Original Article
Interaction of snake venom cardiotoxin (a membrane-disruptive polypeptide) with
human erythrocytes
Yee-Hsiung Chen 1,2, Ruey-Fen Liou 2, Chien-Tsung H u 1, Chung-Ching Juan I and Jen Tsi Yang 3
1Institute of Biochemical Sciences, National Taiwan University and 2Institute of Biological Chemistry, Academia Sinica, Taipei, Taiwan 107, China;
3Cardiovascular Research Institute, University of California, San Francisco, CA 94143, USA
Keywords: cardiotoxin, membrane-disruptive polypeptide, red blood cells
Abstract
The action of 7.2/xM cardiotoxin on 0.25% human erythrocytes in a plasma extender solution was studied by the interaction of toxin with intact red blood cells and subsequent hemolysis of the cells. The binding of toxin to cells was completed within 10 min, whereas the membrane rigidity was weakened in a non-lytic period for about 25 min. The toxin molecules bound almost exclusively to the membrane. The bound toxin could not be liberated with either 0.5% Triton X-100 or 0.1 N NaOH. The degree of binding was slightly reduced in the presence of 10 mM mono- and divalent inorganic salts. The action of toxin might weaken the in situ association of several proteins that are linked with band 3 protein o f the membrane, thus making the cells fragile and altering the shape of the cell to a smooth sphere.
Introduction
Cardiotoxin (CTX) o f snake venom is non- neurotoxic; it acts on cell membranes of different origins. CTX primarily induces cardiac arrest but also causes muscle contraction, membrane depolar- ization and cytolysis (1). Thus, it has been described as a cytoxin, a cytolysin, a direct lytic fac- tor, a membrane-active polypeptide or a membrane-disruptive polypeptide ( 2 - 5 ) . CTX in the venom of Taiwan cobra (Naja naja atra) is a ba- sic polypeptide of 60 amino acid residues with an isoelectric point of 10.8 (6). Its biological activities have intuitively been ascribed to its basicity. How- ever, CTX can intercalate into liposomes and there- by weaken the hydrophobic interactions between hydrocarbon chains in the lipid bilayer (7, 8). Thus, hydrophilic interactions o f basic amino acid residues of CTX with the membrane may be a necessary but not sufficient condition for mem- brane disintegration.
The molecular mechanism of CTX-induced
hemolysis is still obscure, although CTX is known to affect the activities of several membrane-bound enzymes; for instance, treatment of osmotic ghosts of RBC markedly increased the activities of glyceraldehyde-3-phosphate dehydrogenase, adeny- late kinase, 3-phosphoglycerate kinase and aldolase (3, 9). Previously, we have shown that the interac- tion of CTX with h u m a n erythrocytes in plasma ex- tender solution had a non-lytic period during which the membrane structure was weakened and the hydrolysis of phospholipids in the membrane by phospholipase A 2 was potentiated (2). (In nor- mal saline a CTX dosage similar to that used in plasma extender solution would conceal this latent period, whereas a reduced dosage would make the hemolysis too slow to follow). In this work we stud- ied the binding characteristics of CTX to intact red blood cell (RBC) and examined the disintegration of the membrane structure by the CTX action, which led to changes in membrane fragility and cell morphology in the non-lytic period.
To whom correspondence should be addressed: Dr. Yee-Hsiung Chen, Institute of Biochemical Sciences, National Taiwan University, Taipei, Taiwan 107.
