Corresponding author: Dr. Shih-Hua Fang, Institute of Athletics, National Taiwan University of Sport, No. 16, Sec. 1, Shuang-Shih Rd., Taichung 40404, Taiwan, R.O.C. Tel: +886-4-22213108 ext. 2176, Fax: +886-4-22250756, E-mail: shfang@ntupes.edu.tw
Received: March 19, 2015; Revised: June 2, 2015; Accepted: June 17, 2015.
2015 by The Chinese Physiological Society and Airiti Press Inc. ISSN : 0304-4920. http://www.cps.org.tw
Salivary Immuno Factors, Cortisol and
Testosterone Responses in Athletes of a
Competitive 5,000 m Race
Chia-Yang Li1, 2, Gi-Sheng Hsu3, Katsuhiko Suzuki4, Miau-Hwa Ko5, and Shih-Hua Fang3
1Department of Genome Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung 80708 2Center for Infectious Disease and Cancer Research, Kaohsiung Medical University, Kaohsiung 80708
3Institute of Athletics, National Taiwan University of Sport, Taichung 40404 4Faculty of Sport Sciences, Waseda University, Tokorozawa 359-1192, Japan
and
5Department of Anatomy, School of Medicine, China Medical University, Taichung 40402 Taiwan, Republic of China
Abstract
The exercise-stress model can be a model of temporary immunosuppression that occurs after severe physical and psychological stress. It also allows for the study of interactions between the endo- crine and the immune systems. This study examined changes in salivary hormonal and immune factors in athletes in response to physical and psychological stress in a 5,000 m running competition. Eighteen endurance-trained runners (9 males and 9 females) participated in this study. All participants com- pleted a competitive 5,000 m race. Saliva samples were collected 10 min before (PRE) and 10 min after (POST) the competition. Saliva was analyzed for α-amylase activity, concentrations of salivary immuno- globulin A (SIgA), lactoferrin, cortisol, testosterone and total protein. Although the concentrations of salivary TP, SIgA, lactoferrin, cortisol and α-amylase activity were significantly increased immediately after a competitive 5,000 m race, the secretion rates of these factors were not significantly altered in both male and female groups. Additionally, basal levels of SIgA and α-amylase activity were significantly higher in female runners than in male runners. This gender difference still existed after the race. The secretion rates of testosterone decreased significantly after the race in the male, but not in the female group. Moreover, testosterone-to-cortisol (T/C) ratios were significantly lower post-competition compared to pre-competition in both male and female athletes. The T/C ratio had been used as a performance index for athletes. Whether there are correlations between these changes of their physiological characteristics and better running performance need further investigations.
Key Words: cortisol, gender differences, physical and psychological stress, salivary immune factors, testosterone
Introduction
Interactions between exercise stress and the im- mune system provide a unique opportunity to link basic and clinical physiology and to evaluate the role of underlying stress and immunophysiological mech- anisms. Saliva contains numerous host defense factors,
such as immunoglobulin A (IgA), anti-microbial pep- tides and α-amylase, which play key roles in the mucosal immunity against microbial infection (39). Salivary IgA (SIgA) prevents the adherence of mi-crobes to the mucosal surface and, thus, plays an important role in mucosal immunity (20). Alpha-amylase has been shown not only to be as an anti-
bacterial protein by inhibiting bacterial growth and colonization in the oral cavity (24), but is also a bio- marker of sympathetic nervous activity (34). Lacto- ferrin acts as an antibacterial protein by sequestering iron and directly interacting and damaging bacterial membrane (14). Salivary secretion of these host de- fense factors was shown to be affected by high-intensity exercise. In addition, some studies have indicated that SIgA concentrations and secretion rates are higher in athletic men compared to athletic women at rest (9), and also immediately prior to prolonged cycling ex-ercise (1). Another study demonstrated that women had higher secretion rates of SIgA than men pre- exercise; lactoferrin values increased after 45 min at 75% VO2 max running exercise in both sexes (8).
It remains unclear whether gender would influence salivary hormonal and immunological factors.
