgenic lines (Fig. 2). Thus, the disease resistance to the virulent pathogens was due to pflp transgene and not as a result from rearrangement of the transgene at the specific locus of integration. These results indicated that pflp transgenic tobacco has acquired disease resistance and this disease resistance was obtained by the induction of HR.
Earlier we reported that rice and the orchid Oncidium which overexpressed an PFLP transgene from sweet pepper
Fig. 7. H2O2was induced in transgenic tobacco by virulent pathogen infections. The wild type (Wt) and transgenic tobacco plants (T-SPFLP10, T-SPFLP18)
were infiltrated with 100 ml of E. carotovora subsp. carotovora (Ecc) and Pseudomonas syringae pv. syringae (Pss) (1.0!106cfu/ml) under light(L) and dark(D) conditions individual. H2O2in the infiltration areas of tobacco leaves was extracted at the time points indicated. Data were expressed as mean of
showed increased disease resistance.[25,38]. In this study, to confirm that this disease resistance was generally specific, another bacterial pathogen, E. carotovora subsp. carotovora was challenged in pflp transgenic tobacco. E. carotovora subsp. carotovora is a wide host range bacterial plant pathogen that rapidly rots affected tissue. Although it has an inner typeIII secretion system region coding for the elicitor harpin, it never induces HR because the amount of secreted harpin is too low[4,23,29,34]. When E. carotovora subsp. carotovora inoculated in the pflp transgenic tobacco, the HR marker gene hsr203j were also activated (Fig. 6A). This result implies that not only P. syringae pv. tabaci but also E. carotovora subsp. carotovora could induce HR resistance in pflp transgenic tobacco. We also demonstrated that pflp transgenic tobacco was more sensitive to low amount of harpin (Fig. 3). We surmised that pflp transgenic tobacco could be induced HR more easily by low amounts of harpin and thus show an HR against virulent bacterial pathogens.
Ferredoxin-I plays as a role of photosynthetic electron transport by supplying electrons for the reduction of NADPC
to NADPH and under ertain circumstances can be involved in
the production of AOS [1]. PFLP is able to increase the
generation of AOS in tobacco suspension cells[7]. In this paper we measure the H2O2 generated in pflp transgenic
tobacco after virulent pathogen infection. When inoculated with the virulent pathogens Erwinia carotovora subsp. carotovora, the accumulation of H2O2was highly induced
in pflp-transgenic lines. This result was similar to an avirulent pathogen Pseudomonas syringae pv. syringae inoculated in the wild type tobacco (Fig. 7). H2O2generation in the HR is
usually considered to be highly associated with the light condition[42], In this study, the H2O2accumulation of pflp
transgenic tobacco plants induced by the virulent pathogen never occurred under dark conditions. These results imply that the HR induced by the virulent pathogen in pflp transgenic tobacco is light dependent.
Exploitation of plant endogenous defense mechanism is a useful strategy to create disease resistance traits [6,9]. A number of reports have indicated that expression of elicitors or avr gene products in transgenic plants can trigger an HR that generates broad-spectrum disease resistance to virulent pathogens[2,10,13,15,36]. The potential application of this strategy is limited, because HR is a programmed cell death process in which the plant depletes many energy resources to synthesize defense-related compounds. Our strategy has the advantage that overexpression of pflp in transgenic plants does not trigger any macroscopic or microscopic spontaneous HR directly before pathogen infection. How-ever, overexpression of pflp in transgenic plant to protect from bacterial infection was limited by the internal
regulatory elements of PFLP in the young seedling [14].
In young seedlings, ferredoxin could not accumulate very well in the transgenic tobacco even under control of 35S promoter (data not shown).
In summary, we have demonstrated the utility of pflp in transgenic tobacco to increase disease resistance. HR was
induced in pflp transgenic tobacco against two different genera of virulent pathogens. Using this approach, trans-genic plants of more broad disease resistance could be generated for the future.
Acknowledgements
We would like to thank Dr Pontier from Laboratoire de Biologie Moleculaire des Relations Plantes/Microorga-nismes, France for providing the hsr203jclone. This work was supported by grants to T.-Y. Feng from Academia Sinica, Taiwan, Republic of China.
References
[1] Biehler K, Fock H. Evidence for the contribution of the Mehler-peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol 1996;112:265–72.
[2] Bu¨schges R, Hollricher K, Panstruga R, Simons G, Wolter M, Frijters A, Van Daelen R, Van der Lee T, Diergaarde P, Groenendijk L, Toepsch S, Vos P, Salamini F, Schulze-Lefert P. The barley Mlo gene: a novel control element of plant pathogen resistance. Cell 1997;88:695–705.
