Population Biology of the Swimming Crab Portunus sanguinolentus in the Waters off
Northern Taiwan
Author(s): Hui-Hua Lee and Chien-Chung Hsu
Source: Journal of Crustacean Biology, Vol. 23, No. 3 (Aug., 2003), pp. 691-699
Published by: The Crustacean Society
Stable URL:
http://www.jstor.org/stable/1549896
Accessed: 02/11/2009 04:11
Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at
http://www.jstor.org/page/info/about/policies/terms.jsp
. JSTOR's Terms and Conditions of Use provides, in part, that unless
you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you
may use content in the JSTOR archive only for your personal, non-commercial use.
Please contact the publisher regarding any further use of this work. Publisher contact information may be obtained at
http://www.jstor.org/action/showPublisher?publisherCode=crustsoc
.
Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed
page of such transmission.
JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of
content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms
of scholarship. For more information about JSTOR, please contact support@jstor.org.
The Crustacean Society is collaborating with JSTOR to digitize, preserve and extend access to Journal of
Crustacean Biology.
POPULATION BIOLOGY OF THE SWIMMING CRAB PORTUNUS
SANGUINOLENTUS IN THE WATERS OFF NORTHERN TAIWAN
Hui-Hua Lee and Chien-Chung Hsu
(HHL) (CCH, corresponding author) Institute of Oceanography, National Taiwan University, P.O. Box 23-13, Taipei, Taiwan 106 (hsucc@ccms.ntu.edu.tw)
ABSTRACT
The growth, mortality, and reproduction of Portunus sanguinolentus were studied using size-frequency data obtained from crabs collected in pots in the waters off northem Taiwan from October 2000 to March 2001, and October 2001 to January 2002. The Bhattacharya's method and seasonal von Bertalanffy growth curve were used to estimate growth parameters. The growth curve for males was Lt= 204.75 x {1 - e-[087t+0.4(0.87/2n)sin2n(t)]} and the curve for females was Lt = 194.25 X {1- e-[0.97t+0.4(0.97/2n)sin27n(t)]}. A size-converted catch curve was used to estimate the instantaneous total mortality rate (Z), and Pauly's empirical equation was used to estimate the instantaneous natural mortality rate (M). For males, Z = 3.16/year and M = 1.65/year. For females, Z = 3.37/year and M = 1.8/ year. The instantaneous fishing mortality rate (F) was 1.51/year and 1.57/year, and the exploitation rate (E) was 0.48 and 0.47 for males and females, respectively. The exponential relationships were presented for relationships of fecundity in number and weight of egg mass in terms of carapace width and body weight. Those relationships were statistically significant (P < 0.01), indicating that the fecundity increased with the size from 4.05 X 105 to 2.44 X 106 eggs.
The swimming crab Portunus sanguinolentus
(Herbst, 1783) is widely distributed in ocean
waters from East Africa, through the Indo-
Pacific region, to the Hawaiian Islands (Ste-
phenson and Campbell, 1959). Juveniles and
adult males typically inhabit sandy and muddy
bottoms in nearshore waters, about 10-30 m
deep (Chapgar, 1957; Sumpton et al., 1989). In
contrast, females are abundant in 40-80 m
depths (Wenner, 1972; Campbell and Fielder,
1986). In Taiwan, P. sanguinolentus only oc-
curs in the waters around the north and south-
west parts of the island.
There have been numerous studies of
P. sanguinolentus taxonomy (Chapgar, 1957;
Stephenson and Campbell, 1959; Dai and Yang,
1991), maturation
(Sumpton et al., 1989; Jacob
et al., 1990; Reeby et al., 1990), and re-
production (Ryan, 1967; Campbell and Fielder,
1986; Sukumaran and Neelakantan, 1997).
However, there has been only little information
about P. sanguinolentus in the waters of Taiwan
(Huang, 1993; Hsu et al., 2000). The yield of
this economically important and productive
species has declined substantially in recent
years (Anonymous, 2000), and the monthly
relative abundance also shows a declining trend
(unpublished data). To understand the popula-
tion dynamics of P. sanguinolentus around
Taiwan is urgent, and locally collected data
used to study its growth, mortality, and re-
production are needed.
In this paper, growth study was performed to
estimate growth parameters and to understand
the life span. Then, mortality was estimated to
understand causes of population reduction.
Finally, fecundity was estimated based on
carapace width and body weight to determine
recruitment. Therefore, the objective of this
study was to estimate growth, mortality, and
reproduction of P. sanguinolentus living in the
waters off northern Taiwan.
MATERIALS AND METHODS
From October 2000 to March 2001, and October 2001 to January 2002, monthly samples of P. sanguinolentus were collected using circular crab pots in the offshore waters of northern Taiwan (25020'-25050'N and 120?40'-121?20'E). Pot is made of a rigid frame (diameter is 550 mm and height is 240 mm) with meshes (upper mesh size is 35 mm and lower mesh size is 40 mm) and three entrances are inserted on the side (mesh size of entrance is 15 mm). The entrances are designed to prevent crabs escaping. Before setting, pots are baited with frozen mackerels in the center of the pot.