Physical exercise has been shown to influence the secretion of steroid hormones, such as cortisol (C) and testosterone (T). Salivary cortisol represents the bio- logically active, free fraction of blood cortisol in re- sponse to exercise (26) and the concentration of this hormone in the saliva accounts for 70% of the non- bound blood cortisol (2). Cortisol is a catabolic hor- mone secreted from the adrenal cortex in response to physical and/or psychological stress, which stimulates muscle protein degradation (6). Significant increases in cortisol levels were observed in school children and different types of athletes during and after strenuous exercise (3). On the other hand, testosterone is an anabolic hormone which increases muscle mass and strength by enhancing muscle protein synthesis, and is an important stress hormone. Indeed, testosterone concentrations increased after an acute bout of exer- cise (12). Other studies noted that testosterone concen- tration decreased in response to prolonged endurance exercise (30). Moreover, some studies reported that testosterone levels had not changed after exercise (27, 28). The testosterone/cortisol (T/C) ratio is considered to reflect anabolic and catabolic balance. Therefore, depending on type, intensity, frequency and duration of a preceding exercise as well as an athlete’s hydra- tion state, levels of anabolic and/or catabolic hormones and the catabolic state of the body are changed (22). When the T/C ratio decreased by more than 30%, it has been proposed to indicate a state of overreaching (23). Investigators have also noted lower T/C ratios in elite performers than in medium performers (28). Therefore, the T/C ratio has also been used as a per- formance index for athletes.
There is a limited research on hormonal and im- mune responses of experienced (>4 years) endurance- trained runners. Vuorimaa et al. reported that both cortisol and testosterone concentrations of well-trained middle-distance runners were significantly elevated 20 min after continuous or intermittent ex-
ercise (36). A previous study has found gender differ- ences in metabolic substrates and endocrine responses to stress during prolonged post-exercise and recovery period (34). A recent report also demonstrated that saliva flow rates and the secretion rates of lactoferrin, lysozyme and amylase, but not SIgA, are significantly higher in males than in females during a 16-week, 11 h per week moderate-vigorous physical activity in winter (11). In contrast, SIgA was significantly lower in women than men no matter at rest or after perform- ing 2 h 65% VO2 max cycling (1). However, the acute
effects of 5,000 m competitive race on endurance-trained runners and gender differences have not been investigated. The aim of this study was to examine and compare the effects of 5,000 m running competi-tion on salivary hormone and immune responses be- tween male and female athletes.
Materials and Methods
Participants
Eighteen endurance-trained runners (nine males and nine females) from the college team of the Na-tional Taiwan University of Physical Education and Sport volunteered to participate in this study. The criteria for inclusion into this study included en-gagement in intense athletic conditioning for over four years, and healthy physical conditions without history of cardiovascular or endocrine/immune-related diseases. Participants who need to take any medica- tion during the period of this study were excluded. All participants signed an informed consent form after having been fully informed of the risks and the purpose of the study. The study was reviewed and ap- proved by the Human Ethics Committee of the Na-tional Taiwan University of Sport.
Determination of Physical Characteristics
The physical characteristics of endurance-trained runners were measured as in our previous study (33). An eight-electrode bioimpedance analyzer InBody 3.0 (Biospace, Seoul, Korea) was used to measure the body weight. Body height was measured using a stadiometer (Holtain, UK) to the nearest 0.1 cm in standing posi- tion and without shoes. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m). Prior to entering the study, VO2 max
(ml⋅kg-1⋅min-1) of each subject had been measured with
the Bruce Protocol that provides excellent accuracy and a standardized testing procedure for all partici- pants on a treadmill (Medtrack ST65, Quinton, Seattle, WA, USA) (19). The breath-by-breath gas analysis was performed using a Vmax 29C gas analyzer (Sen-sormedics, Yorba Linda, CA, USA). VO2 max was
