INTRODUCTION
In Taiwan's aquaculture, Japanese eel Anguilla japonica is a commercially valuable species. To date, the migration and reproduction of eels have still remained puzzles requiring further investigation. Female Japanese eels possess sexually immature ovaries which the most developed oocytes were in the pre-vitellogenic stage (the appearance of the oil drop) and GSI keep on 0.46% to 3.98% (Ijiri et al., 1998; Yoshikawa, 1995) for a long time. In fact, due to the deficiency of endocrine gonadotropic function, female Japanese eels cannot spontaneously undergo further gonadal development (Dufour et al., 1983; Dufour et al., 1988; Fontaine et al., 1976;
Olivereau and Olivereau, 1979). By multiple injections of salmon or carp pituitary extract, artificially maturation of Japanese eel has proven successful for the obtaining of eggs and larvae (Ijiri et al., 1998; Yamamoto and Yamauchi, 1974; Yamauchi et al., 1976). Furthermore, leptocephali larvae and glass eel were reared from a few weeks to more than 250 days (Satoh et al., 1992; Tanaka et al., 2001; Tanaka et al., 2003; Yamauchi et al., 1976). However, both the quality of eggs or low survival rate of larvae, these studies still remained the uncertainty from the reproductive physiology by artificial maturation. Particularly, exogenous hormone treatment should consider an actual endocrine mechanism of ovarian development throughout the process of Institute of Fisheries Science National Taiwan University
* Corresponding author. E-mail: swlou@ccms.ntu.edu.tw
Influence of Exogenous Gonadotropin and Sexual Steroids on
Ovary Development in Japanese eel
Anguilla japonica
Yung-Song Wang and Show-Wan Lou* (Received, May 1, 2007; Accepted, June 15, 2007)
ABSTRACT
This study examines Japanese eel ovary responded to the administration of exogenous sexual hormone throughout the reproductive process. In short-term experiment, ovarian follicle exhibited sensitivity to salmon pituitary homogenate (SPH) and sexual steroids (E2 and MT) in the early vitellogenic stages. The apoptotic signals were exclusively localized in the outer theca layer which was detected from in situ TUNEL, furthermore, the appearance of the apoptotic signal in the early vitellogenic stage seems to correlate with a reduction of oocyte numbers in long-term experiment. In long-term experiment, the mean GSI and total oocyte numbers indicated that multiple injections of E2 or MT were not of much benefit to promote the gonadal development of those fish. Despite SPH treatment was an effective and indispensable method to hasten artificially sexual maturation, and the size-frequency distribution indicated that the most developed oocytes of those were growing from the vitellogenic stage to the final maturational phase. However, the treatment of SPH plus E2 failed to promote the ovarian development and exhibited a remarkable reduction of total oocyte numbers. Of interest is the synergistic effect of SPH plus MT revealed that MT, a potent, aromatizable androgen, provides a potential usefulness to participate the process of vitellogenesis when it combines with SPH treatments, furthermore, the synergistic effect actually enhances the survival and synchronous development of ovarian follicle throughout the reproductive process.
vitellogenesis.
