Manuscript number 201310008
Title: Increased risk of Parkinson’s disease in patients with end-stage renal disease: a
retrospective cohort study (Revision)Short title: Parkinson’s disease and End-stage renal disease
I-Kuan Wang, MD 1,2,3, Cheng-Li Lin, MS4,5, Yi-Ying Wu, PhD6, Che-Yi Chou, MD2,
Shih-Yi Lin, MD2, Jiung-Hsiun Liu, MD2, Tzung-Hai Yen, PhD7,8, Chiu-Ching Huang, MD2,
Fung-Chang Sung, PhD, MPH4,5*
1Institute of Clinical Medical Science, China Medical University College of Medicine,
Taichung, Taiwan
2Division of Nephrology, China Medical University Hospital, Taichung, Taiwan
3Department of Internal Medicine, China Medical University College of Medicine, Taichung,
Taiwan
4Management Office for Health Data, China Medical University Hospital, Taichung, Taiwan 5Department of Public Health, China Medical University, Taichung, Taiwan
6Department of Medical Laboratory Science and Biotechnology, China Medical university,
Taichng, Taiwan.
7Division of Nephrology, Chang Gung Memorial Hospital, Taipei, Taiwan. 8Chang Gung University College of Medicine, Taoyuan, Taiwan.
Correspondence:
Fung-Chang Sung, PhD, MPH Professor
Institute of Clinical Medical Science, China Medical University 91 Hsueh Shih Road
Taichung 404, Taiwan Tel: 886-4-2206-2295 Fax: 886-4-2233-9216
E-mail: fcsung1008@yahoo.com
Conducting the statistical analysis: Cheng-Li Lin, MS
Words count: 94 characters in title, 195 words in the Abstract, 2260 words in the Text, 4 tables, 1 figure and 33 references
Funding: National Sciences Council, Executive Yuan, Taiwan (Grant Numbers NSC 100-2621-M-039-001), China Medical University Hospital (Grant Number 1MS1), and Taiwan
Department of Health Clinical Trial and Research Center for Excellence (Grant Numbers DOH102-TD-B-111-004 and DOH102-TD-C111-005).
Competing interests
Abstract
Background: Studies on dyskinesia and Parkinson’s syndrome, associated with basal
ganglion lesions in patients with chronic kidney disease or end-stage renal disease (ESRD) have been limited to most case reports or case series studies. This population-based study investigated the risk of Parkinson’s disease in patients with ESRD.
Methods: From a universal insurance claims database of Taiwan, we established a cohort of
8,325 adults with newly diagnosed ESRD from 1997 to 2010 without Parkinson’s disease history. A control cohort of 33,382 insured peoplewithout histories of kidney disease and Parkinson’s disease was also selected, with frequency matched with age, sex and index date of the patients with ESRD. Both cohorts were followed-up until the end of 2010.
Results: The Parkinson’s disease incidence was 1.55-fold higher in the cohort with ESRD than the comparison cohort (48.8 vs. 31.7 per 10,000 person-years) with an adjusted hazard ratio of 1.73 (95% confidence interval, 1.39-2.15). Sex-specific and age-specific analysis showed a higher relative risk for women and younger patients with ESRD compared to the control cohort.
Conclusions: ESRD is significantly associated with an increased risk of Parkinson’s disease.
Close surveillance for Parkinson’s disease should be considered for patients with ESRD.
Introduction
Parkinson’s disease (PD) is a second most common neurodegenerative disorders after
Alzheimer’s disease, characterized by movement disorders such as bradykinesia, resting tremor, rigidity, and postural instability . The pathogenesisof the disease is associated with progressive loss of the neurons of substantia nigra and other brain structures . The prevalence of PD increases with age with a range from 1% to over 4% in the elderly . Epidemiological studies have demonstrated that PD reduces life expectancy with the hazard ratios of mortality ranged from 1.5 to 2.7, compared with population without the disease .