confirmed when the following criteria were met: [1] a respiratory exchange ratio higher than 1.20; [2] a plateau in VO2 max despite an increase in running
speed; and [3] visible exhaustion. Saliva Collection
Saliva samples were collected 10 min prior to (PRE) and 10 min after (POST) the 5,000 m race that was held at 15:00, and at least 2 h had passed after meal and brushing the teeth. In order to minimize possible contamination (e.g. by food), all participants were asked to thoroughly rinse their mouth with sterile distilled water and then spit out the water. Ten minutes later, they were seated and unstimulated whole-saliva specimens were collected for two (PRE) and five (POST) minutes. All salivary samples appeared clear with no visible color, although there was still possibilities of invisible contamination. Volume of the saliva collected was computed by weighting the tube immediately after collection, and the saliva density was assumed to be 1.00 g/ml. Salivary flow rate was calculated as volume (ml)/collection time (min). Saliva specimens were stored in sterile plastic containers at -80°C until use. Assays
A Bio-RAD protein assay kit (Bio-RAD, Hercules, CA, USA) was used to measure the salivary TP con-centrations. Concentrations of SIgA, lactoferrin, cortisol, testosterone and α-amylase activity were measured as described before (33). In brief, an enzyme-linked immunosorbent assay (ELISA) was used to determine the SIgA concentrations. Primary and secondary antibodies were anti-human IgA anti- body (I-9889, Sigma, Poole, UK) and anti-human IgA conjugated with horseradish peroxidase (A3062, Sigma, Poole, UK), respectively. Lactoferrin levels were detected with the sandwich ELISA method. Pri- mary antibody was sheep anti-human lactoferrin (ab36303, Abcam, Cambridge, UK). Secondary anti- body and tertiary antibody were rabbit anti-human lactoferrin (ab15811, Abcam, Cambridge, UK) and alkaline phosphatase-conjugated goat anti-rabbit IgG (816122, ZYMED, South San Francisco, CA, USA), respectively. The α-amylase activity was determined using a kinetic reaction assay kit (Salimetrics LLC, State College, PA, USA) according to the manufac-turer’s instructions. Commercial ELISA kits (DRG Instruments, GmbH, Marburg, Germany) were used to measure cortisol and testosterone concentrations. All samples were measured in triplicates and data were expressed as absolute concentrations and pro-tein was assayed relative to salivary flow rate (37). The inter-assay coefficient of variation was 2% for SIgA, 2.5% for lactoferrin and α-amylase, and 1.5%
for cortisol and testosterone. The intra-assay coeffi- cient of variation (CV) for the measurements of SIgA, lactoferrin, amylase activity, cortisol and testosterone was 3, 3, 4, 4 and 4%, respectively.
Statistical Analysis
All data are expressed as mean ± SD. Statistical comparisons before and after the race were analyzed using paired t-test. Intergroup differences were treated with one-way analysis of variance. Significant dif- ference was set atP < 0.05. A Tukey post hoc analysis was used when significant differences were found for the main effects.
Results
Anthropometrical and Physical Parameters
Nine males (age: 19.3 ± 0.7 years) and nine fe- males (age: 19.1 ± 0.5 years) endurance-trained runners of national level participated in this study. Participant characteristics and their 5,000 m running time are summarized in Table 1. Although the male partici- pants were taller and heavier (P < 0.05) than the female participants, their BMI values were not significantly different. Male athletes show higher VO2 max values
and better exercise performances (P < 0.01) than fe- males.
Salivary Total Protein and Defense Factors
The concentrations of salivary TP, SIgA, lac-toferrin as well as the α-amylase activity increased significantly (P < 0.05) after the 5,000 m race com- pared to the pre-race values in both male and female athletes (Table 2). In addition, the basal levels of α-amylase (P < 0.05) and SIgA (P < 0.01) were sig- nificantly higher in female runners than in male run- ners. This gender difference still existed after the race (Table 2, P < 0.05). After the race, the salivary flow
Table 1. Anthropometrical and physical parameters of the athletes
Group Male Female
Number of athletes 9 9 Age (years) 19.3 ± 0.7 19.1 ± 0.5 Height (cm) 171.8 ± 1.6 160.3 ± 1.8# Body weight (kg) 57.8 ± 1.5 49.9 ± 1.7# BMI (kg/m2) 19.6 ± 0.3 19.4 ± 0.3 VO2 max (ml/kg/min) 59.9 ± 3.8 47.5 ± 3.9##
5000 m running time (min) 17.1 ± 1.7 20.4 ± 1.9##
Values are mean ± SD. BMI, body mass index. #P < 0.05, ##P < 0.01 significantly different from Male.