The ovarian follicle is the basic functional unit in the vertebrate ovary, and is comprised of an oocyte and its surrounding granulosa, theca and epithelial cell population. In teleosts, the pituitary-derived gonadotropic hormones stimulate the vitellogenesis of ovary by activating the synthesis and secretion of steroid hormones (primarily testosterone and estrogen) in the oocyte-follicle layers. Testosterone, which is derived from cholesterol in theca cells, is converted to estrogen by aromatase in the granulosa cells (Campbell et al., 1976; Wingfield and Grimm, 1977; Kagawa et al., 1982). Thereafter, vitellogenin (Vtg), the precursor molecule for yolk, is synthesized by hepatocytes under estrogenic control (Wallace and Selman, 1981). In the induction of European eel (Olivereau and Olivereau, 1979; Dufour et al., 1983; Burzawa-Gerard and Dumas-Vidal, 1991), estradiol stimulated Vtg synthesis and secretion into the circulation, but the most developed oocytes were in the tertiary yolk globule stage because of deficient pituitary gonadotropin. In addition to gonadotropin, it is unclear that the actual action of sexual steroids involved in ovarian follicle growth and development of Japanese eel. The objective of the present study was to reveal the reproductive physiology of Anguilla. japonica under artificial maturation and to evaluate the interaction of exogenous gonadotropin (salmon pituitary homogenate) and sexual steroids (estradiol-17β and 17α-methyltestosterone) treatment on mechanisms controlling gonadal development. First, the terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) technique detects the expression and location of apoptosis of ovarian follicular cells in the early vitellogenic stage (short-term experiment). Second, we try to compare the stimulating functions of SPH and sexual steroid for gonadal development during the course of the experiment (long-term experiment).
MATERIALS AND METHODS Experimental Animals
Pond-cultivated sexually immature female Japanese eel Anguilla japonica (bodyweight, 450–550g) were purchased from a local aquaculture farm in Lukang, Taiwan. All these eels were reared in circulated freshwater tanks of 3400 L, and were gradually acclimated to seawater in the same tank at 20oC. One hundred fishes were separated into six groups for short- and long-term experiments. Both experiments were administered to each group: (1) control, (2) salmon pituitary homogenate (SPH), (3) estradiol-17β (E2) alone, (4) salmon pituitary homogenate plus estradiol-17β (SPH+E2), (5) 17α-methyltestosterone (MT) alone and (6) salmon pituitary homogenate plus 17α-methyltestosterone (SPH+MT). The vehicle was given to control fish. The injection scheme was shown as Table 1. All of fishes received weekly intraperitoneal injections over 12 weeks and a part of fishes of each group tried to detect the signal and location in ovarian follicular cells after fourth weekly injection in short-term group.
Table 1. Description of experimental approach
Group Number of fish Treatment and Dose (B.W.)
SPH Steroids Control 15 - -SPH 25 20 mg/kg -E2 15 - 3 mg/kg SPH + E2 15 20 mg/kg 3 mg/kg MT 15 - 3 mg/kg SPH + MT 15 20 mg/kg 3 mg/kg
In situ end labeling of apoptosis (short-term experiment)
Following 4 weekly induction, in situ TUNEL analyses were performed to localize apoptotic cells in ovarian follicles of the randomly selected female eel, and were scarified and ovary were removed immediately after perfusion and placed in fresh 4% paraformaldehyde in PBS for 1 h at room temperature. The fresh 4% paraformaldehyde-fixed ovarian samples were dehydrated in a graded ethanol series, embedded in paraffin, and sectioned (6 µm) onto charged microscope slides (Superfrost Plus; Fisher Scientific). Sections were deparaffinized for 60 min at 60°C, cleared in a xylene substitute, rehydrated in a graded ethanol series, and washed in 0.1 M PBS. The sections were permeabilized with Proteinase K (50 µg/ml) for 20 min at 37oC, washed twice with 0.1 M PBS, and then washed in 0.1 M glycine in 0.1 M PBS, pH 7.4, for 5 min at RT, washed in 0.1 M PBS for 1 min, and then incubated in 0.25% acetic anhydride (Sigma; St Louis, MO) in 0.1 M triethanolamine, pH 8.0, and 0.9% NaCl for 10 min at RT. After a wash in sterile dH2O for 10 min, sections were washed in 0.1 M PBS, pH 7.4, for 5 min, treated with 0.3% H2O2 for 30 min, rinsed in 0.1 M PBS for 5 min, and then incubated in TUNEL reaction mixture using the In Situ Cell Death Detection Kit, POD (Roche) in a humidified chamber for 1 hr at 37oC. The tissue was then rinsed in 0.1 M PBS three times for 5 min and incubated in Converter-POD (Peroxidase; Roche) for 30 min at 37oC, rinsed in 0.1 M PBS three times for 5 min, and color-developed with SIGMA FAST, a 3,3’-diaminobenzidine tetrahydrochloride (DAB) POD substrate solution (Sigma), in a dark chamber. After performing slight counterstaining with hematoxylin, the samples were dehydrated and mounted. Controls for the TUNEL procedure were treated in the same manner as the test samples except that the TdT enzyme was omitted from the reaction mixtures in both kits and was replaced with dH2O. No labeling was found in the controls.
