Solution Structure of a Kunitz-type Chymotrypsin Inhibitor Isolated
from the Elapid Snake Bungarus fasciatus*
Received for publication, July 3, 2001, and in revised form, August 17, 2001 Published, JBC Papers in Press, September 18, 2001, DOI 10.1074/jbc.M106182200
Chinpan Chen‡§, Chun-Hua Hsu‡¶, Ning-Yuan Su‡, Yu-Ching Lin储, Shyh-Horng Chiou¶储**, and Shih-Hsiung Wu¶储‡‡
From the Institutes of ‡Biomedical Sciences and储Biological Chemistry, Academia Sinica, Taipei 115, Taiwan and the ¶Institute of Biochemical Sciences, National Taiwan University, Taipei 106, Taiwan
Bungarus fasciatus fraction IX (BF9), a chymotrypsin
inhibitor, consists of 65 amino acid residues with three disulfide bridges. It was isolated from the snake venom of B. fasciatus by ion-exchange chromatography and belongs to the bovine pancreatic trypsin inhibitor (BPTI)-like superfamily. It showed a dissociation con-stant of 5.8ⴛ 10ⴚ8Mwith␣-chymotrypsin as measured by a BIAcore binding assay system. The isothermal ti-tration calorimetry revealed a 1:1 binding stoichiometry between this inhibitor and chymotrypsin and appar-ently no binding with trypsin. We further used CD and NMR to determine the solution structure of this venom-derived chymotrypsin inhibitor. The three-dimensional NMR solution structures of BF9 were determined on the basis of 582 restraints by simulated annealing and en-ergy minimization calculations. The final set of 10 NMR structures was well defined, with average root mean square deviations of 0.47 Å for the backbone atoms in the secondary structure regions and 0.86 Å for residues The side chains of Phe23, Tyr24, Tyr25, Phe35, and Phe47
exhibited many long-range nuclear Overhauser effects and were the principal components of the hydrophobic core in BF9. To gain insight into the structure-function relationships among proteins in the BPTI-like super-family, we compared the three-dimensional structure of BF9 with three BPTI-like proteins that possess distinct biological functions. These proteins possessed similar secondary structure elements, but the loop regions and
-turn were different from one another. Based on
resi-dues at the functional site of each protein, we suggest that the flexibility, rigidity, and variations of the amino acid residues in both the loop and -turn regions are related to their biological functions.
Proteins that belong to the bovine pancreatic trypsin inhib-itor (BPTI)1-like superfamily are present in a variety of living organisms. They display significant differences in amino acid sequences and in biological functions, but they all possess three disulfide bridges, which supposedly contribute to the overall stability of this class of proteins. In general, the BPTI-like superfamily is classified into two families based on protein structure: (a) small Kunitz-type inhibitors and BPTI-like tox-ins and (b) soft tick anticoagulant protetox-ins.
To date, structural studies have been extensively carried out on the BPTI-like superfamily proteins. For small Kunitz-type inhibitors, a number of x-ray crystal structures (1, 2) as well as the solution structure of BPTI (3) have been reported. Both NMR and x-ray three-dimensional structures of the human Kunitz-type protease inhibitor domain of the Alzheimer’s amy-loid-protein precursor (4, 5) have been solved, as have the NMR solution structures of the Kunitz-type domain from the human type VI collagen␣3(VI) chain (C5) (6) and of a Kunitz-type protease inhibitor from the sea anemone Stichodactyla helianthus (7). Most BPTI-like toxins isolated from snake venom are Ca2⫹ or K⫹ channel blockers, e.g. ␣-dendrotoxin, dendrotoxin K (DTK), dendrotoxin I, calcicludine, and the smaller subunit of-bungarotoxin from various snake sources (8, 9). The three-dimensional structures of these BPTI-like toxins have been determined using NMR and/or x-ray crystal-lography (10 –13). In the family of soft tick anticoagulant pro-teins, the NMR solution structure of tick anticoagulant protein (TAP), a highly selective inhibitor of blood coagulation factor Xa that exhibits no inhibitory activity against other common serine proteases such as trypsin, chymotrypsin, or thrombin, has been solved (14, 15). Ornithodorin, a potent and highly selective thrombin inhibitor, also belongs to this family, and the ornithodorin-thrombin complex crystal structure has been reported (16).
Bungarus fasciatus fraction IX (BF9), which consists of 65 amino acid residues with three disulfide bridges, is the fraction IX component isolated from the venom of B. fasciatus. It is a Kunitz-type protease inhibitor that possesses inhibitory action only against chymotrypsin and belongs to the BPTI-like super-family (17). Fig. 1 shows the sequence alignments of BF9 with seven other proteins in the BPTI-like superfamily, including three small Kunitz-type inhibitors (BPTI, C5, and S. helian-thus proteinase inhibitor), three BPTI-like toxins (dendrotoxin I, DTK, and calcicludine), and a soft tick anticoagulant protein (TAP). The sequence identity of BF9 to the Kunitz-type inhib-itors and BPTI-like toxins is⬃35–50%, but the sequence
iden-* This work was supported by Academia Sinica and the National Science Council (Taipei, Taiwan). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The atomic coordinates and structure factors (code 1JC6) have been deposited in the Protein Data Bank, Research Collaboratory for Struc-tural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
The resonance assignment of BF9 at 310 K and pH 3.0 has been deposited in the BioMagResBank under accession number 5050.
§ To whom correspondence may be addressed: Inst. of Biomedical Sci-ences, Academia Sinica, 128 Academia Rd., Section 2, Taipei 115, Taiwan. Tel.: 886-2-2652-3035; Fax: 886-2-2788-7641; E-mail: bmchinp@ccvax. sinica.edu.tw.
** To whom correspondence may be addressed: Inst. of Biological Chemistry, Academia Sinica, 128 Academia Rd., Section 2, Taipei 115, Taiwan. Tel.: 886-2-2785-5696 (Ext. 7010); Fax: 886-2-2653-0014; E-mail: shchiou@gate.sinica.edu.tw.
‡‡ To whom correspondence may be addressed: Inst. of Biological Sciences, Academia Sinica, 128 Academia Rd., Section 2, Taipei 115, Taiwan. Tel.: 886-2-2785-5696 (Ext. 7101); Fax: 886-2-2653-9142; E-mail: shwu@gate.sinica.edu.tw.
1The abbreviations used are: BPTI, bovine pancreatic trypsin
inhib-itor; DTK, dendrotoxin K; TAP, tick anticoagulant protein; BF9, B.
fasciatus fraction IX; TOCSY, total correlation spectroscopy; NOESY,
nuclear Overhauser effect correlation spectroscopy; NOE, nuclear Over-hauser effect.
© 2001 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A.