Materials and methods
Materials
The crude venom o f Taiwan cobra
(Naja naja
atra)
was supplied by Chen Hsin Tong Chemical Co., Taipei. CM-Sephadex C-25, Sephadex G-25 and Sepharose 4B were obtained from Pharmacia. Heparin, Tris, NaDodSO4, chymotrypsin and neu- raminidase were obtained from Sigma. Iodogen (1,3,4,6-tetrachloro-3o~,6oL-diphenylglycoluril) was obtained from Pierce and Na125I f r o m New En- gland Nuclear. Moriamin, a plasma extender, was supplied by China Chemical & Pharmaceutical Co., Taipei; its composition in m M was 6.4 ArgSBHC1, 22.6 Gly, 3.10 HissBHCI hydrate, 6.87 Ile, 15.6 Leu, 16.9 LysSBHC1 dihydrate, 8.05 Met, 8.79 Phe, 7.56 Thr, 1.47 Trp, 8.55 Val, 0.857 (or 6.00%) Dextran T70 and 274 D-sorbitol. All other chemicals were o f reagent grade. Water was double- distilled.Preparation of CTX
C T X was isolated from crude snake venom on a CM-Sephadex C-25 column (10). It was iodinated by the chloroglycoluril method (11, 12). Twenty mg of C T X and 5 mCi o f Na125I in 1.0 ml of 10 m M phosphate buffer ( p H 7.4) reacted in a vessel, which had been plated with Iodogen, for 20 min at r o o m temperature. The solution and two rinses of the vessel with the buffer were p r o m p t l y applied to a CM-Sephadex C-25 column (1.5 × 40 cm) pre- equilibrated with 0.2 M N a C I - 0 . 0 5 M Tris ( p H 8.6). The u n b o u n d iodide was washed o f f the column with the Tris buffer. The radioactive frac- tions on the column were linearly eluted with 0.2 to 0.4 M NaC1 in 0.05 M Tris buffer, collected and desalted on a Sephadex G-25 column.
Preparation of erythrocytes
Fresh h u m a n blood was centrifuged at 1500 g for 5 min at 4 ° C to remove the buffy coat. The RBCs were washed at least three times with phosphate-buffered saline at 4 ° C and were used immediately. Cardiotoxin-pretreated erythrocytes (CPEs) were prepared by incubating 0.25% RBCs with 7.2 IzM C T X in the plasma extender solution for 15 min at 37 °C (2). The reaction mixture was
diluted with two volumes o f cold phosphate- buffered saline. CPEs were collected by centrifuga- tion at 4 °C and washed twice with the cold saline solution to remove any free CTX.
Intact RBCs were also treated with chymotrypsin to cleave band 3 protein (13, 14) or with neur- aminidase to remove sialic acid (15) on the external cell surface.
Hemolysis assay
The hemolysis of 0.25% RBC by C T X in plasma extender solution at 37°C was followed spec- trophotometrically on a Cary 14 spectrophotome- ter equipped with a jacketed cell holder through which water was circulated to control the tempera- ture (2).
Binding assay
Aliquots of 0.25% flesh RBC containing about 2 × 107 cells and appropriate amounts o f 1:9 125I- labeled and unlabeled C T X in 1.0 ml of plasma ex- tender solution were incubated at 37 °C for various times within the non-lyric period in plastic tubes that had been coated with silicone. The cells were collected, washed at least three times each for 15 rain at 4 ° C and broken up in 2 m l of 1% N a D o d S O 4. The radioactivity of the hemolyzate was counted in a Packard A5000 g a m m a counter. The cells were counted in a Coulter ZF-6 cell count- er. Each binding experiment was repeated at least three times and its standard deviation was less than 10%.
One ~g of 125I-CTX had 84000 to 98000 cpm; thus, the 1:9 hot and cold C T X gave 8400 to 9 800 cpm per #g of total C T X and was routinely used in the assays. The nonspecifically bound C T X was minor as determined by measuring the counts of a mixture of 10 tzg/ml of 125I-CTX and 10 m g / m l o f unlabeled C T X that had been in- cubated with 0.25% RBC; the counts did not ex- ceed 1 0 - 1 6 % of the total counts of b o u n d C T X obtained with 10 izgSBml o f 125I-CTX alone and during the 15-min incubation, there was no hemol- ysis; see Fig. 1 under Results.