Movement disorders also appear in uremic patients with symptoms ofmainly asterixis, myoclonus and restless leg syndrome . Asterixis and myoclonus seen in patients withuremic encephalopathy may reflect cortical involvement . The restless leg syndrome is a common disorder not only in PD patients but also inpatients with chronic kidney disease(CKD) for at least 20% of prevalence with response to dopaminergic treatment . It is therefore possible that there is an association between PD and CKD. However, limited case reports or case seriesstudies have reporteddyskinesia and Parkinson’s syndromes in association with the basal ganglion lesions for patients with CKD or end-stage renal disease (ESRD) . Therefore, the present study aims to investigate the risk of PD in incident ESRD patients using
population-based claim data, derived from the Taiwan National Health Insurance program.
Materials and Methods
Data sources
This study used claims dataretrieved from the National Health Insurance Research Database (NHIRD), obtained from Taiwan’s national health insurance program. The insurance program provides health careto approximately 99 % of 23.74 million people and
has contracted with 97% of hospitals and clinics by the end of 2009. The NHIRD contains all reimbursement claim records from 1996-2010 for 1,000,000 persons randomly selected from all insured persons in Taiwan. Information available from the database included all registered medical facilities, ambulatory cares, inpatient cares, dental services, prescription drugs, and registration files with scrambled identifications. The diseases were identified according to International Classification of Disease, 9th revision, Clinical Modification (ICD-9-CM).
This retrospective observational study complied with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of China Medical University (CMU-REC-101-012). Since this study involved retrospective review of existing data, the Institutional Review Board of China Medical University has specifically waived the need for consent. All data was de-identified and analyzed anonymously.
Study Subjects
We identified 8,959 patients with newly diagnosed ESRD (ICD-9-CM codes 585) during 1997-2010from the Registry for Catastrophic Illness Patient Database, which included those who require long-term renal replacement therapy such as dialysis or kidney transplant. The date that ESRD was diagnosed was designated as the index date. Patients with a history of PD diagnosed before index date (N = 481), with missing information on age or sex (N = 64), and aged less than 20 years were excluded (N = 89). The remaining 8,325 patients with ESRD were included in the ESRD g roup . For every ESRD patients, we randomly selected 4 insured people without any the history of PD or kidney disease, matched by age, sex, and the month of index day.
Outcome Measures
Each study subject was followed until PD (ICD-9-CM codes 332) was diagnosed or censored because of kidney transplantation, death, loss to follow-up, termination of
insurance, or December 31, 2010. The diagnos is of PD was made by neurologists with at least two successive records of diagnosis in the claims data. The comorbidities that might associate with PD to be included in this study were coronary artery disease (CAD; ICD-9-CM codes 410 to 413, 414.01 to 414.05, 414.8, and 414.9), congestive heart failure (CHF; ICD-9-CM codes 428, 398.91, 402.x1), stroke (ICD-9-CM codes 430 - 438), hyperlipidemia (ICD-9-CM codes 272), hypertension (ICD-9-CM codes 401- 405), diabetes (ICD-9-CM codes 250), head injury (ICD-9-CM codes:850-854, 959.01), identifiedat the baseline.
Statistical Analysis
Our data analyses first compared the distribution of socio-demographic factors and comorbid conditions between the ESRD group and the control group. We used the Chi-square test to examine categorical variables and t-test for continuous variables. The incidence density rates of PD were estimated in person-years for both groups.Univariate and
multivariate Cox proportional hazards models were used to assess the risk of developing PD associated with ESRD, compared with the control cohort. Hazard ratio (HR) and 95% CI were estimated in the Cox models. The follow-up period was partitioned into 2 segments (year ≤1, and >1 years), and the multivariable Cox proportional hazard regression analyses was also used to assess whether the risk of PD changedover time. A plot of Kaplan-Meier analysis was performed to show the probability of persons remaining without PD, and the log-rank test was applied to examine the difference between the ESRD cohort and the control cohort. All statistical analyses were performed by SAS version 9.2 (SAS Institute Inc, Cary, NC) and the Kaplan-Meier survival curve was plotted using R software (R Foundation for Statistical Computing, Vienna, Austria). The level of statistical significance was set at p<0.05.