This paper is available on line at http://www.jbc.org
45079
at National Taiwan University on June 11, 2009
www.jbc.org
tity between BF9 and TAP is much smaller (13.3%). Laskowski and Kato (18) pointed out that the P1 site residue in the trypsin inhibitor is a positively charged residue (Arg or Lys) and in the chymotrypsin inhibitor usually is a large hydrophobic residue (Leu, Phe, or Tyr). The P1 reactive-site residue of BF9 has been identified, however, to be Asn17 (17). Recently, the P1 site residues His and Asn for chymotrypsin binding were also re-ported (19). It is therefore interesting to know the structural differences that are responsible for the distinct biological func-tions among the proteins in the BPTI-like superfamily. The Kunitz-type protease inhibitor possessing specific chymotryp-sin inhibition is very rare, and the detailed three-dimensional structure of this kind of protease inhibitor has not yet been reported. In this work, we used surface plasmon resonance and isothermal titration calorimetry to obtain the binding constant for BF9 and␣-chymotrypsin. In addition, we applied CD and NMR techniques to determine the solution structure of BF9. We then made a structural comparison between BF9 and BPTI, DTK, and TAP to shed some light on structure-function rela-tionships in these homologous proteins with distinct functions.
EXPERIMENTAL PROCEDURES
Sample Preparation—BF9 was isolated and purified from the snake
venom of B. fasciatus by ion-exchange chromatography on CM-cellulose and by gel filtration on Sephadex G-50 (17). Unless otherwise stated, all reagents and solvents were obtained commercially as reagent grade and used without further purification.
Surface Plasmon Resonance—Association and dissociation of BF9
and␣-chymotrypsin were measured with a BIAcore X instrument (BIA-core AB, Uppsala, Sweden). BF9 was coupled to a carboxymethyl-dextran CM5 sensor chip with an amine coupling kit containing N-eth-yl-N⬘-(3-dimethylaminopropyl)carbodiimide HCl, ethanolamine HCl, and N-hydroxysuccinimide. After the chip was activated by 35l of
N-ethyl-N⬘-(3-dimethylaminopropyl)carbodiimide HCl/N-hydroxysuc-cinimide mixture, BF9 was coupled to the CM5 sensor surface using 35 l of BF9 (5 M) in BIAcore binding buffer (10 mMHEPES, 150 mM
NaCl, and 3 mMEDTA (pH 7.4) containing 0.005% surfactant P2O). The
binding assay was performed with a constant flow rate of 30l/min at 25 °C with␣-chymotrypsin concentrations in the range of 10–500 nM. 5
MNaCl was used to regenerate the chip. For fitting of the binding
kinetics, BIAevaluation Version 3.0 software (BIAcore AB) was applied, and the 1:1 Langmuir binding model was chosen.
Isothermal Titration Calorimetry—Titration calorimetry
experi-ments were performed with a VP-ITC titration calorimetric system (Microcal Inc., Northampton, MA). 0.04 mMBF9 solution in 20 mM
HEPES (pH 7.5) in the calorimetric cell was titrated with 0.3 mM
␣-chymotrypsin dissolved in the same buffer in a 250-l injection sy-ringe at 30 °C. The raw calorimetry data were collected and analyzed by Origin Version 5.0 data analysis software (Microcal Inc.). The binding isotherms were fitted to the one-site binding model, giving values for the stoichiometry (N) of the interaction, the enthalpy of binding (⌬H), and the binding constant (K).
CD Experiments—CD experiments were carried out using an Aviv
202 instrument (Aviv, Lakewood, NJ) calibrated with ( ⫹)-10-cam-phorsulfonic acid at 25 °C. In general, a 2-mm path-length cuvette with 20MBF9 peptide in 20 mMphosphate buffer was used for CD
exper-iments, and all protein solutions were made up to 1 ml. The CD spectra of BF9 at different temperatures and pH values were recorded. Each of the CD spectra was obtained from an average of three scans with 1-nm bandwidth. The spectra were recorded from 180 to 260 nm at a scanning rate of 38 nm/min with a wavelength step of 0.5 nm and a time constant of 100 ms. After background subtraction and smoothing, all the CD data were converted from CD signal (millidegree) into mean residue
elliptic-ity (degrees cm2dmol⫺1). The secondary structure content was
esti-mated from the CD spectra according to the methods of CONTIN-LL, SELCON3, and CDSSTR (20).
NMR Experiments—The NMR measurements were carried out on a
Bruker AMX-500 or AVANCE-600 spectrometer (Bruker, Karlsruhe, Germany). Samples for NMR experiments contained 0.35 ml of 2 mM
BF9 in 50 mMphosphate buffer at pH 3.0, 3.9, and 7.0. pH values were measured with a dissolved-oxygen (DO) microelectronic pH-vision Model PHB-9901 pH meter equipped with a 4-mm electrode. All re-ported pH values were direct readings from the pH meter without correction for isotope effect. For monitoring the exchange rates of labile protons, the concentrated sample in H2O was lyophilized only once and
redissolved in D2O (99.99% D). NMR spectra were acquired
immedi-ately and thereafter at appropriate time intervals. All chemical shifts were externally referenced to the methyl resonance of 2,2-dimethyl-2-silapentane-5-sulfonate (0 ppm). Double quantum filtered COSY (21), TOCSY (22), and NOESY (23) spectra were collected with 512 t1
incre-ments and 2048 complex data points. All spectra were recorded in time-proportional phase-sensitive mode (24). Low temperature studies employed a temperature-controlled stream of cooled air using a Bruker BCU refrigeration unit and a B-VT 2000 control unit. Water suppres-sion was achieved by 1.4 s of pre-saturation at the water frequency or by the gradient method (25). All spectra were collected with 6024.1- and 7788.16-Hz spectral widths for AMX-500 and AVANCE-600, respectively.
The data were transferred to an SGI O2200-MHz R5000SC work
station (Silicon Graphics, Mountain View, CA) for all processing and further analysis using the Bruker XWINNMR and AURELIA software packages. All data sets acquired were zero-filled to equal points in both dimensions prior to further processing. A 60°-shifted skewed sine bell window function was used for all NOESY and TOCSY spectra and a 20°- or 30°-shifted skewed sine bell function was used for all COSY spectra. To help with resolving spectral overlap, data were collected at different temperatures.
Torsional Angle Restraints and Stereospecific Assignment—The
3
JNH␣coupling constants were estimated from the residual intensity of
the anti-phase cross-peak in double quantum filtered COSY spectra recorded in H2O. torsional restraints of ⫺130 ⫾ 30° for
3J
NH␣coupling
constants⬎8 Hz and ⫺60 ⫾ 30° for3J
NH␣coupling constants⬍6 Hz
were used for structure calculation. We obtained a total of 20 torsional restraints mainly located in the␣-helix and -strand regions. The torsional restraints were used for structure generations starting from the early stage when NOE correlations were also consistent. The ste-reospecific assignments were derived using the method of Hyberts et al. (26). The stereospecific assignment of-methylene allowed us to assign the1torsional angle restraints to 60⫾ 30°, 180 ⫾ 30°, or ⫺60 ⫾ 30°.
To ensure the accuracy of stereospecific assignments, we obtained only 13 prochiral assignments with certainty. We found that the stereospe-cific assignments agreed well with our generated structures in the early stage. Thus, in the later stage of structure generation, we also added1
and prochiral assignments as restraints in the structure calculation.