Developmental Stage of ovarian follicles (long-term experiment)
All treatments of fish were sacrificed and sampled on the seventh day after the 12 weekly injection and the gonadosomatic index (GSI, [total ovary weight/ total body weight] ×102) was calculated. After all the excess tissues were removed and drained, the ovaries were weighed in 0.5 g. Pieces of ovaries from the proximal, middle and distal parts of each lobe of one fish were sampled and histologically examined. Moreover, the individual excised ovary of each treatment was immersed in Gilson’ s fluid (Simpson, 1951) for one month, in the proportion one part ovary to two parts fixative (v/v), to separate the individual oocytes from the tissue matrix using fine forceps. Approximately 10% of the ovarian tissue was used to determine the numbers and size of ovarian follicles present. Total number (N) of ovarian follicles was estimated from the following equation: N= (number of ovarian follicles greater than 0.15 mm for 0.5 g ovary weight) × (weight of ovary preserved in Gilson’s fluid) / (0.5 g ovary). Ovarian follicles diameter measured using a Profile projector model and two hundred randomly selected follicles more were also measured than 0.15 mm from each part to determine the agreement of ovarian follicles diameter frequency distribution. Since no evidence of development differentiation has been found, the distal part of left lobe was chosen as the sampling portion for oogenesis observation. The size-frequency histograms of ovarian follicles present in the left ovary were determined in the given individual of each group with the most developed ovary.
Statistical Analysis
All values were presented as mean ± standard error of the mean (SEM). Statistical differences between the control group and hormone treated groups in gonadosomatic index, oocyte size and oocyte number were determined by one-way analysis of variance (ANOVA, p < 0.05) followed by Duncan’s multiple range tests.
RESULTS Effects of short-term experiment
Changes in the GSI and the profile of ovary development are shown in Table 2. The mean GSI of controls was 0.92 ± 0.18%. In controls, and the most developed oocytes were stagnated from growing in the previtellogenic stage, and it was no signal of apoptosis in follicular layer (Fig. 1A) detected from in situ TUNEL method. In both E2 and MT group, the mean GSI had no statistical difference in comparison with controls after 4 weekly injections (E2: 1.31 ± 0.25%; MT: 0.82 ± 0.30%). The previtellogenic follicles revealed that those treatments also did not induce further ovarian development; on the other hand, in situ TUNEL showed that the apoptotic signals were exclusively localized in the outer theca layer of ovarian follicle (Fig. 1B and 1C).
Under the exogenous pituitary gonadotropin, the mean GSI (4.68 ± 0.70%, p < 0.05) in SPH group showed a significant increase than among groups. The most developed oocytes were progressing from the previtellogenic stage to the primary yolk globule stage (early vitellogenic stage), but the apoptotic signals were found in the outer theca layer (Fig. 1D). All fish in SPH plus E2 group showed an increase in the mean GSI (1.64 ± 0.21%; no statistical difference compared with control group), and the most developed oocytes were still in previtellogenic stage. The apoptotic signals were found in the outer theca layer of ovarian follicle (Fig.
1E). In SPH plus MT group, the mean GSI was 3.55 ± 0.63% (p < 0.05), and it also had a significant increase compared among groups. The most developed oocytes, as SPH plus MT group, were progressing from the previtellogenic stage to the secondary yolk globule stage, however, the apoptotic signal was rarely detected in the ovarian follicular layers (Fig. 1F).