Other analytical procedures
(16) were extracted into CHC13 (17) and the phos- phorous content of the dried lipids was determined (18). Sialic acids in the membrane were liberated by N-acetylneuraminidase digestion (15) and assayed (19). The membrane proteins were determined after the ghosts were solubilized in 1% NaDodSO4 (20); the ghost proteins were detected by electrophoresis on 3% p o l y a c r y l a m i d e - 0 . 4 % agarose gels con- taining 0.2% NaDodSO 4 (21). Once the gels were fixed, stained and destained (22), the Coomassie blue-stained bands were scanned at 560 nm with a GS 300 desitometer (Hoeffer Scientific Instru- ments) equipped with a Hitachi recorder. The pro- tein bands were numbered according to convention (23 - 2 5 ) .
Results
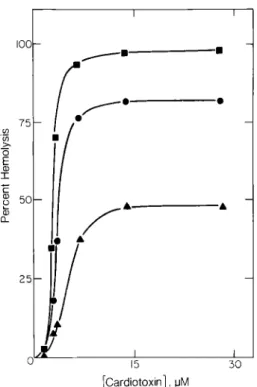
Kinetics of ,~emolysis
The percent hemolysis of 0.25% suspension of RBC mediated by CTX at 37 °C increased with in- creasing CTX concentration at all times studied. Hemolysis approached a plateau when a concentra- tion of about 7.2 I~M CTX was added at each reac- tion time studied (Fig. 1). Our protocol was there- fore to study the events associated with the action of 7.2 #M CTX on 0.25% RBC in plasma extender solution at 37 °C, unless stated otherwise. Previous- ly, we reported a non-lytic period of the CTX- induced hemolysis, which decreased with increas- ing CTX concentration. Under our protocol this la- tent period lasted about 25 to 30 rain (2). Chymotrypsin- or neuraminidase-treated RBC had the same latent period as that of intact RBC (Fig. 2). The kinetics of hemolysis of the enzyme- treated and intact RBC also coincided within ex- perimental errors, suggesting no change in hemoly- sis by cleaving the external polypeptide fragments o f band 3 protein or sialic acids from the external surface of the cells.
Binding of CTX to erythrocytes
125I-CTX was as active as the unlabeled toxin. At each CTX dosage, the maximum binding of CTX to RBC at 37°C (based on bound 125I bounts) was completed in about 10 min, and was independent of the CTX concentration used (Fig. 3). A plot of the initial rate of binding versus
75 U3 O E ! sc o [3= IOC f w / J o
/
I o 15 I I I 30 [Cardiotoxin], uMFig. 1. Hemolysis of 0.25o70 (v/v) erythrocytes by cardiotoxin
in plasma extender solution at 37°C. Time of incubation: • = 2 h ; • = 4 h ; • = 6 h .
the CTX concentration was linear with a slope of approximately 0.035 ~M CTX bound per/~M total CTX per min (not shown). The curve gradually leveled o f f at CTX above 2/zM and the rate of binding approached a plateau o f about 0.13 ~M CTX b o u n d per min at 7.2 ~M total CTX. Essen- tially the same results were obtained for the interac- tion of RBC with CTX in the absence and presence of 10 mM inorganic salts (data not shown).
The uptake of CTX by RBC was found almost exclusively in the cell membrane; more than 95% of the bound 125I-CTX could be recovered in the ghost pellets. The ghost proteins could be extracted selectively with 0.5% Triton X-100 in 56 mM sodi- um borate at pH 8.0 (24) or with 0.1 N N a O H (25), whereas the bound CTX in the cell ghost was non- extractable by either the surfactant or N a O H and was retained in the ghost residues after extraction. Thus, the toxin bound almost exclusively to the Triton- and NaOH-insoluble portion o f the cell membrane. Gel electrophoretic study also indicated that the binding of CTX to RBC did not affect the extraction o f ghost proteins by Triton X-100 and N a O H solutions.