Basic hydrographic data were obtained from the National Center for Oceanic Research database (National Taiwan Uni- versity, Taipei, Taiwan). In the study area, the water depth was around 80 m, and the substrate was sandy. Bottom temper- atures ranged from 20 ? 0.29?C in winter to 25 t 1.53?C in summer. The annual average temperature was 23 ? 3.2?C, and salinity averaged 34 + 0.39 psu (practical salinity units). Nearly all crabs captured in each pot were identified, sexed, and measured for carapace width (CW). Precision
JOURNAL OF CRUSTACEAN BIOLOGY, VOL. 23, NO. 3, 2003
vernier calipers were used in the field to measure CW (the distance from the left tip to the right tip of the posterior margin of the carapace) to the nearest 0.01 mm (Mitutoyo digimatic caliper). Each month, from October 2000 to March 2001, 20 gravid females were selected and brought to the laboratory to study reproduction. During the main spawning season, from October 2001 to January 2002, the number of gravid females in each sample was counted. Altogether, 3298 females and 1504 males were measured, and 117 gravid females with extruded eggs were collected and frozen.
Prior to measuring, each frozen, gravid female was thawed for about 4 h in the laboratory. For each crab, the CW was measured as described above. The wet weight of body, with eggs, was determined to the nearest 0.1 gram with an electronic balance (Snowrex digital scale KF-600). Then the eggs were carefully removed from the pleopods, wiped with tissue paper, and weighed. To maintain tonicity (Wenner et al., 1987) for measurements, eggs were preserved in a mixture of 30% water, 30% ethanol, 30% acetone, and 10% glycerol.
Growth
First, the ELEFAN I subroutine of the FiSAT software package (Gayanilo and Pauly, 1997) was used to estimate a seasonalized version of the von Bertalanffy growth parameters. The growth model with a seasonal fluctuation is:
L, = Lo { 1 -e [KX(t-to)+CX(K/2nt)Xsin27r(t-ts)] }, ( ) where Lt (mm) is the predicted length at time t, Loo (mm) is the asymptotic length, t is a given instant age, to is the theoretical age when carapace width is zero, ts is the starting point of the growth oscillation, K (1/year) is the intrinsic growth rate, C is the amplitude of the growth oscillation and ranges from 0 to 1. The growth performance index ((p') was used to compare von Bertalanffy growth of P. sanguino- lentus with that of other crab species, in which (p' = log K + log Lo (Pauly and Munro, 1984). Then, Bhattacharya's method was used to determine the number of age groups in monthly samples (Bhattacharya, 1967).
Hotelling's T2, calculated with the SAS/IML module, was used to compare male and female growth curves based on the parameters Lo, K, C, ts, and to (Bernard, 1981; Quinn and Deriso, 1999). When the T2 statistic was significant, indicating the growth of males and females was significantly different, simultaneous Roy-Bose confidence intervals were computed to determine the most important parameter causing the difference between the sexes (Bernard, 1981).
Mortality
The instantaneous total mortality rate (Z, 1/year) was estimated based on the size-converted catch curve (King, 1995) using seasonal von Bertalanffy growth parameters:
ln( sAt ) fa ,, (2) where N, is the number of individuals of size class i, At is the time needed to grow through size class i, ti is the relative age of size class i, cx and P3 are parameters to be estimated. Thus, the instantaneous total mortality rate is Z = -13.
The instantaneous natural mortality rate (M, 1/year) was estimated with Pauly's empirical equation (Pauly's, 1980):
ln(M) = -0.0152 - 0.279 ln(Loo) + 0.6543 ln(K)
+ 0.463 ln(T), (3)
where In is the natural logarithm operator; Lo (cm) and K (1/ year) are growth parameters (described above) and T (?C) is
the mean annual habitat temperature. Thus, the instanta- neous rate of fishing mortality (F, 1/year) is F = Z-M, and the exploitation rate (E) is E = F/Z (Quinn and Deriso, 1999).
Reproduction
The proportion of gravid females, which were used as mature females, was fitted to a logistic equation as described by Quinn and Deriso (1999):
Pmax
P(L) = + e-KX(L-y)' 1 (4) where P(L) is the cumulative proportion of gravid females in CW upper class limit (L) and K, y, and Pmax are parameters estimated by the log-linear least square method (Zar, 1995). Pmax is the asymptotic cumulative proportion of gravid females as L -p oo, K is the curvature, and y is the CW at the inflection point.
Before pooling all the monthly data to fit equation (4), a Kruskal-Wallis' one-way analysis of variance (SAS, Version 8.02) was used to examine the randomness of the monthly data, which were assumed to be from one population. The proportion of gravid females did not vary significantly from month to month (X2 (o.o5,3) = 0.547, P > 0.05). Therefore, we assumed all samples were randomly collected from the same population, and all monthly samples were combined by size intervals to fit the logistic curve (equation 4).
The gravimetric method was used to estimate number of newly deposited eggs extruded by gravid females. The diameters of 30, randomly selected eggs were measured. The relationships between the number of eggs or the weight of the extruded eggs and female carapace width or weight can be expressed by:
Y = a .X, (5)
where Y denotes either the number of eggs or the weight of the eggs; X is either carapace width or the body weight of the female crab from whence the eggs came; and ot and 13 are estimated parameters.