Hydrogen Bond and Disulfide Restraints—The amide proton
ex-change rates were identified from residual amide proton signals ob-served in several TOCSY spectra recorded at 310 K and pH 3.0 and 7.0. The first spectrum was recorded within 5 h after the lyophilized sample was redissolved in D2O. The amide proton exchange rates were
catego-rized into three classes: fast, medium, and slow. Hydrogen bond forma-tion or solvent exclusion from the amide protons was assumed to ac-count for the slow and medium exchanging amide protons. Most of these protons have been identified to be associated with particular secondary structures. For better convergence, a number of hydrogen bonds in-volved in the secondary structure were included as distance restraints in the final stage of structure generation, i.e. an O–N distance of between 2.5 and 3.3 Å and O–HN distances of 1.8 and 2.5 Å between NH protons and the backbone carbonyl oxygen atoms were assigned to the slow and medium exchanging protons, respectively, in the latter stage FIG. 1. Sequence of BF9 aligned with those of seven BPTI-like superfamily proteins for which three-dimensional solution structures have been determined. These are BPTI (3), C5 (6, 37), the S. helianthus proteinase inhibitor (ShPI) (7), dendrotoxin I (DTI) (12),
DTK (11), calcicludine (CALC) (13), and TAP (15). The three disulfide bridges are shown by brackets.
at National Taiwan University on June 11, 2009
www.jbc.org
of structure determination. In the final stage of structure calculation, the hydrogen bonds between NHiand OCjin the-sheet structures
were included as restraints only if the-sheet inter-strand NHi/NHj,
NHi/C␣Hj⫹1, and C␣Hi⫺1/C␣Hj⫹1NOE cross-peaks were observed. The
disulfide bonds used in the structure calculation were Cys7–Cys57,
Cys16–Cys40, and Cys32–Cys53. Covalent bonds between the sulfur
atoms of disulfide bridges were modeled by restraining the distances between the two sulfur atoms to 1.80 –2.30 Å.
Tertiary Structure Calculations—Distance restraints of BF9 were
derived primarily from the 200-ms NOESY spectrum recorded in the aqueous solution at 310 K and pH 3.0. Comparison was made with the 100-ms NOESY spectrum to assess possible contributions of the NOEs from spin diffusion. Peak intensities were classified as large, medium, small, and very small, corresponding to upper bound inter-proton dis-tance restraints of 2.5, 3.5, 4.5, and 6.0 Å, respectively. An additional correction of 1.0 Å was added for methylene and methyl groups. Back-bone dihedral restraints were inferred from3J
NH␣coupling constants,
with restrained to ⫺130 ⫾ 30° for3J
NH␣⬎ 8 Hz and ⫺60 ⫾ 30° for 3J
NH␣⬍ 6 Hz. The structure determination was performed using 517
distance restraints, of which 127 were intra-residue, 172 were sequen-tial, and 218 were medium- and long-range inter-proton distances; and 63 were additional restraints, including 32 hydrogen bonds, 20 tor-sional angles, and 131torsional angles. All simulated minimization
and dynamic annealing calculations were carried out with the program X-PLOR Version 98 (27) on an SGI O2work station. The Insight II
(Molecular Simulation Inc., San Diego, CA), MOLMOL (28), and GRASP (29) programs were used to visually observe sets of structures and to calculate and make the electrostatic surface potential of the final three-dimensional models. The distributions of the backbone dihedral angles of the final converged structures were evaluated by the repre-sentation of the Ramachandran dihedral pattern, revealing the devia-tions from the sterically allowed (, ) angle limits using MOLMOL and PROCHECK-NMR (30).
RESULTS
Binding Studies with ␣-Chymotrypsin—To determine the dissociation constant for BF9 with ␣-chymotrypsin, surface plasmon resonance measurements were carried out. BF9 was coupled to a carboxymethyl-dextran CM5 sensor chip with an amine coupling kit. Binding was observed upon injection of different concentrations of ␣-chymotrypsin (Fig. 2). The pla-teau values reached after completion of the association reaction were analyzed by a Langmuir binding isotherm. A dissociation constant of 5.8⫻ 10⫺8Mwas obtained. Another complementary method used to study the binding of BF9 to ␣-chymotrypsin was isothermal titration calorimetry. Fig. 3 shows the experi-mental data for titration of BF9 with ␣-chymotrypsin using
isothermal titration calorimetry. The analysis of the data re-vealed a 1:1 stoichiometry of the two binding partners, a Kd
value of 2.3⫻ 10⫺7M, and an apparent⌬H of ⫺6.45 kcal/mol. There is apparently no binding between BF9 and trypsin as measured by the same assay system.
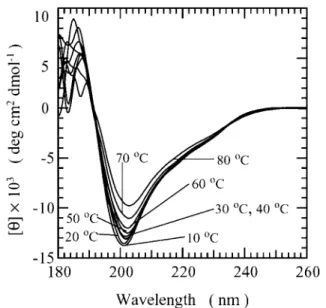
BF9 Is a Highly Stable Protease Inhibitor—We performed CD experiments on BF9 at different pH values (pH 3.5–7.0) and at different temperatures (0 – 80 °C). We found that BF9 possessed similar conformations at different pH values. The CD spectra from 0 to 80 °C at pH 3.5, as shown in Fig. 4, revealed a decrease in negative band intensity when the tem-perature was increased, whereas the minimum stayed almost the same at 203 nm. This indicated that the majority of the secondary structure still existed even at 80 °C. Thus, BF9 is a highly thermostable protein, similar to BPTI, which has a denaturation temperatureⱖ95 °C at pH 4.6 (6). The contents of the secondary structures of BF9 estimated using CONTIN-LL, SELCON3, and CDSSTR (20) are listed in Table I. The con-tents are comparable, and the average concon-tents of the␣-helix, -strand, -turn, and unordered forms are 17.9, 22.7, 24.2, and 35.2%, respectively.
Resonance Assignments and Secondary Structure Determina-tion—With high thermostability and well dispersed NMR data, BF9 is an excellent candidate for NMR structural studies. Initially, we carried out NMR studies at neutral pH. We could not obtain confident and complete resonance assignments due
FIG. 2. Representative overlaid sensorgram for kinetic study
of ␣-chymotrypsin binding to BF9 measured by a BIAcore X system. BF9 was immobilized on a CM5 sensor chip by amine coupling.
The␣-chymotrypsin was injected over the sensor chip at concentrations ranging from 10 to 500 nM: trace a, 10 nM; trace b, 30 nM; trace c, 50 nM;
trace d, 100 nM; trace e, 200 nM; trace f, 300 nM; trace g, 400 nM; trace h, 500 nM. Raw binding data were analyzed by BIAevaluation Version 3.0 Software and fit to a 1:1 Langmuir binding model. RU, response units.