80 60 0 E '-r 4 0 -
§
2 0 - o - c m ~ c l o6 I I I 0 0 0 0 I I I 6 0 120 180 Time, minFig. 2. Kinetics of hemolysis of 0.25% erythrocytes by 7.2 #M
cardiotoxin in p l a s m a extender solution at 37°C. Symbols: • = control RBC; • = chymotrypsin-treated RBC; o = neuraminidase-treated RBC. E 8 c x 2 O O o m
7""
A / A ~ & •Y
/
f
~O..-.O-'O-O I I0 I l • I A ,I, ,k u [ ] 13 0 0 0 0 I I 20 30 Time, minFig. 3. Time dependence of the binding of cardiotoxin to
0.25% (v/v) erythrocytes at 37 °C. Concentrations of CTX in #M: o = 0 . 1 ; [] = 0 . 7 ; • = 2 . 1 ; • = 4 . 3 ; • = 7 . 2 .
Effect of salt on hemolysis
The addition of 10 mM inorganic salts consider- ably inhibited the CTX-induced hemolysis (2). The same was true for the hemolysis of CPE that had been treated with 10 mM inorganic salts (Table 1). The percent hemolysis was significantly reduced in all cases. CPE prepared in the presence of salts seemed to lyse only slightly less than CPE with salts added after its preparation. In the former the salts and unbound CTX were washed o f f from the collected CPE. Thus, inorganic salts inhibited hemolysis regardless of whether they were added before (and subsequently removed) or after the preparation o f CPE.
The degree o f binding o f CTX to RBC was slightly reduced by adding 10 mM NaC1, KC1, CaCI z and MgCI2, respectively, during the prepara- tion of CPE, but it was unaffected if the salts were added after CPE was prepared (Table 1). The bind- ing was also unaltered by treatment of RBC with chymotrypsin or neuraminidase (data not shown).
Composition of CPE ghosts
The ghosts prepared from C P E retained about 91 _+ 2% sialic acid, 87 _+ 3% lipid phosphorus and 75 _+ 9% protein of the untreated erythrocytes. The electrophoretic patterns of proteins of CPE and untreated erythrocytes differed in three aspects (Fig. 4), First, the CPE ghost had a large complex containing about 3% o f the bound ]25I-CTX at the cathodic end of the gel that was absent in the con- trol ghost. Second, bands 1 and 2 (spectrin), band 2.1 (andrin), band 3 and band 5 (actin) in the CPE ghost were considerably smaller than the cor- responding ones in the control ghost; 73% of bands 1 and 2, 84°70 of band 3 and nearly all band 4.1 of the control erythrocytes were retained in the CPE ghost, whereas band 4.2 and band 6 (glyceraldehyde-3-phosphate dehydrogenase) were completely lost in the CPE ghost [the bands of pro- teins were numbered according to Branton (23)]. Third, several fine bands in the band 2 series be- came more prominent in the CPE ghost than in the control.
Morphology and fragility of RBC
Table l. Effect of inorganic salts on the hemolysis of
cardiotoxin-pretreated erythrocytes (CPE) and the binding af- finity of cardiotoxin (CTX) a,
Salt added (10 mM) Percent Relative amount hemolysis b of bound CTX c
None 45.4_+5.5 100.0_+ 4.1 During CPE preparation d
NaC1 25.3_+2.7 96.9_+ 1.5 KCI 25.5+_3.1 95.4+_ 1.3 CaC12 18.6 -+ 0.7 85.8 -+ 3.2 MgCI 2 20.3+2.4 83.9_+ 1.1 After CPE preparation e
NaC1 27.9_+1.7 103.3_+ 2.0 KC1 31.7+_3.4 107.0± 4.5 CaC12 25.3+_3.1 106.8+_10.6 MgCI 2 25.0_+0.7 101.6+_ 3.0
a CPE was prepared by incubating 0.25% RBC in plasma ex- tender solution with 7.2/zM CTX in the non-lyric period for 15 rain at 37 °C and washed free of unbound CTX. b Incubation of unlabeled CPE suspension for 6 h at 37 °C; if
free C T X was not washed away, the percent hemolysis was close to 100 (see Fig. 1).
c Incubation of labeled CPE suspension for 15 rain at 37 °C. d The plasma extender solution in footnote a contained 10 mM
salt.
e CPE in footnote a was resuspended in plasma extender solu- tion containing 10 mM salt.