RESULTS
Growth
The von Bertalanffy growth equations with
seasonal fluctuations (Fig. 1) were:
(1) Males: Lt= 204.75
X { 1 - e-[0.87t+0.4(0.87/27r)sin2ir(t)]}
((p' =2.25);
(2) Females: L,
=194.25
(6)
X { 1 - e-[0.97t+0.4(0.97/2r)sin27r(t)]}
((p' =2.28),
(7)
Loo
and K were the only parameters
that differed
between sexes. Female growth rate (K = 0.97/
year) was greater than male growth rate (K=
0.87/year), but males reached a larger asymp-
totic size (204.75 mm) than females (194.25
mm). Loo and K were significantly negatively
correlated (males: r = -0.826, P < 0.01;
females: r - -0.787, P < 0.01). Thus, females
reach maximum size in less time than males.
Furthermore, the results obtained by Bhatta-
charya's method indicated almost all monthly
samples might include two age-classes, and the
longevities of male and female P. sanguinolen-
tus are asymptotically over 11.4 and 10.2 years,
respectively, corresponding to the asymptotic
carapace widths (Lxo) in growth equations (6)
and (7), respectively.
Hence, to determine whether the growth rates
and asymptotic sizes of male and female P.
sanguinolentus differed, the test statistic, Hotel-
ling's T2 (Bernard, 1981), was computed.
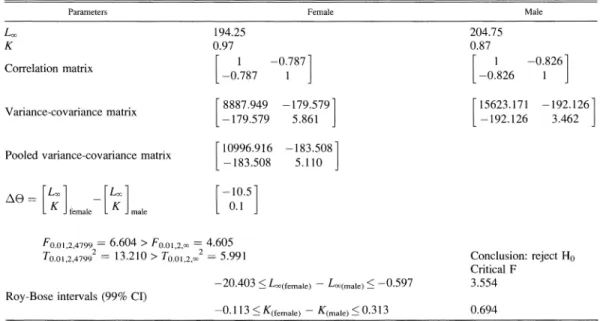
Female and male Loo and K were significantly
different (reject Ho:
e(female) -= (male), P <0.01; Table 1). However, the 99% Roy-Bose
confidence interval for K(female)- K(male) in-
dicated that K is not significantly different
between sexes, but the 99% confidence interval
for Loo(female) - Loo(male) is different between
sexes. In conclusion, females do not grow
significantly faster than males, but they do
achieve their maximum size, which is signifi-
cantly smaller than the maximum size of males,
in significantly less time (CW range: males 90-
193 mm and females 68-182 mm).
Mortality
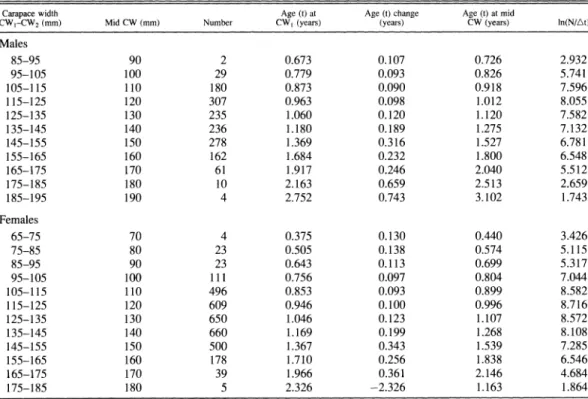
Based on the carapace width-frequency
distribution (Table 2), the size-converted catch
250 - 200 - --- Females Males ,. 150 -S H 100 50 0 1 2 3 4 Age (years)
Fig. 1. Seasonal von Bertalanffy growth curves based on carapace width frequency data for male (-) and female (- - -) P. sanguinolentus.
curve was used to estimate instantaneous
total mortality for both sexes (Fig. 2). The
instantaneous total mortality rate (Z) was 3.16/
year for males and 3.37/year for females. Using
Pauly's empirical equation and an average
annual habitat temperature
of 23?C, the natural
mortality rate (M) was 1.65/year for males and
1.8/year for females. The fishing mortality rate
(F) was 1.51/year for males and 1.57/year for
females, and the exploitation rate (E) was 0.48
Table 1. Hotelling's T2 calculation to test for equality of parameters Lxo and K for male and female P. sanguinolentus in the waters off northern Taiwan. HO: O(female) = ((male) versus HI: )(female) #7 {(male)-
Parameters Female Male
Loc 194.25 204.75
K 0.97 0.87
Correlation matrix [ -0.787 [ 0826
-0.787 1 -0.826 1
?Variancecovariance Varlance-covaance matrix matrlx 8887.949 -179.5791 [15623.171 -192.126
L[-179.579 5.861 -192.126 3.462
F
10996.916 -183.5081 Pooled variance-covariance matrix 108 50-183.508 5.110 A * [ 1 [Kim [-10.5] [ K female [ K male - [ 00 Fo.01,2,4799 = 6.604 > Fo.01,2,o = 4.605
To0.ol,2,47992 13.210 > To.o01,2,o2 = 5.991 Conclusion: reject Ho Critical F
-20.403 < Lo(female) - Lo(male) < -0.597 3.554 Roy-Bose intervals (99% CI)
JOURNAL OF CRUSTACEAN BIOLOGY, VOL. 23, NO. 3, 2003
Table 2. Size-converted catch curves for male (upper panel) and female (lower panel) P. sanguinolentus based on carapace width frequency data.