FIG. 3. Isothermal titration calorimetry data of BF9 titrated
with␣-chymotrypsin. Experiments were carried out in an VP-ITC
system at 30 °C with stirring at 300 rpm. A, raw data in microcalories/s
versus time showing heat release upon injections of 0.3 mM
␣-chymo-trypsin into a 1.4-ml cell containing 0.04 mMBF9; B, integration of the raw data yields the heat/mol versus molar ratio. The best values of the fitting parameters are 0.95 for N, 4.3 ⫻ 106
M⫺1 for K, and⫺6.45
kcal/mol for⌬H.
at National Taiwan University on June 11, 2009
www.jbc.org
to several missing backbone and side chain amide protons, presumably due to their fast exchange with H2O. To lower the exchange rate, we performed NMR experiments at pH 3.9 and 3.0, and the missing protons all appeared and were identified. Based on the NMR data acquired at different pH values and temperatures, the resonance assignments were accomplished using the standard procedures (31), in which spin systems were identified based on COSY and TOCSY experiments, and
sequential connectivities were obtained from NOESY
experiments.
The fingerprint region of a well resolved TOCSY spectrum with partial annotation is shown in Fig. 5. We met with several difficulties when assigning resonance due to the presence of the same chemical shifts for both C␣H and CH in Thr5 and of a higher CH chemical shift (4.03 ppm) than a C␣H chemical shift (3.77 ppm) in Ser27. Table II lists the nearly complete chemical shift assignments for BF9 at 310 K and pH 3.0. We found several unusual chemical shifts. For example, C␣H pro-tons of Cys53and Ile50possessed upfield shifts at 1.89 and 3.05 ppm, respectively. We observed the amide proton of Tyr25at a downfield shift of 10.55 ppm and identified CH2(0.24 and 0.47 ppm) and C␥H2 (0.38 and 0.67 ppm) of Pro11in the upfield regions. Interestingly, we also found these unusual shifts, with the exception of HN(Phe26) and CH
2 (Tyr52) in TAP, in the corresponding residues of other BPTI-like proteins.
Fig. 6 shows the summary of the NMR parameters for BF9 at pH 3.0. The C␣H chemical shift index indicated that Arg3–Lys7 and Ile50–Ala58 formed ␣-helical structures and that Leu19– Tyr24, Lys31–Tyr37, and Asn45–Thr49exhibited-strand confor-mations. Based on the␣-helical NOEs, we observed a regular ␣-helix in the C-terminal Ile50–Cys57segment, which was in good agreement with chemical shift index results. The chemical shift index method also predicted the existence of an␣-helix in the N-terminal Arg3–Lys7segment. However, we observed only an␣-helical NOE, d␣N(6, 9), in this segment, indicating that this region likely forms a turn-like or 310helix conformation. According to the observed long-range NOEs and the deduced hydrogen bonds consistent with the -sheet structure, as shown in Fig. 7, we identified that a double-stranded antipar-allel -sheet, spanning Ala22–Asn26 for the first strand and Lys31–Asn36 for the second, occupies a central place in the sequence. Furthermore, we observed a-turn conformation in Ser27–His30between the
1- and2-strands. By contrast, there were only three inter-residue NOEs between1and3. Among
these crossover NOEs, d␣N (24, 47) and d␣␣ (24, 46) were present only at a higher mixing time, and their intensities were very weak. Therefore, it is likely that only Phe47 associated with Phe23to form a one-residue third strand in an antiparallel manner, which is a feature that was previously reported for other proteins in the BPTI-like superfamily.
We observed an intensive d␣␦(i, i⫹1)NOE for all three proline residues (Pro4, Pro11, and Pro21) in BF9, indicating that the
trans-conformation is predominant. To check the rigidity and to identify the hydrogen bonds, we carried out an amide proton exchange study of BF9 at 310 K and pH 3.0 and 7.0. We observed 22 amide protons at pH 3.0 (Asn8, Leu9, Ile20, Ala22, Phe23, Tyr24, Tyr25, Asn26, His30, Lys31, Gln33, Phe35, Asn45, Asn46, Phe47, Thr49, Cys53, Gln54, Arg55, Thr56, Cys57, and Ala58) that possessed medium or slow exchange rates. By con-trast, only 10 amide protons at pH 7.0 (Phe23, Tyr24, Tyr25, Asn26, Lys31, Gln33, Phe35, Phe47, Cys53, and Gln54) showed medium and slow exchange rates. These residues with medium and slow amide proton exchange rates were all located in polypeptide segments for which the spatial structure was well defined by the NMR data. The residues are mostly amide protons that form characteristic hydrogen bonds in the regular secondary structure elements. Comparison of the exchange rate data revealed that the two-strands, the one-residue third -strand at Phe47, and the C-terminal␣-helix at Ile50–Gln54 possessed high stability based on our observation of slow ex-change rates in these regions at both pH values.
Three-Dimensional Solution Structure of BF9 —A set of 582 restraints was used for simulated annealing and energy mini-mization calculations using the program X-PLOR. Among these restraints, 517 were inter-proton distances, 32 were hy-drogen bonds, and 33 were dihedral angles. Ten structures with the lowest target function and minimal distance and tor-sional angle restraint violations in the final stage were chosen to represent the ensemble of NMR structures. These structures were consistent with both experimental data and standard covalent geometry and displayed no violations⬎0.5 Å for dis-tance restraints. Superposition of each structure with the mean structure yielded average root mean square deviations 0.86 Å for the backbone atoms in residues 3–58 and 0.47 Å for the backbone atoms in the well defined secondary structure regions Pro21–Asn26, Lys31–Asn36, and Ile50–Cys57, as shown in Fig. 8A. Due to the presence of fewer NOE distance restraints, the loop regions (Glu12–Leu19and Gly38–Ala44) are less well de-fined, as shown in Fig. 8B. The residues in these loop regions all possess fast amide proton exchange rates, further indicating a high flexibility in these regions.
The structural statistics on the final set of structures are given in Table III. Analysis of the ensemble of 10 structures using PROCHECK-NMR revealed that 97% of the residues lie in the most favored and additional allowed regions of the Ra-machandran/ dihedral angle plot (Fig. 9). The distribution of, , and 1dihedral angles (data not shown) further dem-onstrated the rigidity of the secondary structure regions. The solution structures of BF9 depict the well known Kunitz-type inhibitor fold. The core of the domain, comprising the second-ary structure elements and two of the three disulfide bonds (Cys7–Cys57and Cys32–Cys53), was exceptionally well defined. The side chains of Phe23, Tyr24, Tyr25, Phe35, and Phe47 exhib-ited an extremely large number of long-range NOEs, and close inspection of the three-dimensional structure revealed that these residues are the principal components of the hydrophobic core in BF9.