Fig. 5. Phase contrast micrography of (A) intact erythrocytes
and (B) cardiotoxin-pretreated erythrocytes.
Fig. 4. NaDodSOn-polyacrylamide-agarose gel electrophoresis of proteins in erythrocyte ghosts. Patterns: (A) control RBC and (B)
cardiotoxin-pretreated erythrocytes (both having an equal number of cells). The bands are numbered according to Branton (23). The arrow indicates protein aggregates of cardiotoxin-pretreated erythrocytes.
I 0 - 0 "-r (J 0.. I I I I _---0
/--
/
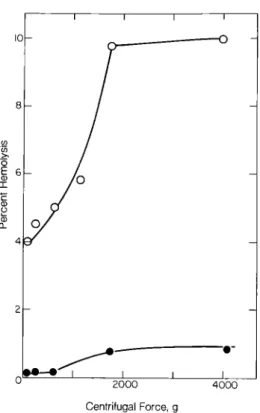
I i I 2 0 0 0 4 0 0 0 Centrifugal Force, gFig. 6. Percent hemolysis of erythrocytes after sedimentation
at 4°C. Symbols: • = control erythrocytes; o = cardiotoxin- pretreated erythrocytes.
were discoid-shaped (Fig. 5A) and were converted to smooth spheres in the presence of CTX (Fig. 5B). Further, CPE was more fragile and less resistant to mechanical friction than untreated RBC and it was easily broken up under centrifugal forces (more than 600 g) (Fig. 6). However, the dis- integration o f cells could be avoided if two volumes of cold phosphate-buffered saline were added to one volume of the more viscous plasma extender solution before sedimentation.
Discussion
CTX was almost exclusively attached to the membrane o f intact RBC as evidenced by more than 95% recoverable CTX associated with the ghost; it may not penetrate the plasma membrane and enter the cytoplasm. The CTX-RBC interac- tion led to structural changes in the cell membrane, as is evident from morphology (Fig. 5) and fragility (Fig. 6) studies. The binding of CTX to RBC
differs from that to the membrane of other types o f cells; for instance, the slow binding requiring about 10 rain (Fig. 3) contrasts the rapid binding of iso- lated axonal membrane (26). The binding was also unaffected by the presence o f inorganic salts (Ta- ble 1); this again contrasts the inhibition o f the CTX binding to heart cell membrane by high con- centration of sodium or calcium ions (27).
Spectrin (bands 1 and 2) and actin (band 5) are two major proteins o f the membrane skeleton, which form cytoskeleton networks. A fraction of band 3 protein attached to the shell of membrane skeleton forms a tertiary complex with band 2.1 and 4.2 proteins (28-32). Band 6 protein binds elec- trostatically to a polyacidic region of band 3 pro- tein (33) (this study used isolated ghost) and is also believed to bind to F-actin and the actin-spectrin complex. During the preparation of CTE ghosts, a considerable portion of band 2.1 protein, 16% o f band 3 protein and nearly all band 4.2 and 6 pro- teins were liberated under a strong centrifugal force (39000 g) (Fig. 4). Concomitant removal of these four proteins implies that the CTX action may weaken the in situ attachment points that connect the lipid bilayer of the membrane and integral pro- teins to the cytoplasmic shell. The CTX action does not seem to affect the binding affinity of band 4.1 protein to the membrane skeleton (the CPE ghost retained nearly all of this protein).