Effects of long-term experiment
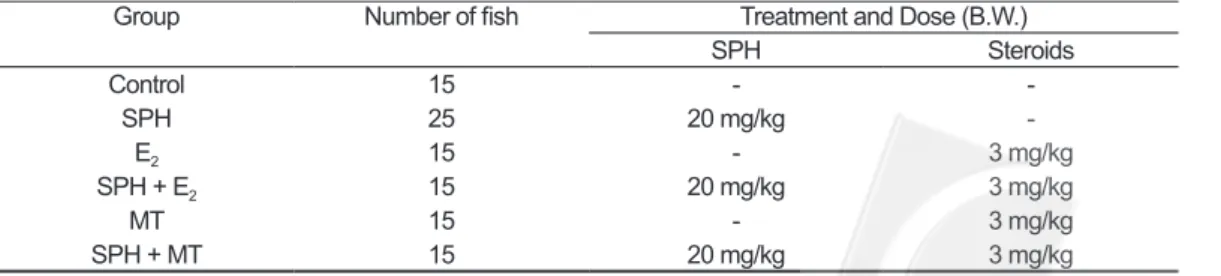
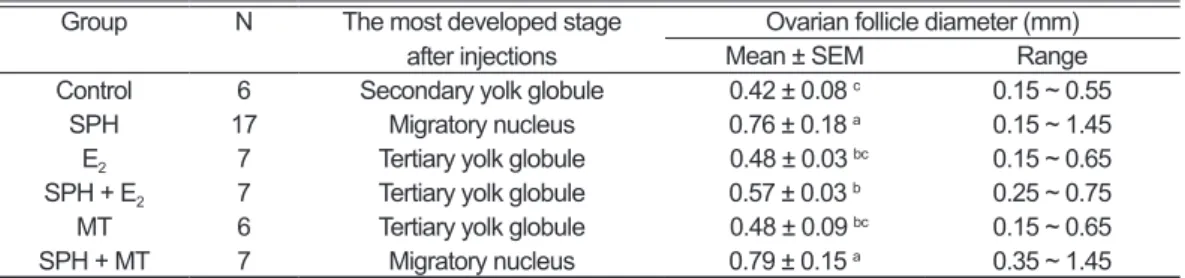
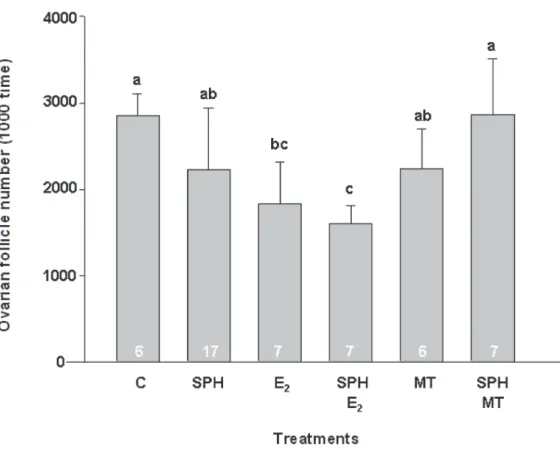
After the long-term induction (12 weekly injections), approximately 33% of all fish were excluded from this experiment. All of them either had died during the course of the experiment, or were immature (it was hard to find and identify gonads of male or female) and male eel. From histological observation, the most developed oocytes of controls were under the secondary yolk globule stage (Table 3). The ovaries contained a unimodal population with a mean diameter of 0.42 ± 0.08 mm (ranged from 0.15 to 0.55 mm, Fig. 2), and the mean number of ovarian follicles was 2,856,960 ± 250,424 (Fig. 3). On the other hand, the treatments of the E2, MT, and SPH plus E2 group showed that the most developed oocytes were progressing to complete the vitellogenesis (Table 3), extending from 0.15 (primary yolk globule) through 0.75 mm (tertiary yolk globule), after 12 weekly injections. Similarly, those ovaries also contained a unimodal population with a mean diameter of 0.48 ± 0.03, 0.48 ± 0.09 and 0.57 ± 0.03 mm (Fig. 2), respectively. Although the mean diameter Table 2. Changes in gonadosomatic index (GSI) and developmental stage after short-term
induction
Group N GSI (g x 100/g B.W.) The most developed stage after injections
Control 3 0.92 ± 0.18 Primary yolk globule
SPH 3 4.68 ± 0.70* Secondary yolk globule
E2 3 1.31 ± 0.55 Primary yolk globule
SPH + E2 3 1.64 ± 0.21 Primary yolk globule
MT 3 0.82 ± 0.30 Primary yolk globule
SPH + MT 3 3.55 ± 0.63* Secondary yolk globule
Values are means ± SEM. N = number of individuals. SPH: salmon pituitary homogenate; E2: estradiol-17β;
of ovarian follicles had no significant differences between those treatments and controls, apparently, the mean number of E2 (1,829,401 ± 478,401) and SPH plus E2 group (1,597,175 ± 216,264) were significantly fewer (p < 0.05, Fig. 3) than in
control eels.