Carapace width Age (t) at Age (t) change Age (t) at mid
CWI-CW2 (mm) Mid CW (mm) Number CWl (years) (years) CW (years) In(N/At) Males 85-95 90 2 0.673 0.107 0.726 2.932 95-105 100 29 0.779 0.093 0.826 5.741 105-115 110 180 0.873 0.090 0.918 7.596 115-125 120 307 0.963 0.098 1.012 8.055 125-135 130 235 1.060 0.120 1.120 7.582 135-145 140 236 1.180 0.189 1.275 7.132 145-155 150 278 1.369 0.316 1.527 6.781 155-165 160 162 1.684 0.232 1.800 6.548 165-175 170 61 1.917 0.246 2.040 5.512 175-185 180 10 2.163 0.659 2.513 2.659 185-195 190 4 2.752 0.743 3.102 1.743 Females 65-75 70 4 0.375 0.130 0.440 3.426 75-85 80 23 0.505 0.138 0.574 5.115 85-95 90 23 0.643 0.113 0.699 5.317 95-105 100 111 0.756 0.097 0.804 7.044 105-115 110 496 0.853 0.093 0.899 8.582 115-125 120 609 0.946 0.100 0.996 8.716 125-135 130 650 1.046 0.123 1.107 8.572 135-145 140 660 1.169 0.199 1.268 8.108 145-155 150 500 1.367 0.343 1.539 7.285 155-165 160 178 1.710 0.256 1.838 6.546 165-175 170 39 1.966 0.361 2.146 4.684 175-185 180 5 2.326 -2.326 1.163 1.864
for males and 0.47 for females. To determine
total mortality rate (Z) of male and female P.
sanguinolentus differed, the analysis of co-
variance (ANCOVA) using age as the covariate
revealed that there is a statistically significant
difference (P < 0.0001). Accordingly, total
mortality rates were higher for females than
males.
Reproduction
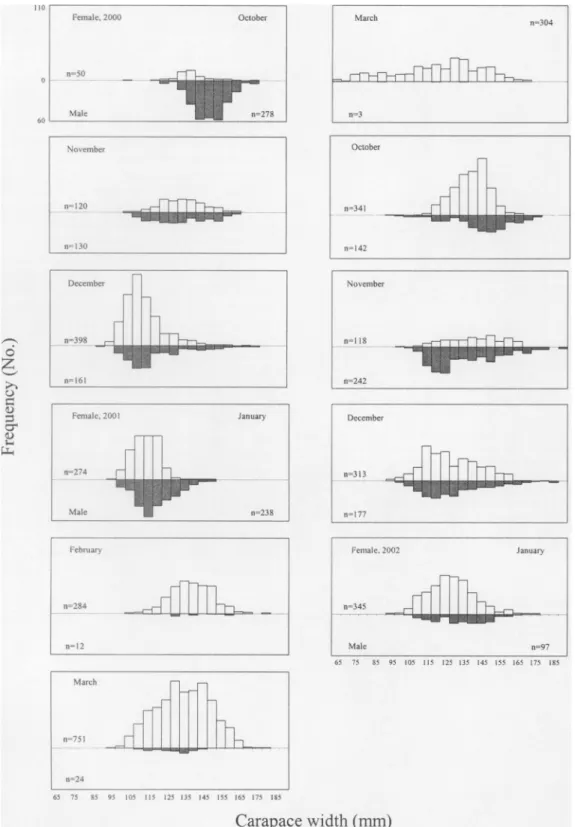
Based on the female proportion in the
population (the number of females in total
catch) estimated from monthly samples, nearly
all crabs caught in February and March were
female (Fig. 3). Carapace width ranged from 90
mm to 193 mm for males and from 68 mm to
182 mm for females (Fig. 4). From 20% to 30%
of the females caught each month were gravid
and had extruded eggs (Fig. 4). The monthly sex
ratio (the number of females divided by the
number of males) was tested using X2 test, and
the value was highly significant
(X
= 1300.54 >
X o.ooo1,o1
= 35.557) to reject the null hypothesis
that the sex ratio is 1:1.
The proportion of gravid females among
monthly samples was not significantly different
(Kruskal-Wallis,
20.05,3= 0.547, P > 0.05).
Therefore, the samples were pooled and a logis-
tic curve (Fig. 5) was:
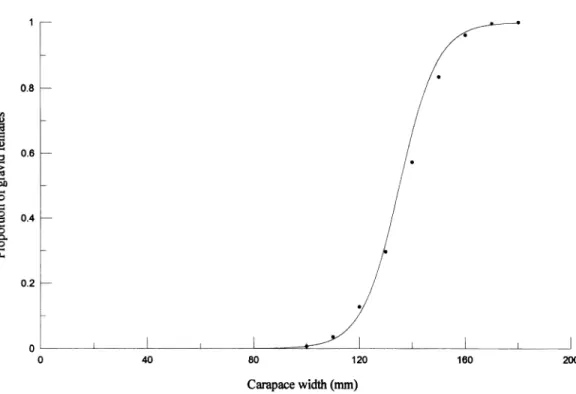
1
P(L)- 1 +
e-0'141(L-135.27) '(8)
Further, the mean carapace width for ovigerous
females
(L50%)was obtained as 135.27 mm
from equation (8) assuming P(L) = 0.5.