CTX caused the appearance of aggregates con- taining 3°70 of the bound CTX at the cathodic end of electrophoretic gel (Fig. 4). We suspect that this complex may be the aggregates of spectrin and ac- tin by direct action of the basic CTX on the cytoskeleton of RBC. This is in line with the reduc- tion of band 1, 2 and 5 proteins in CPE (Fig. 4) and also the fact that basic polypeptides can precipitate an extractable mixture of spectrin and actin and contract the intact spectrin meshwork (34). This tentative conclusion must be confirmed by future studies. The formation of aggregates could not be attributed to the Ca2+-mediated crosslinking of erythrocyte proteins (35, 36) because the action of CTX on RBC proceeded in a medium devoid of any inorganic salts.
Since the bound CTX was recoverable in the Triton-insoluble shells and it was unaffected by chymotrypsin digestion o f RBC (although the cleaved fragment may remain membrane-bound and probably still associated), the binding of CTX
to the external fragment of band 3 protein can be ruled out. Likewise, removal of sialic acid residues of the glycoprotein on the outer cell surface with neuraminidase did not affect the CTX binding (data not shown).
Triton X-100 preferentially releases from ghost cells all glycerides and glycoproteins, including those that can be stained by the periodic acid-Shift reagent, phosphatic acid, lysophosphatidylcholine and phosphatidyl serine (24, 25). A large portion of band 4.2 and 6 proteins and more than 60% of band 3 protein were also extractable by this surfac- tant. The membrane skeleton, i.e. the Triton- insoluble residues, retained a certain amount of lipids and proteins, mainly band 1, 2, 4.1 and 5 pro- teins forming a basic complex of cytoskeleton. The lipids contained about 34°7o phosphatidylcholine, 27°7o phosphatidylethanolamine, 3°7o phosphatidyl- serine, 83% sphingomyelin and 40% cholesterol of the intact membrane. On the other hand, the NaOH solution liberated those proteins in the Triton-insoluble residues, but not lipids. The fact that the bound CTX on RBC could not be liberated by either the Triton X-100 solution or 0.1 M NaOH suggests that the lipids in the membrane skeleton may provide most of the binding sites. If this is the case, lack of inhibition by inorganic salts for the CTX binding implies a hydrophobic interaction in the nonpolar domain of the skeleton lipids [it is noted that CTX maintains its conformation in sol- vents less polar than water (37) and can intercalate into the lipid layer (7, 8)]. Because the bound CTX can interact with CTX antibody (2), most binding sites may reside in the nonpolar region of the outer lipid bilayer of the membrane skeleton, where phosphatidylcholine and sphingomyelin are mainly located (38). The binding of basic CTX molecules to the outer lipid bilayer will affect an electrostatic repulsion and may cause the cell to expand and transform the cellular shape (Fig. 5). This in turn could decrease the/n situ interaction between lipid and protein molecules in the intrinsic domain of the membrane and weaken their attachment to the membrane. The loosening of several band proteins from the cell membrane by the CTX action also seems to support this explanation. Inorganic salts and CTX antibody may shield the charges on CTX molecules and diminish electrostatic repulsion. Thus, these compounds may be partly responsible for their inhibitory effect on the CTX-induced
weakening of the membrane (2).
Alternatively, CTX may make small holes in the membrane bilayer, which exclude sorbitol and Dex- tran more than saline. The cells would then slowly swell and lyse by a colloid-osmotic mechanism; that is, such small holes allow microsolutes to equi- librate but retain hemoglobin and its counterions within the cell as a fixed unbalanced osmotic load. This hole model has recently been proposed for the study of the interaction of chloropromazine with erythrocyte membrane (39). However, there is a ma- jor difference in the hemolytic activity between chloropromazine and CTX. Chloropromazine, which is also basic, lyses the cells even at 0 - 4 ° C , whereas CTX did not below 20 °C (2). The colloid- osmotic mechanism would also favor hemolysis upon the addition of inorganic salts, whereas our results seemed to suggest an inhibitory effect of in- organic salts.