Results
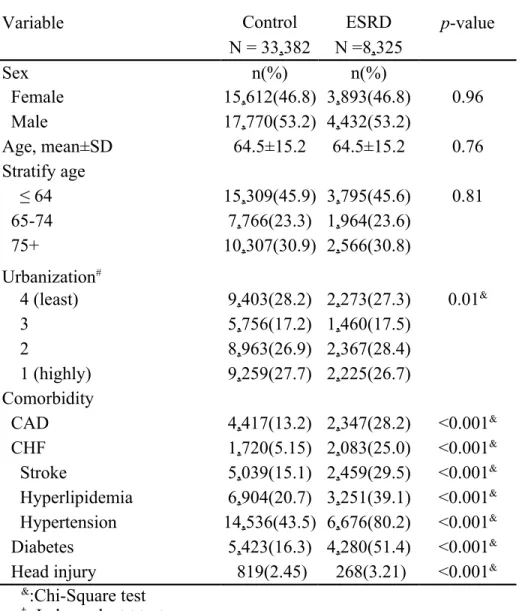
In this study, 8,325 patients with ESRD, including 7,902 hemodialysis patients and 423 peritoneal dialysis patients, and 33,382 subjects as controls without any kidney disease were presented (Table 1). The mean follow-up periods were 2.56±3.24 years in the ESRD cohort and 5.51±3.81 in the control cohort. In addition, the follow-up periods for PD onset were 2.82±2.48 in the ESRD cohort and 4.12±3.0 in the control cohort. Both cohorts dominated with men (53.2% vs. 46.8%) and subjects less 65 years old. Compared to the control cohort, the ESRD cohort tended to live in higher urbanized areas (55.1% in urbanization levels 1 and 2), and was more prevalent with comorbidities of CAD (28.2% vs. 13.2%), CHF (25.0% vs. 5.15%), stroke (39.1% vs. 20.7%), hypertension (80.2% vs. 43.5%), diabetes (51.4% vs. 16.3%), and head injury (3.21% vs. 2.45%).
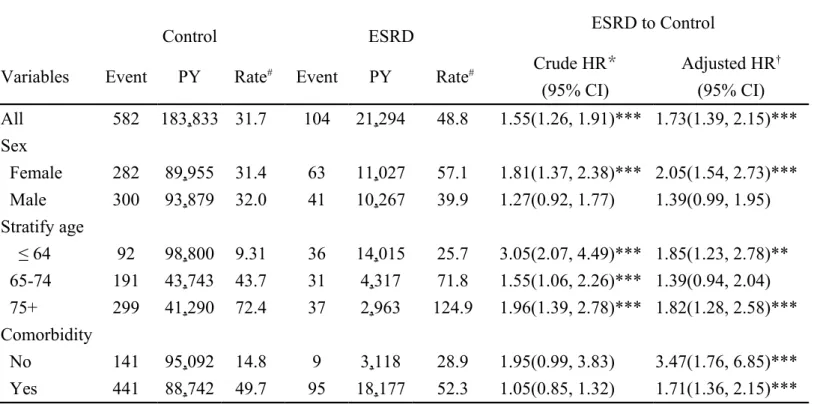
Table 2 shows the overallincidence rate of PD was 1.55 times higher in the ESRD cohort than in the control cohort (48.8 vs. 31.7 per 10,000 person-years), with an adjusted HR of 1.73 (95% CI = 1.39-2.15).The sex-specific comparisons revealed women with ESRD had higher crude HR of PD than men (crude HR =1.81, 95% CI = 1.37-2.38 vs. crude HR = 1.27, 95% CI = 0.92-1.77), and adjusted hazard ratio as well (adjusted HR = 2.05, 95% CI = 1.54-2.73 vs. adjusted HR = 1.39, 95% CI = 0.99-1.95). The crude HR was highest in those aged 64 years and younger (crude HR = 3.05, 95% CI = 2.07- 4.49), with an adjusted HR of 1.85 (95% CI = 1.23-2.78). In patients without comorbidity, the incidence rate of PD was 1.95 times higher in the ESRD cohort than in the control cohort (28.9 vs. 14.8 per 10,000 person-years), with an adjusted HR of 3.47 (95% CI = 1.76-6.85). The old age group may increase the HR of PD. An additional validation analysis by excluding those aged more than 75 years also demonstrated that the ESRD cohort was still at a higher risk of PD with an overall adjusted HR of 1.64 (95% CI = 1.22-2.22) (data not shown).