Hydrogen Bond Networks and Surface Structure—An exam-ination of the atomic positions in the above 10 solution struc-tures provides information about the hydrogen bonds. In this
FIG. 4. CD spectra of 20MBF9 in 20 mMphosphate buffer at
pH 3.5 shown as a function of temperature. deg, degrees.
at National Taiwan University on June 11, 2009
www.jbc.org
regard, one hydrogen bond was considered to be formed if the hydrogen distance to the acceptor atom was⬍2.5 Å and the O-H-N angle was⬎125°. Table IV lists the backbone–backbone hydrogen bonds identified in ⬎30% of the 10 NMR solution structures. Most of the hydrogen bonds are situated in the -sheet and C-terminal ␣-helix regions, with some of the hy-drogen bonds located outside of well defined secondary struc-ture regions. For example, in the N-terminal region, we iden-tified two hydrogen bonds, Asn8NH–Thr5 CO and Leu9NH– Phe6 CO. The formation of two hydrogen bonds at Asn8 and Leu9 is consistent with exchange rate results showing that Asn8 and Leu9 possess a medium and slow exchange rate, respectively (see Fig. 6). Also, it further indicated that the N-terminal Thr5–Leu9segment formed a 3
10␣-helical confor-mation. In the-turn region (Ser27–His30), we saw two hydro-gen bonds, His30NH–Asn26CO and His30NH–Ser27CO. BF9 is a basic protein (pI 9.22) with four arginine, five lysine, one aspartate, and two glutamate residues. At neutral pH, it car-ries an overall positive charge, but the distribution of the charge is highly asymmetric. There are two groups of positively charged side chains situated close to each other at the “base” of the molecule: one close to the N terminus (Lys1and Arg3) and the other in the second-strands (Lys31and Lys34). There are also several uncompensated positive charges scattered over the surface of the molecule (Arg15and Lys48).
DISCUSSION
Using surface plasmon resonance and isothermal titration calorimetry, we confirmed that there is characteristic and spe-cific binding between BF9 and chymotrypsin. In contrast, the binding property was not observed between BF9 and trypsin under the same conditions using both approaches. The
discrep-ancy in the dissociation constant values measured by BIAcore binding assay and isothermal calorimetry is probably due to different buffers used in these two methods. We found that reproducibility was greater in the BIAcore assay system com-pared with calorimetry. On the other hand, the binding stoi-chiometry was easier to obtain by the calorimetry method. These two methods were complementary in our study of the binding constant. Therefore, we believe that the value of 5.8⫻ 10⫺8M obtained by BIAcore binding assays is more accurate than the valued obtained by isothermal calorimetry. Note that the chymotrypsin inhibitors from the hemolymph of silkworm larva (Bombyx mori) (32) and from the venom of Vipera am-modytes amam-modytes (33) possess dissociation constants of 4.3⫻ 10⫺9and 1.3⫻ 10⫺8M, respectively.
CD spectra at different temperatures and pH values demon-strated that BF9 is a protein of high thermostability and that its conformation is independent of pH in the range of 3.0 –7.0. It is noteworthy that the results from secondary structure estimation by CD (as shown in Table I) are very close to those from structural analysis by NMR, as described below.
The three-dimensional solution structure of BF9 is shown to be composed mainly of a double-stranded antiparallel-sheet and a C-terminal␣-helix. A one-residue -strand was observed at Phe47based on NOE connectivities and amide proton ex-change rates. We found a -turn structure at Ser27–His30, which connects the two major-strands. Based on its three-dimensional structure, the unusual chemical shifts we ob-served for BF9 were due to an aromatic ring current effect. The upfield shifts for the C␣H resonance of Ile50and Cys53are due to the ring current effect of Phe23and Phe47, respectively. The aromatic ring of Tyr24 is the major factor that causes the unusual chemical shifts for Pro11. The aromatic ring of Phe47 causes the downfield shift of the Tyr25amide proton. Moreover, these aromatic residues form a very rigid hydrophobic core in BF9. The sequence alignment in Fig. 1 shows that the residue corresponding to Phe23in BF9 in other proteins also possesses an aromatic residue, with the exception of a charged residue (Glu) in TAP. It is likely that this charged residue causes some change in the location and environment of the hydrophobic core in TAP. Consequently, the orientations of aromatic residues are different, and the unusual chemical shifts are hence not observed in TAP.
To further evaluate the structural differences among the proteins in the BPTI-like superfamily, we also compared the amide proton exchange rates of BF9 with those reported for five other proteins in the BPTI-like superfamily, as shown in Fig. 10. These exchange rates were all determined under acidic conditions (pH 3.0⬃5.2) and at temperatures between 30 and 38 °C. We have used these data to compare the structural differences among the different proteins. Interestingly, the me-dium and slow exchanging amide protons in these proteins are all located in the N-terminal 310␣-helix, -sheet, and C-termi-nal␣-helix regions, with a few located close to the secondary structure, similar to BF9.
The exchange data are in good agreement with the formation of the C-terminal ␣-helix for all proteins. In the N-terminal turn-like or 310␣-helix regions, two residues possess slow ex-change rates in four (BF9, BPTI, calcicludine, and TAP) of six
TABLE I
Secondary structure contents of BF9 estimated from CD spectrum acquired at pH 3.5 and 30 °C using three different methods
Methods ␣Ra ␣D R D T U
CONTIN-LL 0.055 0.122 0.115 0.095 0.262 0.351 SELCON3 0.081 0.114 0.134 0.087 0.228 0.362 CDSSTR 0.045 0.120 0.163 0.090 0.237 0.345
a␣
R, regular␣-helix; ␣D, distorted␣-helix; R, regular-strand; D, distorted-strand; T, turns; U, unordered.
FIG. 5. Fingerprint region of a TOCSY spectrum of BF9 ac-quired at 310 K and pH 3.0. The assignments are indicated by
one-letter amino acid codes and residue numbers. The C␣H of Cys53
located at 1.89 ppm is not shown in the spectrum, and CH2is labeled
instead. The side chain N⑀H/C␣H cross-peaks of Arg residues are
la-beled with asterisks. The side chain C␦2H/CH2cross-peaks of His 28and
His30are also annotated.