In this work RBC was suspended in plasma ex- tender solution instead of saline buffer for reasons mentioned previously (2). Most important, the non-lytic period of RBC was prolonged so that the intermediate events leading to cell lysis could be ex- amined. The observed discoid-shaped cells were identical with those found in whole blood (data not shown). However, it is possible that our results could differ somewhat from those under physiolog- ical condition, but the events associated with the CTX action should remain unaffected.
Abbreviations
CTX: cardiotoxin; CPE, cardiotoxin-pretreated erythrocyte; RBC, red blood cell.
Acknowledgments
This work was supported by grant NSC74-0203-B001-02 from the National Research Council, Taiwan, China and in part by United States Public Health Service Grant GM-10880-26.
References
1. Chang CC: The action of snake venom on nerve and mus- cle. In: Lee CY (ed). H a n d b o o k of Experimental Pharma-
cology, Vol. 52. Springer-Verlag Berlin, Heidelberg Press, 1979, pp 309-376.
2. Chen YH, Hu CT, Yang JT: Membrane disintegration and hemolysis of human erythrocytes by snake venom cardi- otoxin (a membrane-disruptive polypeptide). Biocbem Int 8(2):329- 338, 1984.
3. Condrea E: Hemolytic effects of snake venom. In: Lee CY (ed). Handbook of Experimental Pharmacology, Vol. 52. Springer-Verlag Berlin, Heidelberg Press, 1979, pp 448 - 472.
4. Tu A: Nonneurotoxin basic proteins (cardiotoxins, cytotoxins, and others). In: Venoms: Chemistry and molecular biology. John Wiley & Sons, New York, 1977, pp 301 - 320.
5. Yang CC: Chemistry and evolution of toxins in snake ven- oms. Toxicon 12:1-43, 1974.
6. Chen YH, Pan BT, Lee CP: The hydrogen ion titration of Taiwan cobra neurotoxin and cardiotoxin. Biochim Bi- ophys Acta 702:193- 196, 1982.
7. Bougis P, Rochat H, Pieroni G, Verger R: Penetration of phospholipid monolayers by cardiotoxins. Biochemistry 20:4915-4920, 1981.
8. Chen YH, Lai MZ, Kao LS: Destruction of liposome vesi- cles by Taiwan cobra cardiotoxin. Biochem Int 3(4):385 - 390, 1981.
9. Condrea E: Membrane-active polypeptides from snake ven- om: cardiotoxins and haemocytotoxins. Experientia 30:121 - 129, 1974.
20. Lo TB, Chen YH, Lee CY: Chemical studies of Formosan cobra (Naja naja atra) venom• Part 2. Chromatographic separation of crude venom on CM-sephadex and prelimi- nary characterization of its components. J Chinese Chem Soc 13:25-37, 1966.
12. Fraker P J, Speck JC Jr: Protein and cell membrane iodina- tions with a sparingly soluble chloromide, 2,3,4,6-tetrachloro-3c~,6a-diphenylglycoluril. Biochem Bi- ophys Res Commun 80:849-857, 1978.
12. Markwell MAK, Fox CF: Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using
1,3,4,6-tetrachloro-3a,6c~-diphenylglycoluril. Biochemistry 17:4807-4817, 1978.
13. Dupre AM, Rothstein A: Inhibition of anion transport as- sociated with chymotryptic cleavages of red blood cell band 3 protein• Biochim Biophys Acta 646:471- 478, 1981. 14. Steck TL, Ramos B, Strapazon E: Proteolytic dissection of
band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry 15:1154- 1161, 1976.
15. Seaman GVF, Knox R J, Nordt FT, Regan DH: Red cell ag- ing. I. Surface charged density and sialic acid content of density-fractionated human erythrocytes. Blood 50:1002 - 1011, 1977.