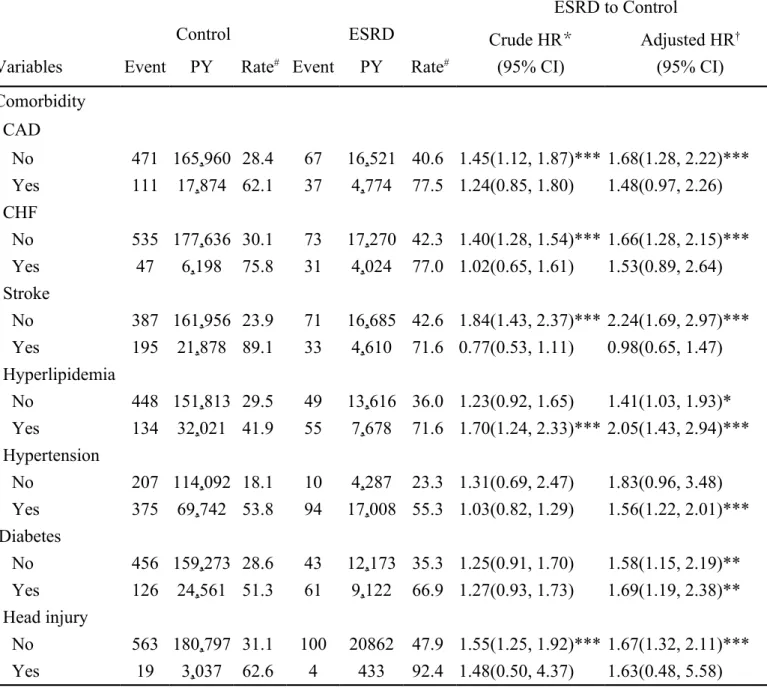
The incidence rate of PD was higher for those with comorbidities in both cohorts (Table 3). The risk of PD was higher among patients with diabetes (adjusted HR = 1.69, 95% CI = 1.19–2.38) in the ESRD cohort than in the control cohort. Analysis by the follow-up time showed that the incidence of PD appeared earlier in the ESRD cohort than in the control cohort. The incidence rates within one year of follow-up were 53.7 vs. 26.2 per 10,000 person-years for the ESRD cohort and the control cohort, respectively, with an adjusted HR is 2.04 (95% CI=1.26-3.29) (Table 4). Kaplan-Meier analysis showed that the cumulative PD incidence after 14 years of follow-up was greater in the ESRD cohort than in the control (log-rank test, p<0.0001).
Discussion
Among the case studies on Parkinson’s syndrome in patients with ESRD, most were
conducted for Asian populations . The present study accounted for 73% increased risk of PD for patients with ESRD. An additional validation analysis by excluding those aged more than 75 years also demonstrated 64% increased risk. The comorbidity specific relative risk is even greater for ESRD patients without the comorbidity. Lin et al. found recently that CKD patients were more vulnerable to have parkinsonism, with a 1.81-fold higher risk than non-CKD patients .It is likely that the risk of PD associated with ESRD is the consequence of further progress of CKD. This explains why a higher risk of PD appears within one year after the diagnosis of ESRD. The risk remains in later years. Our study is the first original research reporting the association between ESRD and the risk of PD.
Although the pathogenic mechanisms governing dopaminergic cell loss in PD are not fully understood, Greenamyre and Hastings have proposed that abnormal protein processing, oxidative stress, mitochondria dysfunction, inflammation play an important role in the pathogenesis of PD . Exposure to certain dopaminergic neuron toxins, such as 1- methyl -4-
phenyl-1,2,3,6-tetrahydro pyridine (MPTP), herbicide paraquat, pesticide, and heavy metals may increase the development of PD . The mechanisms that lead to PD in patient with uremia are also not well understood. Although the abnormality of uremic encephalopathy is in cortical rather than basal ganglion, metabolic derangements in ESRD patients may predispose to basal ganglion injury. CKD and inadequate dialysis has been associated with basal ganglion injury . Accumulation of metabolic acidosis, parathyroid hormone, and methylguanidine are implicated as uremic toxins due to loss of kidney function . Several studies have observed movement disorders in ESRD patients who are susceptible to aluminum and manganese toxicity . In addition, case studies have reported drugs induced parkinsonism in ESRD patients due to affecting dopaminergic neuronal pathways such as metoclopramide and trazodone . Mitochondria dysfunction, augmented oxidative stress and inflammation that contribute to the pathogenesis of PD exist in ESRD patients as well . In our study, the risk of PD continuously remains longer than one year after the diagnosis of ESRD. This suggests that the mechanism of PD attribute to uremia is more than an acute effect.