Furthermore, ovarian follicles of the SPH and SPH plus MT group indicated that the most developed oocytes were progressing from the vitellogenic stage to the final maturational phase. Both treatments had no Fig. 1. Effects of SPH and sexual steroids treatment on programmed cell death in Japanese eel follicle after
short-term induction. (A): C, control; (B): E2, estradiol-17β; (C): MT, 17α-methyltestosterone; (D): SPH,
salmon pituitary homogenate; (E): SPH + E2; (F): SPH + MT. Apoptotic signals (theca cell; black arrows)
were detected by TUNEL staining and were counterstained with hematoxylin. White arrowheads indicate healthy (non-apoptotic) blood cells (C) and black arrowheads indicate theca cells (F). Original magnification, X 300 (A) and X 1000 (B, C, D, E, and F). N, nucleus; OD, oil drop; YG, yolk globules; Z, zona radiata.
significant differences compared with controls in relation to the mean number of ovarian follicle. Despite the mean diameter ranges of both groups were 0.76 ± 0.18 mm and 0.79 ±
0.15 mm respectively, but there were different in size-frequency distribution. The ovaries of SPH plus MT group contained a unimodal population (ranged from 0.35-1.45 mm) with Fig. 2. Change in frequency distribution of oocytes in Japanese eels injected with SPH and sexual steroids
after long-term induction. C: control; SPH: salmon pituitary homogenate; E2: estradiol-17β; MT:
17α-methyltestosterone.
Table 3. Summary of developmental stage and ovarian follicle diameter after long- term induction
Group N The most developed stage
after injections Mean ± SEMOvarian follicle diameter (mm)Range Control 6 Secondary yolk globule 0.42 ± 0.08 c 0.15 ~ 0.55
SPH 17 Migratory nucleus 0.76 ± 0.18 a 0.15 ~ 1.45
E2 7 Tertiary yolk globule 0.48 ± 0.03 bc 0.15 ~ 0.65
SPH + E2 7 Tertiary yolk globule 0.57 ± 0.03 b 0.25 ~ 0.75
MT 6 Tertiary yolk globule 0.48 ± 0.09 bc 0.15 ~ 0.65
SPH + MT 7 Migratory nucleus 0.79 ± 0.15 a 0.35 ~ 1.45
Values are means ± SEM. N = number of individuals. SPH: salmon pituitary homogenate; E2: estradiol-17β;
approximately 25% of the vitellogenic oocyte and 75% of the maturing oocytes/ovulated eggs after 12 weekly injections. On the other hand, the ovaries of SPH group seem to have a bimodal (ranged from 0.15-1.45 mm) population with approximately 46% of the vitellogenic oocyte and 54% of the maturing oocytes/ovulated eggs.