The extruded egg mass was detached from
the abdomen of each female, and the eggs were
counted. The number of eggs ranged from
405,375 to 2,438,645 (average 1,075,857). Egg
diameter ranged from 234 gtm to 297 itm, with
a mode of 270 jtm (n -3420). Additionally, the
relationship between the number and weight of
the eggs in each extruded egg mass were
exponentially related to female carapace width
and weight (Table 3). Egg number and weight
were significantly positively correlated with
body weight and carapace width (n =- 117, each
P < 0.01).
DISCUSSION
Growth
In this study, seasonal fluctuation was in-
corporated into estimates of P. sanguinolentus
Males
growth
in
the waters off
northern Taiwan.
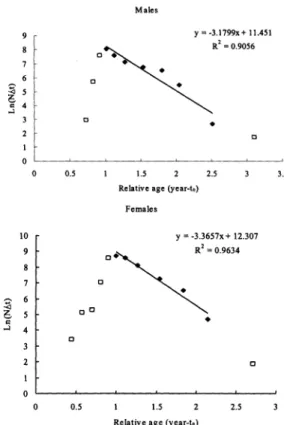
y=-3.1799x+ 11.451Asymptotic carapace widths for males and
R = 0.9056females were estimated as 204.75 mm and
194.25 mm, respectively. These estimates are
?-
a*
greater than those for P. sanguinolentus (163
mm and 173 mm for males and females,
respectively) from waters off the southern
o
Kanara Coast, India (Sukumaran et al., 1986).
However, estimated growth rates (males: 0.87/
0.5 1...
year; females: 0.97/year) in this study were
0 0.5 1 1.5 2 2.5 3 3.5Relative age (year-t) 5
much lower than those (3.54/year for both
sexes) estimated by Sukumaran et al. (1986).
Females
The growth
performance
indexes for
India -y=-3.3657x+ 12.307
population ((p'
=2.76 for males and 2.79 for
. R2= 0.9634 females) were slightly greater than those of the-0
present study, but both were located at the
-
ams,^reasonable range (2 < (p' < 3) within the same
^-
\""~
?
R2=0'9634family
(Pauly and Munro, 1984). Defeo and
0-
? ?
Cardoso (2002) indicated that environmental
o-
Dfactors,
such as water temperature, may affect
crab growth rate. Crab growth may be faster in
?
warm water than in cool water (Leffler, 1972).
As it is so, environmental
differences may result
0 0.5 1 1.5 2 2.5 3 in the discrepancies of growth of P. sanguino- Relative age (year-to)
lentus from those two waters indicated above.
Growth rate differences between males and
'. Size-converted catch curves of male (upper panel) females result mainly from the greater repro- female (lower panel) P. sanguinolentus. The total ductive output of females. When crabs become taneous mortality rates (Z) were estimated from the ual mtu o o en cras H
of the regression line (solid squares); data points
sexually mature, growth often decreases (Hart-
led in the regression line because of data from mean
noll, 1982) because of the significant amount of
with very small sample size (less than 10) and the
energy used for reproduction.
In this study, the
ascending data point representing groups of individ- estimated L50%for females is 135.27 mm
which were not fully recruited (open squares). For,
Z = 3.155/ear and for females, and for females, 3.3657/year Z .
carapace width (about 1.17 years).
After that,females grew slowly (Fig. 1) and seemed
coincident with Hartnoll's points. With greater
100
80
60
40
0 S. 8)20
0
Nov. Dec. Jan. Feb. Mar.
2001
Oct. Nov. Dec. Jan.
2001
2002
Fig. 3. Monthly change in the P. sanguinolentus female proportion in the population, October 2000 to January 2002. 9 8 7 6
1
5 e 4 3 2 1 0 c -4 10 9 8 7 6 5 4 3 2 1 0 Fig. 2 and f instan slope exclu? ages i initial uals malesOct.
2000
JOURNAL OF CRUSTACEAN BIOLOGY. VOL. 23. NO. 3. 2003 Female, 2000 October n=50 Male n=278 November n=120 -g n=130 December n=398 >, n=161 Female, 2001 January n=274 Male n=238 February n=284 _ L i n=12 March - n=751 2 4 n=24 March n=304 n=3 October n=341 n=142 November n=1 18 n=242 December n=313 n=177 Female, 2002 January n=345 Male n=97 65 75 85 95 105 115 125 135 145 155 165 175 185 65 75 85 95 105 115 125 135 145 155 165 175 185
Carapace width (mm)
Fig. 4. The frequency distribution of carapace widths of male and female P. sanguinolentus. Solid squares denote gravid females and numbers indicate samples sizes of gravid females.