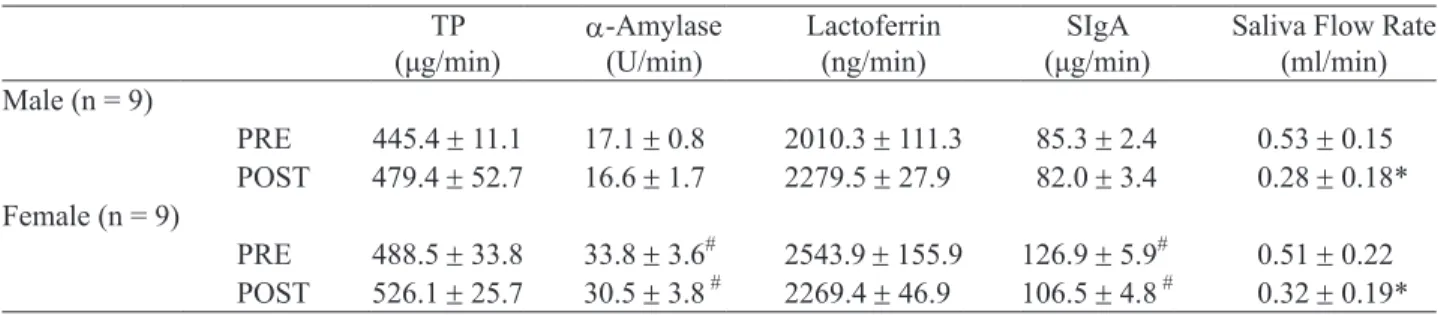
rates dramatically decreased in all athletes (Table 3). However, the secretion rates of total protein, α-amylase activity, lactoferrin and SIgA were not markedly dif- ferent between before and after the race. Consistent with the basal levels of α-amylase and SIgA, the secretion rates of α-amylase activity and SIgA re- mained higher (P < 0.05) in female than in the male athletes, irrespective of pre-exercise or post-exercise levels.
Salivary Hormones
Cortisol concentrations of both the male and
female runners increased significantly (P < 0.001) after the 5000 m running (Table 4), but there were no significant changes in the secretion rates of cortisol. No significant gender differences in concentrations and secretion rates of cortisol were observed. How- ever, the concentrations (P < 0.05), secretion rates (P < 0.01) of testosterone and the T/C ratio (P < 0.05) were much lower in females than males. Further-more, the secretion rates of testosterone dropped dramatically after the race in male (P < 0.05), but not female athletes. The T/C ratio decreased mark-edly (P < 0.05) in both the male and female groups after the race.
Table 2. Concentrations of salivary total protein, lactoferrin, SIgA and α-amylase activity
TP (μg/ml) α-Amylase (U/ml) Lactoferrin (ng/ml) SIgA (μg/ml) Male (n = 9) PRE 97 ± 74 32.3 ± 5.5 3,793 ± 742 161 ± 16 POST 1,748 ± 293* 59.2 ± 9.5* 8,141 ± 155* 293 ± 19* Female (n = 9) PRE 997 ± 180 66.2 ± 16.3# 4,988 ± 709 249 ± 27## POST 1,644 ± 135* 88.6 ± 18.0*, # 7,092 ± 247* 300 ± 25*, #
Values are mean ± SD. TP, total protein; SIgA, salivary immunoglobulin A; PRE & POST, before and after the 5,000 m race, respectively. *P < 0.05 significantly different from PRE. #P < 0.05; ##P < 0.01 significantly different from male
at that time point.
Table 3. Secretion rates of saliva, salivary total protein, lactoferrin, SIgA and α-amylase activity
TP
(μg/min) α-Amylase (U/min) Lactoferrin (ng/min) (μg/min)SIgA Saliva Flow Rate (ml/min) Male (n = 9) PRE 445.4 ± 11.1 17.1 ± 0.8 2010.3 ± 111.3 85.3 ± 2.4 0.53 ± 0.15 POST 479.4 ± 52.7 16.6 ± 1.7 2279.5 ± 27.9 82.0 ± 3.4 0.28 ± 0.18* Female (n = 9) PRE 488.5 ± 33.8 33.8 ± 3.6# 2543.9 ± 155.9 126.9 ± 5.9# 0.51 ± 0.22 POST 526.1 ± 25.7 30.5 ± 3.8 # 2269.4 ± 46.9 106.5 ± 4.8 # 0.32 ± 0.19*
Values are mean ± SD. Abbreviations are as in footnote of Table 2. *P < 0.05 significantly different from PRE; #P < 0.05, ##P < 0.01 significantly different from male at that time point.