[3] Collmer A, Lindeberg M, Petnicki-Ocwieja T, Schneider DJ, Alfano JR. Genomic mining type III secretion system effectors in Pseudomonas syringae yields new picks for all TTSS prospectors. Trends Microbiol 2002;10:462–9.
[4] Cui Y, Madi L, Mukherjee A, Dumenyo CK, Chatterjee AK. The RsmA-mutants of Erwinia carotovora subsp. carotovora strain Ecc71 overexpress hrpNEccand elicit a hypersensitive reaction-like response
in tobacco leaves. Mol Plant Microbe Interact 1996;9:565–73. [5] Dangl JL. The enigmatic avirulence genes of phytopathogenic
bacteria. Curr Top Microbiol Immunol 1994;192:99–118.
[6] Dangl JL, Dietrich RA, Richberg MH. Death don’t have no mercy: cell death programs in plant–microbe interactions. Plant Cell 1996;8: 1793–807.
[7] Dayakar BV, Lin HJ, Chen CH, Ger MJ, Lee BH, Pai CH, Chow D, Huang HE, Hwang SY, Chung MC, Feng TY. Ferredoxin from sweet pepper (Capsicum annuum L,) intensifying harpinPss-mediated
hypersensitive response shows an enhanced production of active oxygen species (AOS). Plant Mol Biol 2003;51:913–24.
[8] Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Graffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J. A central role of salicylic acid in plant disease resistance. Science 1994;266:1247–50.
[9] Dixon RA, Lamb CJ, Masoud S, Sewalt VJ, Paiva NL. Metabolic engineering: prospects for crop improvement through the genetic manipulation of phenylpropanoid biosynthesis and defense responses. Gene 1996;179:61–71.
[10] Dietrich RA, Delaney TP, Uknes SJ, Ward ER, Ryals JA, Dangl JL. Arabidopsis mutants simulating disease resistance response. Cell 1994;77:565–77.
[11] Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science 1993;261:754–6. [12] Gala´n JE, Collmer A. Type III secretion machines: bacterial devices
for protein delivery into host cells. Science 1999;284:1322–8. [13] Ger MJ, Chen CH, Hwang SY, Huang HE, Podile AR, Dayakar BV,
plant enhances resistance against virulent bacterial pathogens by induction of a hypersensitive response. Mol Plant Microbe Interact 2002;15:764–73.
[14] Gallo-Meagher M, Sowinski DA, Elliott RC, Thompson WF. Both internal and external regulatory elements control expression of the pea fed-1 gene in transgenic tobacco seedlings. Plant Cell 1992;4: 389–95.
[15] Greenberg JT, Guo A, Klessig DF, Ausubel FM. Programmed cell death in plant: a pathogen-triggered response activated coordinately with multiple defense functions. Cell 1994;77:551–63.
[16] He SY, Huang HC, Collmer A. Pseudomonas syringae pv. syringae harpinPss: a protein that is secreted via the Hrp pathway and elicits the
hypersensitive response in plant. Cell 1993;73:1255–66.
[17] Holsters M, De Waele D, Depicker A, Messens E, Van Montagu M, Schell J. Transfection and transformation of Agrobacterium tumefa-ciens. Mol Gen Genet 1978;163:182–7.
[18] Horsch RB, Fry JE, Hofmannn NL, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science 1985;227:1229–31.
[19] Hoyos ME, Stanley CM, He SY, Pike S, Pu XA, Novacky A. The interaction of harpinPss, with plant cell walls. Mol Plant Microbe
Interact 1996;9:608–16.
[20] Huang HC, Shuurink R, Denny TP, Atkinson MM, Baker CJ, Yucel I, Hutcheson SW, Collmer A. Molecular cloning of Pseudomonas syringae pv. syringae gene cluster that enable Pseudomonas fluorescens to elicit the hypersensitive response in tobacco plants. J Bacteriol 1988;170:4748–56.
[21] Jana S, Choudhuri MA. Glycolate metabolism of three submerged aquatic angiosperms during aging. Aqua Bot 1982;12:345–54. [22] Keen NT. Gene-for-gene complementarity in plant–pathogen
inter-actions. Annu Rev Genet 1990;24:447–63.