at National Taiwan University on June 11, 2009
www.jbc.org
proteins. This indicates the flexibility of the N-terminal region in BPTI-like superfamily proteins. In the antiparallel-sheet, TAP possesses a distinct exchange rate phenomenon and con-tains more residues with fast exchange rates. Therefore, it seems that the-sheet in TAP is less stable than that in the other proteins. In the BF9 2-strand, Tyr37 displays a fast
exchange rate, whereas the corresponding residue in other BPTI-like proteins shows a slow exchange rate. Moreover, we observed a very broad NMR resonance line width of the amide proton of Tyr37in BF9, and no hydrogen bond was detected in the backbone atoms of Tyr37in any of the 10 NMR structures. These observations indicate that Tyr37 is not located in the
TABLE II
1H chemical shifts for BF9 in aqueous solution at 310 K and pH 3.0, using 2,2-dimethyl-2-silapentane-5-sulfonate resonance (0.00 ppm)
as a reference
Residue Chemical shift
NH C␣H CH C␥H Others ppm Lys1 4.02 1.92, 1.92 1.48, 1.48 C␦H 2, 1.74, 1.74; CH2, 3.03, 3.03 Asn2 8.71 4.73 2.68, 2.77 Arg3 8.28 4.25 1.63, 1.63 1.41, 1.47 C␦H 2, 2.85, 3.03; NH, 7.01 Pro4 4.27 0.90, 0.90 1.72, 1.90 C␦H 2, 3.54, 3.78 Thr5 8.52 3.87 3.87 1.32 Phe6 7.13 4.55 3.10, 3.22 C␦H2, 7.01, 7.01; CH2, 7.41, 7.41; CH, 7.36 Cys7 7.21 4.35 2.06, 2.80 Asn8 7.45 4.98 2.76, 3.04 N␦H 2, 6.86, 7.48 Leu9 7.45 4.39 1.92, 1.92 1.64 C␦H 3, 0.95, 1.06 Leu10 8.30 4.36 1.47, 1.62 1.85 C␦H3, 0.99, 0.99 Pro11 4.28 0.24, 0.47 0.38, 0.67 C␦H 2, 3.06, 3.36 Glu12 7.49 4.74 1.75, 2.02 2.33, 2.33 Thr13 8.92 4.49 4.32 1.40 Gly14 8.55 4.03, 4.31 Arg15 8.17 4.43 1.76, 1.91 1.62, 1.62 C␦H 2, 3.18, 3.18; NH, 7.13 Cys16 8.56 4.55 2.80, 3.35 Asn17 8.04 4.81 2.70, 2.81 N␦H 2, 6.73, 7.41 Ala18 8.00 4.31 1.20 Leu19 7.82 4.35 1.40, 1.53 1.26 C␦H 3, 0.71, 0.82 Ile20 8.40 4.54 1.97 0.94, 0.94 C␦H 3, 1.05; C␥H3, 1.40 Pro21 4.56 1.88, 2.13 1.99, 2.22 C␦H 2, 3.74, 3.83 Ala22 8.52 4.55 0.97 Phe23 9.17 5.86 2.75, 2.88 C␦H 2, 6.82, 6.82; CH2, 7.38, 7.38; CH, 7.36 Tyr24 9.69 5.23 2.71, 2.75 C␦H 2, 6.92, 6.92; CH2, 6.61, 6.61 Tyr25 10.55 4.30 2.74, 3.60 C␦H 2, 7.19, 7.19; CH2, 6.34, 6.34 Asn26 7.89 4.65 2.15, 2.95 N␦H2, 7.32, 7.72 Ser27 8.44 3.77 4.03, 4.03 His28 8.12 4.45 3.35, 3.35 C␦2H, 7.28, C1H, 8.62 Leu29 7.29 4.17 1.44, 1.47 1.13 C␦H 3, 0.75, 0.83 His30 7.75 3.92 3.34, 3.52 C␦2H, 7.20; C1H, 8.56 Lys31 7.05 4.67 1.58, 1.78 1.11, 1.11 C␦H 2, 1.18, 1.18; CH2, 2.94, 2.94 Cys32 8.99 5.45 2.61, 3.50 Gln33 9.27 4.82 1.63, 1.63 2.05, 2.14 NH 2, 6.91, 7.59 Lys34 8.42 4.82 1.62, 1.69 0.98, 0.98 C␦H2, 1.19, 1.19; CH2, 2.90, 2.90 Phe35 9.33 4.85 3.04, 3.19 C␦H 2, 7.02, 7.02; CH2, 7.13, 7.13; CH 6.86 Asn36 8.34 4.85 2.21, 2.60 N␦H 2, 6.75, 7.34 Tyr37 8.84 4.73 2.39, 2.66 C␦H 2, 7.27, 7.27; CH2, 6.72, 6.72 Gly38 8.44 3.44, 4.23 Gly39 8.02 4.20, 4.20 Cys40 7.72 4.89 3.10, 3.53 Gly41 8.97 3.90, 4.01 Gly42 8.70 3.84, 4.38 Asn43 8.63 4.82 2.78, 3.07 N␦H 2, 7.61, 7.80 Ala44 7.85 3.91 0.61 Asn45 7.96 4.86 2.99, 3.18 N␦H 2, 7.90, 7.91 Asn46 6.55 4.85 2.44, 2.44 N␦H2, 6.82, 7.18 Phe47 9.78 5.02 2.74, 3.33 C␦H 2, 7.28, 7.28; CH2, 7.80, 7.80; CH, 7.59 Lys48 8.95 4.63 2.09, 2.09 1.75, 1.78 C␦H 2, 1.64, 1.64; CH2, 3.07, 3.07 Thr49 7.31 4.83 4.47 1.24 Ile50 8.16 3.05 0.65 0.91, 1.00 C␦H3, 0.76; C␥2H3, 0.76 Asp51 7.84 4.28 2.58, 2.63 Glu52 7.58 3.92 2.25, 2.25 2.56, 2.56 Cys53 6.94 1.89 2.92, 3.22 Gln54 8.61 3.70 1.96, 2.16 2.32, 2.55 NH2, 6.77, 7.16 Arg55 8.11 3.92 1.71, 1.78 1.53, 1.53 C␦H 2, 3.11, 3.11; NH, 7.25 Thr56 7.47 4.03 3.92 1.56 Cys57 7.83 4.66 1.90, 2.19 Ala58 7.61 4.28 1.43 Ala59 7.85 4.18 1.27 Lys60 7.92 4.19 1.60, 1.65 1.29, 1.29 Tyr61 8.04 4.59 2.86, 3.12 C␦H 2, 7.02, 7.02; CH2, 7.13, 7.13 Gly62 8.19 3.93, 3.93 Arg63 7.84 4.23 1.74, 1.85 1.63, 1.63 C␦H 2, 3.18, 3.18; NH, 7.13 Ser64 8.49 4.46 3.92, 3.92 Ser65 8.35 4.51 3.90, 3.90
at National Taiwan University on June 11, 2009
www.jbc.org
2-strand and is instead at the edge of this strand. In contrast, the backbone amide proton of Asn45in BF9 possesses a medium exchange rate, whereas the corresponding protons in all other proteins show a fast exchange rate. This suggests that the local structures of Asn45 and its nearby conformation in BF9 as compared with the other proteins are different. In the-turn (Ser27–His30in BF9), the corresponding residues in BPTI and TAP all show a fast exchange rate. Most of the residues in this turn region are also small residues (Gly and Ala) in BPTI and TAP. Thus, the-turn in BPTI and TAP is likely more flexible.
Therefore, there are different exchange rates in the well de-fined secondary structure regions in the BPTI-like superfamily, revealing that the proteins in the BPTI-like family possess different stabilities in their secondary structures and hence distinct denaturing temperatures.
The functional sites of BPTI were found in segments 12–16
FIG. 6. Summary of the amide proton exchange rates, NOE connectivities, C␣H proton chemical shift index, and derived secondary structures. Open and closed circles represent medium and
slow exchanging amide protons, respectively. Bar thickness indicates the intensity of NOE connectivity, with thicker bars representing stron-ger NOEs. Negative bars in the chemical shift index (CSI) indicate upfield shifts of⬎0.1 ppm of the C␣H proton compared with the
ex-pected random-coil C␣H proton chemical shift. Positive bars indicate downfield shifts of⬎0.1 ppm of the C␣H proton compared with the
expected random-coil C␣H value.