Most previous reports of basal ganglion injury for patients with CKD not yet on dialysis or ESRD were diabetics . Cohort studies have suggested that patients with diabetes are at a higher risk for PD . In experimental animal studies, chronic hyperglycemia reduces striatal dopaminergic transmission and enhances the sensitivity of postsynaptic dopamine receptors . Hypoglycemia and hyperglycemia have been reported to induce basal ganglion injury . In our study, the diabetic ESRD patients with diabetes are at near 2-fold higher risk of PD than those without diabetes (66.9 vs. 35.3 per 10,000 person-years). Vascular parkinsonism, defined as parkinsonism due to cerebrovascular disease, is a controversial clinical entity . However, our data also show that ESRD patients with cardiovascular disorders are
characterized with higher incidence of PD. Further data analysis shows that the risk increases with number of these comorbidities. Microvascular and/or macrovasular disorders in diabetic and/or ESRD patients are likely to make basal ganglion more susceptible to toxins,
hypoglycemia/hyperglycemia, metabolic acidosis, hypoxia or hypoperfusion. In addition, among patients without comorbidity, ESRD still increased the risk of PD.
In a case report with literature review, Li et al have indicated that the Parkinson’s syndrome in ESRD is characterized by acute or subacute movement disorder, which is mainly
bradykinesia in presentation . The neurological symptoms and signs improved slowly. They found that 20 percent of patients with complete resolution, 50% improved with residual deficit, and 30% did not improve. The neuroimaging findings are characterized by bilateral hypodense lesions of basal ganglion on CT. MRI showed low density lesions of basal ganglion on T1-weighted images and increased intensity on T2-weighted images . In addition, two of the 24 patients received peritoneal dialysis. The duration of renal replacement therapy at the time of PD onset was 2.4±1.6 years.
This large national cohort study has disclosed ESRD as a risk factor of PD. However, the present study has several limitations. First, information on lifestyle, exposure to pesticides or herbicides, smoking habit, body-mass index, and laboratory measures on serum glucose, urea nitrogen, creatinine, aluminum, manganese, parathyroid hormone, pH value and adequacy of dialysis are unavailable from the claims file. These variables could not be adjusted in the data analysis. Second, the neuroimaging findings are also not available from this database.
However, the diagnosis of PD is based on clinical presentation . Furthermore, PD and other comorbidities were identified using ICD-9-CM codes. Although we could not calculate the validity of diagnostic codes for PD, the high validity of the diagnostic codes of the NHRI database has been reported . In addition, ESRD patients were visited by health care
professionals frequently, the claims data is reliable. However, ESRD patients with PD might be more likely to be coded as such than control patients with this disorder. On the other hand, the mortality of ESRD patients is expected to be high. Therefore, the incidence and risk of PD might be underestimated in ESRD patients.
Conclusions
ESRD is significantly associated with an increased risk of PD. Close surveillance for PD should be considered for ESRD patients. Further studies are needed to investigate the mechanisms underlying the association of ESRD and subsequent PD.
Acknowledgments
The study was supported partly by the National Sciences Council, Executive Yuan, Taiwan (Grant Numbers NSC 100-2621-M-039-001), China Medical University Hospital (Grant Number 1MS1), and Taiwan Department of Health Clinical Trial and Research Center for Excellence (Grant Numbers DOH102-TD-B-111-004 and DOH102-TD-C111-005).
Conflict of interest
The authors declare no conflicts of interest.
References
1 de Lau LM, Breteler MM: Epidemiology of parkinson's disease. Lancet Neurol 2006;5:525-535.