Table 4. Concentrations and secretion rates of cortisol and testosterone in saliva
Group Cortisol (nmol/l) (pmol/min)Cortisol Testosterone (nmol/l) Testosterone (pmol/min) T/C Ratio Male (n = 9) PRE 85.32 ± 20.08 45.22 ± 3.01 0.16 ± 0.12 0.085 ± 0.018 1.83 ± 1.41 POST 134.55 ± 22.31*** 37.67 ± 4.01 0.09 ± 0.08 0.025 ± 0.014* 0.73 ± 0.67* Female (n = 9) PRE 76.44 ± 25.63 38.98 ± 5.64 0.04 ± 0.03# 0.020 ± 0.006## 0.65 ± 0.60# POST 137.15 ± 17.75*** 43.88 ± 3.37 0.03 ± 0.03 0.009 ± 0.006# 0.28 ± 0.21*, #
Values are mean ± SD. Abbreviations are as in footnote of Table 2. *P < 0.05, ***P < 0.001 significantly different from PRE; #P < 0.05, ##P < 0.01 significantly different from male at that time point.
Discussion
The aim of the current study was to examine the effects of the competitive 5,000 m race on the hor-monal and immune responses of male and female endurance-trained athletes. The present study has revealed that: [1] the post-race concentrations of the salivary defense factors α-amylase, lactoferrin and SIgA were significantly increased; however, the secre- tion rates of these factors were not markedly different compared to the pre-race values; [2] the T/C ratios were significantly declined after race; and [3] gender differ- ences in pre-race and post-race levels of α-amylase, SIgA, testosterone and T/C ratio were observed.
Strenuous exercise is known to decrease the flow rate of saliva (16). This is probably one explanation for the increased concentrations of total protein, lacto- ferrin, SIgA and α-amylase activities. Walsh et al. reported that salivary total protein concentration can be used to estimate the hydration status of athletes (38). Either in the condition of dehydration or drying oral surface caused by oral breathing during strenuous exercise (18), salivary total protein concentrations and osmolality were markedly elevated. In our studies, the salivary total protein concentrations were signifi- cantly elevated post-race. It is known that dehydra- tion has an negative impact on saliva flow rate during exercise (38). In order to correct for this factor, secretion rates of the salivary defense factors were divided by salivary flow rates and the values were not markedly different compared to the pre-race values. Therefore, the levels of salivary defense factors be-fore the 5,000 m race were regarded to be not sig-nificantly different from those of post-race. Similar findings have been reported after a competitive mara- thon race (25), a single bout of soccer-specific exercise (31) and repeated bouts of short-term, high-intensity cycling exercise (32) as well as high-intensity inter- mittent exercise, all of which did not affect the SIgA concentration (37). Eleven male runners ran for 2 h at 75% VO2 max and the SIgA concentration decreased
dramatically to below the pre-exercise concentrations (5). Thus, the changes of SIgA are dependent on the intensity, duration and mode of exercise. Additionally, other studies found that α-amylase increases signifi- cantly in response to physiological- and psychosocial- stress conditions, such as marathon (16), written ex- amination (4) and the cold press test (34). However, there is no previous evidence of gender differences in salivary α-amylase in response to physical stressors. Here, we first report that female athletes show higher α-amylase levels than males before or after 5,000 m running. Because α-amylase is also a biomarker of sympathetic nervous activities, it might be implied that the female athletes in this study showed higher sympathetic responses to the competitive 5,000 m race.
In addition, a previous study showed that menstrual phase did not affect SIgA and lactoferrin secretion rates (8). Whether variations of ovarian hormones during the menstrual cycle affect α-amylase secretion required further investigations.