FIG. 7. Definition of the -sheet structure of BF9 is shown based on the NOEs and amide proton exchange rate. Long-range
NOEs between-strands are shown by double-headed arrows. Dashed
lines between backbone amide protons and backbone carbonyl oxygens
indicate hydrogen bonds consistent with slow exchanging HNobserved
in D2O. The amide protons with slow exchange rates are circled.
FIG. 8. A, superimposition of the backbone atoms (N, C␣, C⬘, and O) of 10 NMR structures obtained from simulated annealing and energy minimization calculations. The structures are best fitted to residues 21–26, 31–36, and 50 –57. B, ribbon structure of the mean NMR solution structure of BF9. A spline function was drawn through the C␣atoms, and the radius of the cylindrical rod corresponds to the mean of the global displacements. Only residues 2– 60 are shown in both structures.
TABLE III
Structural statistics of the final set of 10 simulated annealing structures of BF9 A. Constraints used Distance restraints Intra-residue 127 Sequential 172 Medium-range 65 Long-range 153 Total distance 517 Hydrogen bonds 16⫻ 2 Dihedral angles 33 B. Statistics for the final X-PLOR structures
Number of structures in the final set 10 X-PLOR energy (kcal/mol)a
ENOE 25.53⫾ 1.36
Ecdih 14.80⫾ 1.28
Ebond⫹ Eangle⫹ Eimproper 215.51⫾ 5.03
Eelec 2.13⫾ 0.10
EVDW 107.15⫾ 1.41
NOE violations
No.⬎0.5 Å None
r.m.s.bdeviation (Å) 0.048
Deviation from idealized covalent geometry
Angle (°) 7.79⫾ 0.43 Impropers (°) 0.52⫾ 0.02
Bonds (Å) 0.005
Mean global r.m.s. deviation (Å) Backbone (N, C␣, C⬘) Residues 21–26, 31–36, 50–57 0.47⫾ 0.36 Residues 3–58 0.86⫾ 0.23 Heavy atoms Residues 21–26, 31–36, 50–57 1.26⫾ 0.23 Residues 3–58 1.64⫾ 0.38 Ramachandran data
Residues in most favored regions (%) 68.3 Residues in allowed regions (%) 28.7 Residues in generously allowed regions (%) 2.0 Residues in disallowed regions (%) 0.9
aE
cdih, torsion angle energies; Eelec, electrostatic energies; EVDW, van
der Waals repulsive emergies.
bRoot mean square.
at National Taiwan University on June 11, 2009
www.jbc.org
and 36 –38, corresponding to Gly14–Ala18and Gly38–Cys40 in BF9. These functional residues are all located in the loop re-gions and possess fast exchange rates. Different active-site residues were identified in BPTI-like toxins. For example, the toxic site of DTK comprises several positively charged residues in the N terminus (Lys3and Lys6) and Lys24, Lys26, and Lys28 in the-turn (34). For TAP, the first four residues (Tyr1, Asn2,
Arg3, and Leu4) in the N terminus are the active-site residues (35). Furthermore, a secondary binding determinant located in the C-terminal␣-helix was observed in the complex structure of TAP with bovine factor Xa (36). To gain insight into ture-function relationship of BF9, its three-dimensional struc-ture was compared with those of BPTI, DTK, and TAP, which possess different biological functions. Even though these four proteins possess varied degrees of sequence identity, the well defined secondary structure regions, which consist of the -sheet and the ␣-helix, are highly similar, as shown in Fig. 11A. Also, the aromatic residues that facilitate protein stabili-zation by forming a hydrophobic interior are generally con-served in these four proteins. Outside of the regular secondary structure elements, the fold of these four proteins is somewhat different. Because the active-site residues are mostly located in the loop or-turn regions, as described above, one can postu-late that the biological difference in BPTI-like proteins is due to a major change in the surface topology of the solvent-exposed amino acid side chains. As shown in Fig. 11B, the -turn in DTK is more exposed to the aqueous phase and is closer to the N terminus compared with the-turn in BF9 and BPTI. DTK also contains several positively charged residues in these two regions, whereas BF9 and BPTI do not have this characteristic. Thus, the closer location between the-turn and the N-termi-nal region when combined with dense positively charged resi-dues might be a key factor for the unique biological activities of BPTI-like toxins such as DTK. By contrast, for the protease inhibitory activity in BF9 and BPTI, the functional site is located in the two flexible loop regions. The amino acid types in these loop regions likely play an important role in causing different inhibitory activities. The -turn and loop conforma-tions in TAP are very different from the conformaconforma-tions in BF9 (Fig. 11C), BPTI, and DTK. This explains why TAP does not possess protease inhibition and channel blocker activities. In summary, we conclude that the proteins in the BPTI-like
su-FIG. 9. Ramachandran plot of and dihedral angles for the ensemble of 10 NMR structures of BF9 generated using the PROCHECK-NMR program. Triangles in the plots represent the
angles for glycine residues.
TABLE IV
List of backbone– backbone hydrogen bonds identified in⬎30% of the
BF9 structures, as calculated by insight II
NH donor O acceptor Conformers 2nd structure
Asn8 Thr5 10 3 10␣-helix Leu9 Phe6 3 3 10␣-helix Ala22 Ile20 10 Phe23 Phe47 10 -Sheet Tyr24 Gln33 10 -Sheet
Asn26 Lys31 10 -Sheet
His30 Asn26 7 -Hairpin
His30
Ser27
4 -Hairpin Gln33 Tyr24 10 -Sheet
Phe35 Ala22 10 -Sheet
Phe47 Phe23 9 -Sheet
Lys53
Thr49
5
Gln54 Ile50 10 ␣-Helix
Gln54 Asp51 5
Arg55 Asp51 10 ␣-Helix
Thr56
Glu52
10 ␣-Helix Cys57 Cys53 10 ␣-Helix
FIG. 10. Comparison of the amide proton exchange rates of
BF9 with the published exchange rate data for the other five protein members of the BPTI superfamily. The amide protons of
the boxed residues possess medium or slow exchange rates for BF9 and the corresponding residues in the other proteins. The conditions used for exchange rate experiments were as follows: 37 °C and pH 3.0 for BF9; 36 °C and pH 3.5 for BPTI (38); 36 °C and pH 4.6 for the S.
helianthus proteinase inhibitor (ShPI) (7); 38 °C and pH 5.2 for
dend-rotoxin I (DTI) (12); 30 °C and pH 4.5 for calcicludine (CALC) (13); and 36 °C and pH 3.6 for TAP (14). The locations of the secondary structures of BF9 are shown and labeled at the top.