2 Tolosa E, Wenning G, Poewe W: The diagnosis of parkinson's disease. Lancet Neurol 2006;5:75-86.
3 Nussbaum RL, Ellis CE: Alzheimer's disease and parkinson's disease. N Engl J Med 2003;348:1356-1364.
Neurol Neurosurg 2004;107:1-16.
5 Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K: Epidemiology of restless legs syndrome: The current status. Sleep Med Rev 2006;10:153-167.
6 Cupidi C, Piccoli F, La Bella V: Acute reversible parkinsonism in a diabetic-uremic patient. Clin Neurol Neurosurg 2006;108:601-603.
7 da Silva CJ, da Rocha AJ, Jeronymo S, Mendes MF, Milani FT, Maia AC, Jr., Braga FT, Sens YA, Miorin LA: A preliminary study revealing a new association in patients undergoing maintenance hemodialysis: Manganism symptoms and t1 hyperintense changes in the basal ganglia. AJNR Am J Neuroradiol 2007;28:1474-1479.
8 Kim TK, Seo SI, Kim JH, Lee NJ, Seol HY: Diffusion-weighted magnetic resonance imaging in the syndrome of acute bilateral basal ganglia lesions in diabetic uremia. Mov Disord 2006;21:1267-1270.
9 Lee EJ, Park JH, Ihn Y, Kim YJ, Lee SK, Park CS: Acute bilateral basal ganglia lesions in diabetic uraemia: Diffusion-weighted mri. Neuroradiology 2007;49:1009-1013.
10 Lee PH, Shin DH, Kim JW, Song YS, Kim HS: Parkinsonism with basal ganglia lesions in a patient with uremia: Evidence of vasogenic edema. Parkinsonism Relat Disord
2006;12:93-96.
11 Lee YH: Diabetic nephropathy with acute symmetrical changes in the basal ganglia regions. Clin Radiol 2005;60:815-820.
12 Li JY, Yong TY, Sebben R, Khoo E, Disney AP: Bilateral basal ganglia lesions in patients with end-stage diabetic nephropathy. Nephrology (Carlton) 2008;13:68-72. 13 Ohtake T, Negishi K, Okamoto K, Oka M, Maesato K, Moriya H, Kobayashi S: Manganese-induced parkinsonism in a patient undergoing maintenance hemodialysis. Am J Kidney Dis 2005;46:749-753.
14 Okada J, Yoshikawa K, Matsuo H, Kanno K, Oouchi M: Reversible mri and ct findings in uremic encephalopathy. Neuroradiology 1991;33:524-526.
15 Wang HC, Brown P, Lees AJ: Acute movement disorders with bilateral basal ganglia lesions in uremia. Mov Disord 1998;13:952-957.
16 Wang HC, Cheng SJ: The syndrome of acute bilateral basal ganglia lesions in diabetic uremic patients. J Neurol 2003;250:948-955.
17 Lin HL, Lin HC, Chen YH: Increased risks of parkinsonism in the 3 years after chronic renal failure. Int J Clin Pract 2012;66:499-503.
18 Greenamyre JT, Hastings TG: Biomedicine. Parkinson's--divergent causes, convergent mechanisms. Science 2004;304:1120-1122.
19 Arieff AI: Aluminum and the pathogenesis of dialysis encephalopathy. Am J Kidney Dis 1985;6:317-321.
20 Fukunishi I, Kitaoka T, Shirai T, Kino K, Kanematsu E, Sato Y: A hemodialysis patient with trazodone-induced parkinsonism. Nephron 2002;90:222-223.
21 Sethi KD, Patel B, Meador KJ: Metoclopramide-induced parkinsonism. South Med J 1989;82:1581-1582.
22 Sirota RA, Kimmel PL, Trichtinger MD, Diamond BF, Stein HD, Yudis M:
Metoclopramide-induced parkinsonism in hemodialysis patients. Report of two cases. Arch Intern Med 1986;146:2070-2071.