Studies have been performed to examine the im- pact of exercise, competition and examination on cor- tisol responses (3). In addition, cortisol responses to psychological stress in the absence of physical exer-cise are generally recognized (34). An earlier study reported that salivary cortisol concentrations of young male athletes were significantly increased after a 90-min training session (7). However, exercises of low inten- sity and short duration exert minimal effects on the cortisol response (17). Consistent with previous report that cortisol concentrations were higher when runners were in a state of dehydration than euhydration (21), our results showed that cortisol concentrations sig-nificantly increased after the 5,000 m race in both genders. Interestingly, we found that salivary cortisol concentrations before the race were much higher than the baseline level (below 60 nmole/L, data not shown) of these athletes. Cortisol is a hormone secreted in re- sponse to many factors such as physical (e.g. heat and cold), physiological (e.g. high-intensity exercise), and psychological (e.g. competition) stress (2, 6). Similar to previous findings (10, 13, 35), we found much higher post-exercise levels of cortisol, which may be due to these factors. Moreover, no gender difference of ab- solute concentrations and secretion rates of cortisol were found in this study.
A previous study found significant decreases of testosterone concentrations in male runners at 90 min after the start of the relay race, but the testosterone levels of female runners were not affected (15). In addition, taekwondo fighting simulation decreased the levels of testosterone in male fighters, but not in females (29). We had a similar finding in the gender difference of testosterone concentration. Our results also demonstrated that males have four times the salivary testosterone of female before the 5,000 m race, and three times the testosterone of females after the race. In sports science, T/C ratio has been shown to be an indicator of anabolic/catabolic balance, which is affected by intensity of the exercise (32). Previous studies showed that the T/C ratio following taekwondo fighting simulation decreased in both genders (29), and the T/C ratio was significantly lower after 10 min of running at 70% VO2 max (21). Similarity of T/C
ratio response to exercise was also observed in our results. Furthermore, the T/C ratio has also been used as a performance index for athletes (28). However, we further observed that an inverse correlation existed between the decreased percentage of T/C ratio and the 5,000 m running time in the competition for all athletes, but not in individual male nor female group.
This observation may be further supported by increas- ing the number of participants in each group in future studies. Whether changes of the T/C ratio are cor-related to better running performance and the pos-sible mechanisms needs further investigation.
In conclusion, the concentrations of salivary de- fense factors measured post-race were significantly increased. However, the secretion rates of these factors were not markedly different compared to the pre-race values. In addition, the T/C ratios significantly de-clined after the 5,000 m running race in both male and female athletes.
Acknowledgments
We warmly thank all the athletes for their pa-tience and participation in this study. This study was supported by NSC101-2628-H-028-002-MY3 granted by the National Science Council, R.O.C. We thank Cheng-Shiun He for his expert technical assistance. The authors are grateful to Dr. Alexander Wanek for editorial assistance.
References
1. Allgrove, J.E., Geneen, L., Latif, S. and Gleeson, M. Influence of a fed or fasted state on the s-IgA response to prolonged cycling in active men and women. Int. J. Sport Nutr. Exerc. Metab. 19: 209-221, 2009.
2. Bozovic, D., Racic, M. and Ivkovic, N. Salivary cortisol levels as a biological marker of stress reaction. Med. Arch. 67: 374-377, 2013.
3. Budde, H., Windisch, C., Kudielka, B.M. and Voelcker-Rehage, C. Saliva cortisol in school children after acute physical exercise.
Neurosci. Lett. 483: 16-19, 2010.
4. Chatterton, R.T., Jr., Vogelsong, K.M., Lu, Y.C., Ellman, A.B. and Hudgens, G.A. Salivary α-amylase as a measure of endogenous adrenergic activity. Clin. Physiol. 16: 433-448, 1996.
5. Costa, R.J., Fortes, M.B., Richardson, K., Bilzon, J.L. and Walsh, N.P. The effects of postexercise feeding on saliva antimicrobial proteins. Int. J. Sport Nutr. Exerc. Metab. 22: 184-191, 2012. 6. de Kloet, E.R., Joels, M. and Holsboer, F. Stress and the brain:
from adaptation to disease. Nat. Rev. Neurosci. 6: 463-475, 2005. 7. Di Luigi, L., Baldari, C., Gallotta, M.C., Perroni, F., Romanelli,
F., Lenzi, A. and Guidetti, L. Salivary steroids at rest and after a training load in young male athletes: relationship with chronolog-ical age and pubertal development. Int. J. Sports Med. 27: 709- 717, 2006.