FIG. 11. A, ribbon diagrams of BF9, BPTI (accession number PDB-6PTI) (2), DTK (accession number PDB-1DTK) (11), and TAP (accession number PDB-1TAP) (14). The ribbon structures were produced using the MOLMOL program. B, pairwise root mean square deviation com-parisons of the solution structures of BF9 (yellow) and the two homol-ogous proteins, PBB-BPTI (orange) and DTK (white). The root mean square deviations were calculated for the backbone atoms of residues 4 –58, corresponding to residues 2–56 in BPTI and DTK. C, pairwise root mean square deviation comparisons of the solution structures of BF9 (yellow) and TAP (red). The root mean square deviations were calculated for the backbone atoms of residues 31–36 and 50 –57 in BF9, corresponding to residues 32–37 and 52–59 in TAP.
at National Taiwan University on June 11, 2009
www.jbc.org
perfamily all possess a well defined-sheet and a C-terminal ␣-helix secondary structural motif. Because three disulfide bridges are conserved in all BPTI-like proteins, the difference in protein stability among members of the BPTI-like superfam-ily is mainly due to the distinct stability of the secondary structure region. The biological activity is, however, affected by the types, surface structure, flexibility, and rigidity of the amino acids in the loop regions as well as by the-turn region.
REFERENCES
1. Wlodawer, A., Walter, J., Huber, H., and Sjolin, L. (1984) J. Mol. Biol. 180, 301–329
2. Wlodawer, A., Nachman, J., Gilliland, G. L., Gallagher, W., and Woodward, C. (1987) J. Mol. Biol. 198, 469 – 480
3. Berndt, K. D., Guntert, P., Orbons, L. P., and Wu¨thrich, K. (1992) J. Mol. Biol. 227, 757–775
4. Heald, S. L., Tilton, R. F., Jr., Hammond, L. J., Lee, A., Bayney, R. M., Kamarck, M. E., Ramabhadran, T. V., Dreyer, R. N., Davis, G., Unterbeck, A., and Tamburini, P. P. (1991) Biochemistry 30, 10467–10478
5. Hynes, T. R., Randal, M., Kennedy, L. A., Eigenbrot, C., and Kossiakoff, A. A. (1990) Biochemistry 29, 10018 –10022
6. Zweckstetter, M., Czisch, M., Mayer, U., Chu, M. L., Zinth, W., Timpl, R., and Holak, T. A. (1996) Structure 4, 195–209
7. Antuch, W., Berndt, K. D., Chavez, M. A., Delfin, J., and Wu¨thrich, K. (1993) Eur. J. Biochem. 212, 675– 684
8. Dufton, M. J. (1985) Eur. J. Biochem. 153, 647– 654
9. Harvey, A. L., and Anderson, A. J. (1991) Dendrotoxins: Snake Toxins That Block Potassium Channels and Facilitate Neurotransmitter Release in Snake Toxins, pp. 131–164 Pergamon Press Inc., Tarrytown, New York 10. Skarzynski, T. (1992) J. Mol. Biol. 224, 671– 683
11. Berndt, K. D., Guntert, P., and Wu¨thrich, K. (1993) J. Mol. Biol. 234, 735–750 12. Foray, M. F., Lancelin, J. M., Hollecker, M., and Marion, D. (1993) Eur.
J. Biochem. 211, 813– 820
13. Gilquin, B., Lecoq, A., Desne, F., Guenneugues, M., Zinn-Justin, S., and Menez, A. (1999) Proteins Struct. Funct. Genet. 34, 520 –532
14. Antuch, W., Guntert, P., Billeter, M., Hawthorne, T., Grossenbacher, H., and Wu¨ thrich, K. (1994) FEBS Lett. 352, 251–257
15. Lim-Wilby, M. S., Hallenga, K., de Maeyer, M., Lasters, I., Vlasuk, G. P., and Brunck, T. K. (1995) Protein Sci. 4, 178 –186
16. van de Locht, A., Stubbs, M. T., Bode, W., Friedrich, T., Bollschweiler, C., Hoffken, W., and Huber, R. (1996) EMBO J. 15, 6011– 6017
17. Liu, C. S., Wu, T. C., and Lo, T. B. (1983) Int. J. Pept. Protein Res. 21, 209 –215 18. Laskowski, M., Jr., and Kato, I. (1980) Annu. Rev. Biochem. 49, 593– 626 19. Scheidig, A. J., Hynes, T. R., Pelletier, L. A., Wells, J. A., and Kossiakoff, A. A.
(1997) Protein Sci. 6, 1806 –1824
20. Sreerama, N., and Woody, R. W. (2000) Anal. Biochem. 287, 252–260 21. Rance, M., Sorensen, O. W., Bodenhausen, G., Wagner, G., Ernst, R. R., and
Wu¨ thrich, K. (1983) Biochem. Biophys. Res. Commun. 117, 479 – 485 22. Bax, A., and Davis, D. G. (1985) J. Magn. Reson. 65, 355–360
23. Kumar, A., Ernst, R. R., and Wu¨ thrich, K. (1980) Biochem. Biophys. Res. Commun. 95, 1– 6
24. Marion, D., and Wu¨ thrich, K. (1983) Biochem. Biophys. Res. Commun. 113, 967–974
25. Piotto, M., Saudek, V., and Sklenar, V. (1992) J. Biomol. NMR 2, 661– 665 26. Hyberts, S. G., Marki, W., and Wagner, G. (1987) Eur. J. Biochem. 164,
625– 635
27. Bru¨ nger, A. T. (1998) X-PLOR, Version 98, Yale University Press, New Haven, CT
28. Koradi, R., Billeter, M., and Wu¨ thrich, K. (1996) J. Mol. Graph. 14, 29 –32 29. Nicholls, A., Sharp, K. A., and Honig, B. (1991) Proteins Struct. Funct. Genet.
11, 281–296
30. Laskowski, R. A., Rullmannn, J. A., MacArthur, M. W., Kaptein, R., and Thornton, J. M. (1996) J. Biomol. NMR 8, 477– 486
31. Wu¨ thrick, K. (1986) NMR of Protein and Nucleic Acids, pp. 130 –161, Wiley-Interscience, New York
32. Sasaki, T., and Kobayashi, K. (1984) J. Biochem. (Tokyo) 95, 1009 –1017 33. Ritonja, A., Meloun, B., and Gubensek, F. (1983) Biochim. Biophys. Acta 746,
138 –145
34. Smith, L. A., Reid, P. F., Wang, F. C., Parcej, D. N., Schmidt, J. J., Olson, M. A., and Dolly, J. O. (1997) Biochemistry 36, 7690 –7696
35. Dunwiddie C. T., Neeper, M. P., Nutt, E. M., Waxman, L., Smith, D. E., Hofmann, K. J., Lumma, P. K., Garsky, V. M., and Vlasuk, G. P. (1992) Biochemistry. 31, 12126 –12131
36. Wei, A., Alexander, R. S., Duke, J., Ross, H., Rosenfeld, S. A., and Chang, C. H. (1998) J. Mol. Biol. 283, 147–154
37. Sorensen, M. D., Bjorn, S., Norris, K., Olsen, O., Petersen, L., James, T. L., and Led, J. J. (1997) Biochemistry 36, 10439 –10450
38. Wagner, G., and Wu¨ thrich, K. (1982) J. Mol. Biol. 160, 343–361 at National Taiwan University on June 11, 2009
www.jbc.org