110 0 60
6
z
>-t
Q
(D
O
cr
0
;.4 "4 6961
0.8
40 80 120 160 200
Carapace
width
(mm)
Fig. 5. A logistic curve of gravid female P. sanguinolentus and dotted line indicate the carapace width of proportion 0.5 corresponding to L50%.
investment in reproduction, females may be
smaller than males at maturity. Reproductively
active females typically postpone growth, and
their growth rates often lag behind those of
males (Cobb and Caddy, 1989). In this study,
the asymptotic size of female P. sanguinolentus
was smaller than that of males, and, overall,
their growth was significantly slower. This
supports the hypothesis that differences in
reproductive
output may account for differences
in male and female growth (Cobby and Caddy,
1989; Hartnoll, 1982). However, the asymptotic
size of females was larger than that of males in
Indian population. This difference depends on
the observed maximum carapace width due to
the different sample location between Indian
waters and the waters off North Taiwan.
Gayanilo and Pauly (1997) proposed that the
maximum predicted size may be equivalent to
95% of the estimated asymptotic size. Accord-
ing to this viewpoint, the maximum predicted
carapace width in the present analysis should
be 194.5 mm and 184.5 mm for males and
females, respectively, compared with the ob-
served maximum carapace widths that were 193
mm and 182 mm CW for males and females,
respectively, indicating that the present growth
estimations and the corresponding predicted
longevities are reasonable.
Mortality
Total mortality rates for P. sanguinolentus in
the waters off northern Taiwan (males: Z =
3.16/year; females: 3.37/year) were much great-
er than those obtained for P. sanguinolentus in
the Indian Ocean (males: Z = 0.78/year;
females: 0.79/year; Sukumaran et al., 1986).
Part of these differences may be attributed to
different methods of estimation and temper-
atures (Leffler, 1972). The exploitation rates
(males: E = 0.48; females: E = 0.47) indicated
that natural and fishing losses contributed
equally to the decrease in the P. sanguinolentus
population off northern Taiwan. During this
study, P. sanguinolentus natural mortality was
high. This is typical of r-selected species
(Gunderson, 1980), which mature early and
have high fecundity, short life spans, and small
body size. However, these population traits may
be affected by temperature and latitudinal
variation (Defeo and Cardoso, 2002).
The size-converted catch curve is strongly
influenced by population structure such as size
at recruitment
and sample size, which affect the
goodness of fit and the slope of the regression
line (cf. equation 2). There is no information
about P. sanguinolentus recruitment to the crab
pot fishery in the waters off Taiwan. Thus, the
32 C4. 0 0 04 0.6 - 0.4- 0.2- 0 0
JOURNAL OF CRUSTACEAN BIOLOGY, VOL. 23, NO. 3, 2003
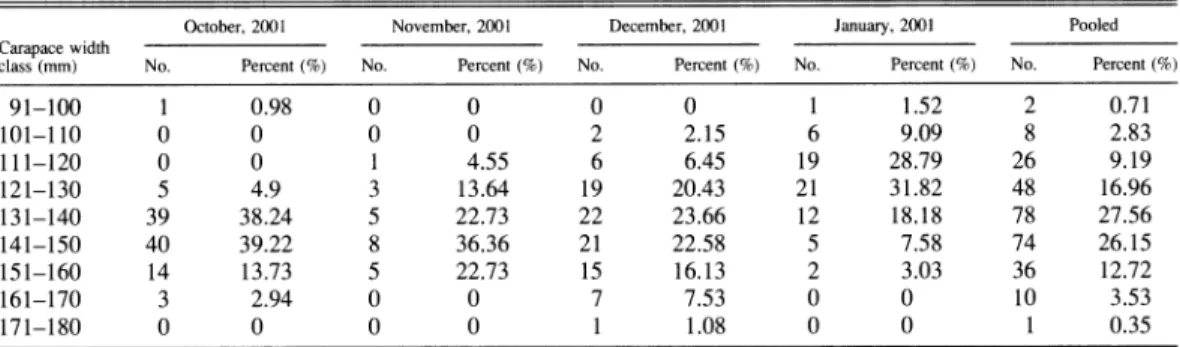
Table 3. The percentage of gravid female P. sanguinolentus from October 2001 to January 2002 by carapace width class. October, 2001 November, 2001 December, 2001 January, 2001 Pooled Carapace width
class (mm) No. Percent (%) No. Percent (%) No. Percent (%) No. Percent (%) No. Percent (%)
91-100 1 0.98 0 0 0 0 1 1.52 2 0.71 101-110 0 0 0 0 2 2.15 6 9.09 8 2.83 111-120 0 0 1 4.55 6 6.45 19 28.79 26 9.19 121-130 5 4.9 3 13.64 19 20.43 21 31.82 48 16.96 131-140 39 38.24 5 22.73 22 23.66 12 18.18 78 27.56 141-150 40 39.22 8 36.36 21 22.58 5 7.58 74 26.15 151-160 14 13.73 5 22.73 15 16.13 2 3.03 36 12.72 161-170 3 2.94 0 0 7 7.53 0 0 10 3.53 171-180 0 0 0 0 1 1.08 0 0 1 0.35
A Kruskal-Wallis test was used to assess the homogeneity of the percentage of gravid females across monthly samples: x2 (0.05, 3) =0.5467, (P >0.05, not significant)
total mortality rate was estimated for males and
females separately based on carapace width
frequency data (Table 2; Fig. 2), which were
measured from all crabs caught during each trip
to increase satisfactorily the sample size avail-
able in the present analysis.