8. Gillum, T., Kuennen, M., Miller, T. and Riley, L. The effects of exercise, sex, and menstrual phase on salivary antimicrobial pro-teins. Exerc. Immunol. Rev. 20: 23-38, 2014.
9. Gleeson, M., Bishop, N., Oliveira, M., McCauley, T. and Tauler, P. Sex differences in immune variables and respiratory infection in-cidence in an athletic population. Exerc. Immunol. Rev. 17: 122-135, 2011.
10. Handziski, Z., Maleska, V., Petrovska, S., Nikolik, S., Mickoska, E., Dalip, M. and Kostova, E. The changes of ACTH, cortisol, testosterone and testosterone/cortisol ratio in professional soccer players during a competition half-season. Bratisl. Med. J. 107: 259-263, 2006.
11. He, C.S., Bishop, N., Handzlik, M.K., Muhamad, A.S. and Gleeson, M. Sex differences in upper respiratory symptoms prevalence and
oral-respiratory mucosal immunity in endurance athletes. Exerc.
Immunol. Rev. 20: 8-22, 2014.
12. Hough, J.P., Papacosta, E., Wraith, E. and Gleeson, M. Plasma and salivary steroid hormone responses of men to high-intensity cycling and resistance exercise. J. Strength Cond. Res. 25: 23-31, 2011. 13. Izquierdo, M., Ibanez, J., Calbet, J.A., Navarro-Amezqueta, I.,
Gonzalez-Izal, M., Idoate, F., Hakkinen, K., Kraemer, W.J., Palacios- Sarrasqueta, M., Almar, M. and Gorostiaga, E.M. Cytokine and hormone responses to resistance training. Eur. J. Appl. Physiol. 107: 397-409, 2009.
14. Jenssen, H. and Hancock, R.E. Antimicrobial properties of lacto-ferrin. Biochimie 91: 19-29, 2009.
15. Lac, G. and Berthon, P. Changes in cortisol and testosterone levels and T/C ratio during an endurance competition and recovery. J.
Sports Med. Phys. Fitness 40: 139-144, 2000.
16. Ljungberg, G., Ericson, T., Ekblom, B. and Birkhed, D. Saliva and marathon running. Scand. J. Med. Sci. Sports 7: 214-219, 1997. 17. Lovallo, W.R., Farag, N.H., Vincent, A.S., Thomas, T.L. and
Wilson, M.F. Cortisol responses to mental stress, exercise, and meals following caffeine intake in men and women. Pharmacol.
Biochem. Behav. 83: 441-447, 2006.
18. MacKinnon, L.T. and Jenkins, D.G. Decreased salivary immuno-globulins after intense interval exercise before and after training.
Med. Sci. Sports Exerc. 25: 678-683, 1993.
19. Manfre, M.J., Yu, G.H., Varma, A.A., Mallis, G.I., Kearney, K. and Karageorgis, M.A. The effect of limited handrail support on total treadmill time and the prediction of VO2 max. Clin. Cardiol.
17: 445-450, 1994.
20. Marcotte, H. and Lavoie, M.C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 62: 71- 109, 1998.
21. Maresh, C.M., Whittlesey, M.J., Armstrong, L.E., Yamamoto, L.M., Judelson, D.A., Fish, K.E., Casa, D.J., Kavouras, S.A. and Cast-racane, V.D. Effect of hydration state on testosterone and cortisol responses to training-intensity exercise in collegiate runners. Int.
J. Sports Med. 27: 765-770, 2006.
22. Meeusen, R., Duclos, M., Foster, C., Fry, A., Gleeson, M., Nieman, D., Raglin, J., Rietjens, G., Steinacker, J. and Urhausen, A. Euro- pean College of Sport Science and American College of Sports Medicine. Prevention, diagnosis, and treatment of the overtrain-ing syndrome: joint consensus statement of the European College of Sport Science and the American College of Sports Medicine.
Med. Sci. Sports Exerc. 45: 186-205, 2013.