[23] Laby RJ, Beer SV. Hybridization and functional complementation of the hrp gene cluster from Erwinia amylovora strain Ea321 with DNA of other bacteria. Mol Plant Microbe Interact 1992;5:412–9. [24] Lamb CJ, Lawton MA, Dron M, Dixon RA. Signals and transduction
mechanisms for activation of plant defenses against microbial attack. Cell 1989;5:215–24.
[25] Liau CH, Lu JC, Prasad V, Hsiao HH, You SJ, Lee JT, Yang NS, Huang HE, Feng TY, Chen WH, Chan MT. The sweet pepper ferredoxin-like protein (PFLP) conferred resistance against soft rot disease in oncidium orchid. Transgenic Res 2003;12:329–36. [26] Lindsay WP, Lamb CJ, Dixon RA. Microbial recognition and
activation of plant defense systems. Trends Microbiol 1993;1:181–7. [27] Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990;250:1002–4.
[28] Mittler R, Herr EH, Orvar BL, van Camp W, Willekens H, Inze D, Ellis BE. Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperre-sponsive to pathogen infection. Proc Natl Acad Sci USA 1999; (96):14165–70.
[29] Mukherjee A, Cui Y, Liu Y, Chatterjee AK. Molecular characteriz-ation and expression of the Erwinia carotovora hrpNEccgene, which
encodes an elicitor of the hypersensitive reaction. Mol Plant Microbe Interact 1997;10:462–71.
[30] Pike SM, Adam AL, Pu XA, Hoyos ME, Laby R, Beer SV, Novacky A. Effects of Erwinia amylovora harpin on tobacco leaf cell membranes are related to leaf necrosis and electrolyte leakage and distinct from perturbations caused by inoculated E. amylovora. Physiol Mol Plant Pathol 1998;53:39–60.
[31] Pontier D, Balague´ C, Bezombes-Marion I, Tronchet M, Deslandes L, Roby D. Identification of a novel pathogen-responsive element in the promoter of the tobacco gene hsr203J, a molecular marker of the hypersensitive response. Plant J 2001;26:495–507.
[32] Pontier D, Tronchet M, Rogowsky P, Lam E, Roby D. Activation of hsr203J, a plant gene expressed during incompatible plant-pathogen interactions, is correlated with programmed cell death. Mol Plant Microbe Interact 1998;11:544–54.
[33] Popham P, Pike S, Novacky A. The effect of harpin from Erwinia amylovora on the plasmalemma of suspension-cultured tobacco cells. Physiol Mol Plant Pathol 1995;47:39–50.
[34] Rantakari A, Virtaharju O, Vahamiko S, Taira S, Palva ET, Saarilahti HT, Romantschuk M. Type III secretion contributes to the pathogenesis of the soft-rot pathogen Erwinia carotovora: partial characterization of the hrp gene cluster. Mol Plant Microbe Interact 2001;14:962–8.
[35] Sambrook J, Fritsch EF, Maniatis T., 2nd ed Molecular Clone. A Laboratory Manual, E6. New York, USA: Cold Spring Harbor; 1989 p. 9.31–9.57.
[36] Shen S, Li Q, He SY, Barker KR, Li D, Hunt AG. Conversion of compatible plant–pathogen interactions into incompatible interactions by expression of the Pseudomonas syringae pv. syringae 61 hrmA gene in transgenic tobacco plants. Plant J 2000;23:205–13. [37] Sutherland MW. The generation of oxygen radicals during host plant
responses to infection. Physiol Mol Plant Pathol 1991;39:79–93. [38] Tang KX, Sun XF, Hu QN, Wu AZ, Lin CH, Lin HJ, Twyman RM,
Christou P, Feng TY. Transgenic rice plants expressing the ferredoxin-like protein (AP1) from sweet pepper show enhanced resistance to Xanthomonas oryzae pv. oryzae. Plant Sci. 2001;160:1035–42. [39] Tampakaki AP, Panopoulos N. Elicitation of hypersensitive cell death
by extracellularly targeted HrpZPsphproduced in planta. Mol Plant
Microbe Interact 2000;13:1366–74.
[40] Tenhaken R, Levine A, Brisson LF, Dixon RA, Lamb C. Function of the oxidative burst in hypersensitive disease resistance. Proc Natl Acad Sci USA 1995;92:4158–63.
[41] Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992;257:85–8. [42] Zeier J, Pink B, Mueller MJ, Berger S. Light conditions influence
specific defence responses in incompatible plant-pathogen inter-actions: uncoupling systemic resistance from salicylic acid and PR-1 accumulation. Planta 2004 in press. PMID15098125.