Reproduction
In the monthly samples, female P. sanguino-
lentus outnumbered males. The inequality of
female proportion in the population or sex ratio
may result from the depth at which most
samples were collected. Females were relatively
more abundant around 80 m in depth, while
males were more abundant from 40 m to 60 m.
In addition, the proportion of females varied
from year to year. This also was observed in the
waters of Queensland, Australia (Sumpton et al.,
1989). Because the proportion of mature
females increases significantly with increased
depth (Wenner, 1972), offshore samples contain
larger proportions of gravid females than
samples from coastal areas.
For the Queensland population, Campbell
and Fielder (1986) found that the female
matured at 75 mm carapace width. Sumpton
et al. (1989) found the smallest mature male and
female were 83 mm and 74 mm carapace width,
respectively, and the smallest female with
recently implanted spermatophores
was 94 mm
CW. In this study, the observed smallest
ovigerous female was 96 mm CW, which is
similar to the smallest female with recently
implanted spermatophores in the Queensland
population.
Egg number and size are significantly
correlated
with female crab weight and carapace
width. In the waters off northern
Taiwan, gravid
female P. sanguinolentus had from 4.1 X 105 to
2.44 X 106 eggs. The mode diameter was 270
ptm. These numbers were very similar to those
for gravid female P. sanguinolentus in Indian
waters, which had from 9.6 X 105 to 2.25 X 106
eggs (Ryan, 1967), and greater than estimates
of 4.4 X 104 to 1.19
X106 eggs for P.
sanguinolentus in the waters off Kamataka,
India (Sukumaran
and Neelakantan, 1997). The
discrepancy may be affected by environmental
factors, including predation, parasitization, and
temperature, which may affect the balance
between the optimal number and size of eggs
(Smith and Fretwell, 1974; Lawlor, 1976).
This study is the first on the growth,
mortality, and reproduction of P. sanguinolen-
tus in Taiwan. However, validation of growth
estimates has not been undertaken
because hard-
parts are lost during molting. As noted, age
determination
is absolutely necessary for nearly
all studies of population dynamics (Wolff and
Soto, 1992; Marques et al., 1994). Therefore,
studies of the growth of cultivated crabs and
tagged wild crabs are needed to develop and
validate methods of age determination. In
mortality analysis, biased estimates of growth
parameters
seriously affect estimates of popula-
tion parameters, such as the instantaneous
natural mortality rate (Lai and Gunderson,
1987; Lai et al., 1996). Hence, more reliable
estimates of natural mortality rate are still
required. Further research is urgently needed
on other population dynamics studies such as
yield per recruit model and length-based models
analyses for estimating crab recruitment and
abundance.
ACKNOWLEDGEMENTS
We appreciate the National Science Council, Taiwan, for financially supporting this project with grant NSC89-2313- B-002-167 to C.-C. Hsu, and Ms. Stacy Kao, Research Assistant at the Institute of Oceanography, National Taiwan 698
University, for collecting and measuring crabs. We also thank the anonymous reviewers for their very constructive and critical comments.
LITERATURE CITED
Anonymous. 2000. Year-Books, 2000. Fisheries Adminis- tration, Council of Agriculture, Taipei, Taiwan. Bernard, D. R. 1981. Multivariate analysis as a means of
comparing growth in fish.-Canadian Journal of Fisheries and Aquatic Sciences 38: 233-236.
Bhattacharya, C. G. 1967. A simple method of resolution of a distribution into Gaussian components.-Biometrics 23: 115-135.
Campbell, G. R., and D. R. Fielder. 1986. Size at sexual maturity and occurrence of ovigerous females in three species of commercially exploited portunid crabs in S.E. Queensland.-Proceedings of the Royal Society Queens- land 97: 79-87.
Chapgar, B. R. 1957. On the marine crabs (Decapoda: Brachyura) of Bombay State.-Journal of the Bombay Natural History Society 54: 399-439.
Cobb, J., and J. F. Caddy. 1989. The population biology of decapods. Pp. 327-374 in J. F. Caddy, ed. Marine Invertebrate Fisheries: Their Assessment and Manage- ment. Wiley Interscience, New York.
Dai, A. Y., and S. L. Yang. 1991. Crabs of the China Seas. China Ocean Press, Beijing. 682 pp.
Defeo, O., and R. S. Cardoso. 2002. Macroecology of population dynamics and life history traits of the mole crabs Emerita brasiliensis in Atlantic sandy beaches of South American.-Marine Ecology: Progress Series 239: 169-179.
Gayanilo, F. C. Jr., and D. E. Pauly. 1997. FAO-ICLARM Stock Assessment Tools (FiSAT): Reference Manual. FAO Computerized Information Series (Fisheries). 8, FAO, Rome. 262 pp.
Gunderson, D. R. 1980. Using r-K selection theory to predict natural mortality.-Canadian Journal of Fisheries and Aquatic Sciences 37: 2266-2271.