23. Meeusen, R., Piacentini, M.F., Busschaert, B., Buyse, L., De Schutter, G. and Stray-Gundersen, J. Hormonal responses in athletes: the use of a two bout exercise protocol to detect subtle differences in (over)training status. Eur. J. Appl. Physiol. 91: 140- 146, 2004.
24. Nater, U.M. and Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology 34: 486-496, 2009. 25. Nieman, D.C., Henson, D.A., Fagoaga, O.R., Utter, A.C., Vinci,
D.M., Davis, J.M. and Nehlsen-Cannarella, S.L. Change in sali-vary IgA following a competitive marathon race. Int. J. Sports
Med. 23: 69-75, 2002.
26. Papacosta, E. and Nassis, G.P. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise sci-ence. J. Sci. Med. Sport 14: 424-434, 2011.
27. Passelergue, P. and Lac, G. Saliva cortisol, testosterone and T/C ratio variations during a wrestling competition and during the post- competitive recovery period. Int. J. Sports Med. 20: 109-113, 1999.
28. Passelergue, P., Robert, A. and Lac, G. Salivary cortisol and testos- terone variations during an official and a simulated weight-lifting competition. Int. J. Sports Med. 16: 298-303, 1995.
29. Pilz-Burstein, R., Ashkenazi, Y., Yaakobovitz, Y., Cohen, Y., Zigel, L., Nemet, D., Shamash, N. and Eliakim, A. Hormonal response to
Taekwondo fighting simulation in elite adolescent athletes. Eur.
J. Appl. Physiol. 110: 1283-1290, 2010.
30. Rossi, P., Buonocore, D., Altobelli, E., Brandalise, F., Cesaroni, V., Iozzi, D., Savino, E. and Marzatico, F. Improving training con-dition assessment in endurance cyclists: effects of Ganoderma
lucidum and Ophiocordyceps sinensis dietary supplementation. Evid. Based Complement. Alternat. Med. 2014: 979613, 2014.
31. Sari-Sarraf, V., Reilly, T., Doran, D.A. and Atkinson, G. The effects of single and repeated bouts of soccer-specific exercise on sali-vary IgA. Arch. Oral Biol. 52: 526-532, 2007.
32. Thomas, N.E., Leyshon, A., Hughes, M.G., Davies, B., Graham, M. and Baker, J.S. The effect of anaerobic exercise on salivary cortisol, testosterone and immunoglobulin (A) in boys aged 15-16 years. Eur. J. Appl. Physiol. 107: 455-461, 2009.
33. Tsai, M.L., Chou, K.M., Chang, C.K. and Fang, S.H. Changes of mucosal immunity and antioxidation activity in elite male Tai-wanese taekwondo athletes associated with intensive training and rapid weight loss. Brit. J. Sports Med. 45: 729-734, 2011. 34. van Stegeren, A.H., Wolf, O.T. and Kindt, M. Salivary alpha amylase
and cortisol responses to different stress tasks: impact of sex. Int. J.
Psychophysiol. 69: 33-40, 2008.
35. Vislocky, L.M., Gaine, P.C., Pikosky, M.A., Martin, W.F. and Rodriguez, N.R. Gender impacts the post-exercise substrate and endocrine response in trained runners. J. Int. Soc. Sports Nutr. 5: 7, 2008.
36. Vuorimaa, T., Ahotupa, M., Hakkinen, K. and Vasankari, T. Dif-ferent hormonal response to continuous and intermittent exercise in middle-distance and marathon runners. Scand. J. Med. Sci.
Sports 18: 565-572, 2008.
37. Walsh, N.P., Blannin, A.K., Clark, A.M., Cook, L., Robson, P.J. and Gleeson, M. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J. Sports Sci. 17: 129-134, 1999.
38. Walsh, N.P., Laing, S.J., Oliver, S.J., Montague, J.C., Walters, R. and Bilzon, J.L. Saliva parameters as potential indices of hydra-tion status during acute dehydrahydra-tion. Med. Sci. Sports Exerc. 36: 1535-1542, 2004.
39. West, N.P., Pyne, D.B., Renshaw, G. and Cripps, A.W. Antimi-crobial peptides and proteins, exercise and innate mucosal immu-nity. FEMS Immunol. Med. Microbiol. 48: 293-304, 2006.