23 Kimmel PL, Phillips TM, Phillips E, Bosch JP: Effect of renal replacement therapy on cellular cytokine production in patients with renal disease. Kidney Int 1990;38:129-135. 24 Raj DS, Boivin MA, Dominic EA, Boyd A, Roy PK, Rihani T, Tzamaloukas AH, Shah VO, Moseley P: Haemodialysis induces mitochondrial dysfunction and apoptosis. Eur J Clin Invest 2007;37:971-977.
25 Raj DS, Dominic EA, Pai A, Osman F, Morgan M, Pickett G, Shah VO, Ferrando A, Moseley P: Skeletal muscle, cytokines, and oxidative stress in end-stage renal disease. Kidney Int 2005;68:2338-2344.
26 Cereda E, Barichella M, Pedrolli C, Klersy C, Cassani E, Caccialanza R, Pezzoli G: Diabetes and risk of parkinson's disease: A systematic review and meta-analysis. Diabetes Care 2011;34:2614-2623.
27 Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J: Type 2 diabetes and the risk of parkinson's disease. Diabetes Care 2007;30:842-847.
28 Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY: Risk of parkinson disease onset in patients with diabetes: A 9-year population-based cohort study with age and sex
stratifications. Diabetes Care 2012;35:1047-1049.
29 Sandyk R, Kay SR, Awerbuch GI, Iacono RP: Risk factors for neuroleptic-induced movement disorders. Int J Neurosci 1991;61:149-188.
30 Hsu JL, Wang HC, Hsu WC: Hyperglycemia-induced unilateral basal ganglion lesions with and without hemichorea. A pet study. J Neurol 2004;251:1486-1490.
31 Lai SL, Tseng YL, Hsu MC, Chen SS: Magnetic resonance imaging and single-photon emission computed tomography changes in hypoglycemia-induced chorea. Mov Disord 2004;19:475-478.
32 Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML: Validation of the national health
insurance research database with ischemic stroke cases in taiwan. Pharmacoepidemiol Drug Saf 2011;20:236-242.
33 Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY: Accuracy of diabetes diagnosis in health insurance claims data in taiwan. J Formos Med Assoc 2005;104:157-163.
Table 1 Demographic characteristics and comorbidities in the cohort with end-stage renal disease and the control cohort
Variable Control ESRD p-value
N = 33,382 N =8,325 Sex n(%) n(%) Female 15,612(46.8) 3,893(46.8) 0.96 Male 17,770(53.2) 4,432(53.2) Age, mean±SD 64.5±15.2 64.5±15.2 0.76 Stratify age ≤ 64 15,309(45.9) 3,795(45.6) 0.81 65-74 7,766(23.3) 1,964(23.6) 75+ 10,307(30.9) 2,566(30.8) Urbanization# 4 (least) 9,403(28.2) 2,273(27.3) 0.01& 3 5,756(17.2) 1,460(17.5) 2 8,963(26.9) 2,367(28.4) 1 (highly) 9,259(27.7) 2,225(26.7) Comorbidity CAD 4,417(13.2) 2,347(28.2) <0.001& CHF 1,720(5.15) 2,083(25.0) <0.001& Stroke 5,039(15.1) 2,459(29.5) <0.001& Hyperlipidemia 6,904(20.7) 3,251(39.1) <0.001& Hypertension 14,536(43.5) 6,676(80.2) <0.001& Diabetes 5,423(16.3) 4,280(51.4) <0.001&
Head injury 819(2.45) 268(3.21) <0.001&
&:Chi-Square test
†: Independent t test
#: The urbanization level was categorized by the population density of the residential area
Table 2 Comparison in the incidence rates of Parkinson’s disease between patients with end-stage renal disease and those without any kidney disease
ESRD to Control
Control ESRD