16. Steck TL, Weinstein RS, Straus JH, Wallach DFH: Inside- out red cell membrane vesicles: preparation and purifica- tion. Science 168:255-256, 1970.
27. Wintrobe ML: Clinical Hematology. Lea and Febiger, Philadelphia, 1974, p 96.
18. Lebel D, Poirier GG, Beaudoin AR: A convenient method for the ATPase assay. Anal Biochem 85:86-89, 1978. 19. Warran L: The thiobarbituric acid assay of sialic acids. J
Biol Chem 234:1971 - 1975, 1959.
20. Lowry OH, Rosebrough N J, Farr AL, Randall B J: Protein measurement with the Folin reagent• J Biol Chem 193:265-275, 1951.
21. Steck TL: Cross-linking the major proteins of the isolated erythrocyte membrane. J Mol Biol 66:295-305, 1972. 22. Fairbanks G, Steck TL, Wallach DFH: Electrophoretic
analysis of the major polypeptides of the human erythro- cyte membrane. Biochemistry 10:2606- 2617, 1970. 23. Branton D, Cohen CM, Tyler T: Interaction of cytoskeletal
proteins on the human erythrocyte membrane. Cell 24:24- 32, 1981.
24. Yu J, Fischman DA, Steck TL: Selective solubilization of proteins and phospholipids from red blood cell membranes by nonionic detergents• J Supramol Struct 1:233-248, 1973.
25. Steck TL, Yu J: Selective solubilization of proteins from red blood cell membranes by protein perturbants. J Supramol Struct 2:220- 232, 1973.
26. Vincent JP, Schweitz H, Chicheportiche R, Fosset M, Balerna M: Molecular mechanism of cardiotoxin action on axonal membranes• Biochemistry 15:3171 - 3175, 1976. 27. Tonsing L, Potgieter DTJ, Louw AI, Visser L: The binding
of snake venom cardiotoxins to heart cell membranes. Bi- ochim Biocphys Acta 732:282-288, 1983.
28. Haest CWM: Interactions between membrane skeleton pro- teins and the intrinsic domain of the erythrocyte mem- brane. Biochim Biophys Acta 694:331-352, 1982. 29. Bennett V, Stenbuck P J: The membrane attachment pro-
tein for spectrin is associated with band 3 in human erythrocyte membranes. Nature 280:468-473, 1979. 30. Steck TL: The organization of proteins in the human red
blood cell membrane. J Cell Biol 62:1 - 29, 1974. 32. Sheetz MP: Integralmembrane proteininteracti0n with tri-
ton cytoskeletons of erythrocytes. Biochim Biophys Acta 557:122-234, 1979.
32. Bennett V, Stenbuck P J: Human erythrocyte ankyrin. J Biol Chem 255:2540- 2548, 1979.
33. Tsai I-H, Murthy SNP, Steck TL: Effect of red cell mem- brane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 257:1438- 1442, 1982.
34. Elgsaeter A, Shotton DM, Branton D: Intramembrane par- ticle aggregation in erythrocyte ghosts. II. The influence of spectrin aggregation. Biochim Biophys Acta 426:201 - 122, 1976.
35. Carraway KL, Triplett RB, Anderson DR: Calcium- promoted aggregation of erythrocyte membrane proteins. Biochim Biophys Acta 379:571- 581, 1975.
36. Lorand L, Weismann LB, Epel DL, Bruner-Lorand J: Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci USA 73:4479 - 4481, 1976.
37. Hung MC, Chen YH: Conformational stability of a snake cardiotoxin. Int J Peptide Protein Res 10:277- 285, 1977. 38. Op den Kamp JAF: Lipid asymmetry in membranes. Ann
Rev Biochem 48:47-71, 1979.
39. Lieber MR, Lange Y, Weinstein RS, Steck TL: Interaction of chlopromazine with the human erythrocyte membrane. J Biol Chem 249:9225- 9234, 1984.