Hartnoll, R. G. 1982. Growth. Pp. 111-196 in D. E. Bliss, ed.-in-chief, The Biology of Crustacea. Vol. 2, L. G. Abele, ed., Embryology, Morphology, and Genetics. Academic Press, New York.
Hsu, C. C., H. C. Chang, and H. C. Liu. 2000. Sex-variant morphometrics of the swimming crab, Portunus sangui- nolentus (Herbst), from the waters off northern Taiwan.- Journal of the Fisheries Society of Taiwan 27: 175-185. Huang, R. F. 1993. Studies on the taxonomy and
distribution of the portunid crabs (Crustacea: Decapoda: Brachyura) in Taiwan.-Doctoral Dissertation, National Taiwan Ocean University, Keelung, Taiwan. 235 pp. [In Chinese.]
Jacob, R., P. N. Prasad, and M. S. Kusuma. 1990. Maturity and dimensional studies in female crabs of Portunus sanguinolentus and P. pelagicus (Decapoda: Portuni- dae).-Indian Journal of Marine Sciences 19: 221-223. King, M. 1995. Fisheries Biology, Assessment and
Management. Fishing News Books, London. 341 pp. Lai, H. L., and D. R. Gunderson. 1987. Effects of ageing
errors on estimates of growth, mortality and yield per recruit for walleye pollock (Theragra chalcogramma).- Fisheries Research 5: 287-302.
, V. T. Gullucci, D. R. Gunderson, and R. F. Donnelly. 1996. Age determination in fisheries: methods and applications to stock assessment. Pp. 82-178 in V. F. Gullucci, S. B. Saila, D. J. Gustafson, and B. J.
Rothschild, eds. Stock Assessment, Quantitative Methods and Applications for Small-Scale Fisheries. CRS, Lewis Publishers, New York.
Lawlor, L. R. 1976. Parental investment and offspring fitness in the terrestrial isopod Armadillidium vulgare (Latr.) (Crustacea: Oniscoidea).-Evolution 30: 775-785. Leffler, C. W. 1972. Some effects of temperature on the
growth and metabolic rate of juvenile blue crabs, Callinectes sapidus, in the laboratory.-Marine Biology 14: 104-110.
Marques, J. C., I. Martins, C. T. Ferreira, and S. Cruz. 1994. Population dynamics, life history, and production of Cyathura carinata (Kroyer) (Isooda: Anthuridae) in the Mondego estuary, Portugal.-Journal of Crustacean Bi- ology 14: 258-272.
Pauly, D. 1980. On the interrelationship between natural mortality, growth parameters and mean environmental temperature in 175 fish stocks.-Journal du Conseil 39: 175-192.
, and J. L. Munro. 1984. Once more on the comparison of growth in fish and invertebrates.- ICLARM Fishbyte 2: 21.
Quinn, T. J. II, and R. B. Deriso. 1999. Quantitative Fish Dynamics. Oxford, New York. 542 pp.
Reeby, J., P. N. Prasad, and M. S. Kusuma. 1990. Size at maturity in male crabs of Portunus sanguinolentus and P. pelagicus.-Fishery Technology 27: 115-119.
Ryan, E. P. 1967. Structure and function of the reproductive system of the crab, Portunus sanguinolentus (Herbst).- Proceedings of the Symposium on Crustacea, Marine Biology Association of India, Part II: 252-544.
Smith, C. C., and S. D. Fretwell. 1974. The optimal balance between size and number of offspring.-American Naturalist 108: 499-506.
Stephenson, W., and B. Campbell. 1959. The Australian portunids (Crustacea: Portunidae). III. The genus Portu- nus.-Australian Journal of Marine and Freshwater Research 10: 84-124.
Sukumaran, K. K., K. Y. Telang, and D. Thippeswamy. 1986. On the fishery and biology of the crab Portunus sanguinolentus (Herbst) along the south Kanara coast.- Indian Journal of Fisheries 33: 188-200.
, and B. Neelakantan. 1997. Sex ratio, fecundity and reproductive potential in two marine portunid crabs, Portunus (Portunus) sanguinolentus (Herbst) and Portu- nus (Portunus) pelagicus (Linnaeus) along the Kamataka coast.-Indian Journal of Marine Sciences 26: 43-48. Sumpton, W. D., G. S. Smith, and M. A. Potter. 1989. Notes
on the biology of the Portunid crab, Portunus sanguino- lentus (Herbst), in subtropical Queensland waters.- Australian Joumal of Marine and Freshwater Research 40: 711-717.
Wenner, A. M. 1972. Sex ratio as a function of size in marine Crustacea.-The American Naturalist 106: 321- 350.
, D. M. Hubbard, J. Dugan, J. Shoffner, and K. Jellison. 1987. Egg production by sand crabs (Emerita analoga) as a function of size and year class (Decapoda, Hippidae).-The Biological Bulletin 172: 225-235. Wolff, M., and M. Soto. 1992. Population dynamics of
Cancer polyodon in La Herradura Bay, northern Chile.- Marine Ecology: Progress Series 85: 69-81.
Zar, J. H. 1995. Biostatistical Analysis. 3rd Edition. Prentice-Hall, Englewood Cliffs, New Jersey. 718 pp. RECEIVED: 23 August 2002.