Variables Event PY Rate# Event PY Rate# Crude HR
* (95% CI) Adjusted HR† (95% CI) All 582 183,833 31.7 104 21,294 48.8 1.55(1.26, 1.91)*** 1.73(1.39, 2.15)*** Sex Female 282 89,955 31.4 63 11,027 57.1 1.81(1.37, 2.38)*** 2.05(1.54, 2.73)*** Male 300 93,879 32.0 41 10,267 39.9 1.27(0.92, 1.77) 1.39(0.99, 1.95) Stratify age ≤ 64 92 98,800 9.31 36 14,015 25.7 3.05(2.07, 4.49)*** 1.85(1.23, 2.78)** 65-74 191 43,743 43.7 31 4,317 71.8 1.55(1.06, 2.26)*** 1.39(0.94, 2.04) 75+ 299 41,290 72.4 37 2,963 124.9 1.96(1.39, 2.78)*** 1.82(1.28, 2.58)*** Comorbidity No 141 95,092 14.8 9 3,118 28.9 1.95(0.99, 3.83) 3.47(1.76, 6.85)*** Yes 441 88,742 49.7 95 18,177 52.3 1.05(0.85, 1.32) 1.71(1.36, 2.15)***
Rate#, incidence rate, per 10,000 person-years; Crude hazard ratio (HR)*: relative
hazard ratio; Adjusted HR† : multivariate analysis adjusting for age, sex, urbanization,
Table 3. Comparison in the incidence rates of Parkinson’s disease among different comorbidities between patients with end-stage renal disease and those without any kidney disease
ESRD to Control
Control ESRD Crude HR*
(95% CI)
Adjusted HR†
(95% CI) Variables Event PY Rate# Event PY Rate#
Comorbidity CAD No 471 165,960 28.4 67 16,521 40.6 1.45(1.12, 1.87)*** 1.68(1.28, 2.22)*** Yes 111 17,874 62.1 37 4,774 77.5 1.24(0.85, 1.80) 1.48(0.97, 2.26) CHF No 535 177,636 30.1 73 17,270 42.3 1.40(1.28, 1.54)*** 1.66(1.28, 2.15)*** Yes 47 6,198 75.8 31 4,024 77.0 1.02(0.65, 1.61) 1.53(0.89, 2.64) Stroke No 387 161,956 23.9 71 16,685 42.6 1.84(1.43, 2.37)*** 2.24(1.69, 2.97)*** Yes 195 21,878 89.1 33 4,610 71.6 0.77(0.53, 1.11) 0.98(0.65, 1.47) Hyperlipidemia No 448 151,813 29.5 49 13,616 36.0 1.23(0.92, 1.65) 1.41(1.03, 1.93)* Yes 134 32,021 41.9 55 7,678 71.6 1.70(1.24, 2.33)*** 2.05(1.43, 2.94)*** Hypertension No 207 114,092 18.1 10 4,287 23.3 1.31(0.69, 2.47) 1.83(0.96, 3.48) Yes 375 69,742 53.8 94 17,008 55.3 1.03(0.82, 1.29) 1.56(1.22, 2.01)*** Diabetes No 456 159,273 28.6 43 12,173 35.3 1.25(0.91, 1.70) 1.58(1.15, 2.19)** Yes 126 24,561 51.3 61 9,122 66.9 1.27(0.93, 1.73) 1.69(1.19, 2.38)** Head injury No 563 180,797 31.1 100 20862 47.9 1.55(1.25, 1.92)*** 1.67(1.32, 2.11)*** Yes 19 3,037 62.6 4 433 92.4 1.48(0.50, 4.37) 1.63(0.48, 5.58)
Rate#, incidence rate, per 10,000 person-years; Crude hazard ratio (HR)*: relative
hazard ratio; Adjusted HR† : multivariate analysis adjusting for age, sex, urbanization,
Table 4. Hazard ratios for Parkinson’s disease in patients with end-stage renal disease compared to those without any kidney disease by the follow-up duration.
Control ESRD
Event PY Rate# Event PY Rate# Crude HR † (95% CI) Adjusted HR† (95% CI) Follow years ≤1 82 31,353 26.2 28 5,213 53.7 2.07(1.35, 3.19)*** 2.04(1.26, 3.29)*** >1 500 152,481 32.8 76 16,081 47.3 1.48(1.16, 1.88)** 1.48(1.14, 1.92)***
Rate#, incidence rate, per 10,000 person-years; Crude hazard ratio (HR)*: relative
hazard ratio; Adjusted HR† : multivariate analysis adjusting for age, sex, urbanization,
and co-morbidities;
Legends
Figure 1. Probability free of Parkinson’s disease for patients with end-stage renal disease (dashed line) or without any kidney disease (solid line).