DISCUSSION
This study examined the effects of exogenous gonadotropic hormone (SPH) and sexual steroids (E2 and MT) on the dynamics of the ovaries during the vitellogenic process. Exogenous sexual hormonal treatment of short-term induction
showed that the apoptosis of ovarian follicle were appeared in the early vitellogenic stage, and the signals seem to clearly localize in the outer theca layer of Japanese eels in this study. Furthermore, all of the results demonstrated that the appearance of the apoptotic signal surmised to connect with the reduction of ovarian follicle number throughout artificial sexual maturation. In vivo and in vitro studies from two species of teleost fish (Wood and Van Der Kraak, 2001, 2002) indicate that the theca/epithelial cells, not the granulosa cells, undergo apoptosis in both vitellogenic rainbow trout Oncorhynchus mykiss and pre-spawning goldfish Carassius auratus. In contrast to teleost, mammalian studies evidenced that ovarian follicle atresia Fig. 3. Effects of SPH and sexual steroids treatment on total number of ovarian follicle after long-term induction.
(A): C, control; (B): E2, estradiol-17β; (C): MT, 17α-methyltestosterone; (D): SPH, salmon pituitary
homogenate; (E): SPH+ E2; (F): SPH+MT. Data are expressed as the mean ± S.E.M. Statistical significance tested by ANOVA and Duncan’s multiple range test: values with the same letter codes are not significantly difference at P < 0.05. The number of animal in each treatment is indicated at the base of the columns.
was a result of apoptosis of granulosa cell population during all developmental stages of reproductive life for normal cyclic recruitment (Billing et al., 1993; Hsueh et al., 1994). Although the findings of teleost and this study may seem to indicate that the theca cell type are susceptible to apoptosis during early vitellogenic stage of teleost, nevertheless, structural differences of follicular cell type have also been considered between mammalian and teleost during early ovarian development. For example, the study in female rhesus monkey Macaca mulatto (Vendola et al., 1998) indicated that the ovaries were only composed of the cuboidal shape characteristic of granulosa cell type during primary follicle stage, and definitive theca cell type only emerges when antral cavities begin to form. On the other hand, the oocyte of Japanese eel possessed poorly developed theca and granulosa cells at the pre- or early vitellogenic stage before exogenous hormonal treatment (Kamei et al., 2006), until the flat-shaped theca cells were well developed at the early to mid-vitellogenic stage following exogenous GTH administration (4-7 weekly injections). Thus, additional studies should clearly identify the functional and structural relationship of follicular cells (theca and granulosa cell) between oviparous and viviparous species.
In addition to stimulating ovarian steroid production (Nagahama et al., 1995) and Vtg uptake (Tyler et al., 1991), gonadotropic hormone, GtH-I or FSH, acts as the major survival factor to inhibit apoptosis for mature vitellogenic and pre-ovulatory follicles (Janz and Van Der Kraak, 1997; Wood and Van Der Kraak, 2002) in rainbow trout ovary. In the present study, it should be noted that crude salmon pituitary homogenate (SPH) was utilized for induced oocyte maturation of sexual immature female eel, and a mixture of GTHs (primarily GtH-II) in SPH explained the responsiveness of ovarian follicles during the course of vitellogenesis. In salmon species, both GTH-I and GTH-II enhance steroidogenesis during the mid-vitellogenic stage (Suzuki et al., 1988), whereas GTH-II appeared to be more effective in stimulating maturation inducing steroid (MIS) than GTH-I
in the post-vitellogenic follicles. In Japanese eel, the duality of gonadotropin (Yoshiura et al., 1999) demonstrates that both GTH-I and GTH-II are differentially expressed in immature and ovulated females, respectively, and may play separate roles in the reproductive process. Apparently, the stimulation of SPH treatment, especially GTH-II, was effective in promoting complete ovarian development from the vitellogenic stage to the final maturational phase in this study, however, the absence/low contents of GTH-I can not prevent the spontaneous onset of degeneration at least in a fraction of early vitellogenic follicles (dormant follicles) cohort. It is unclear at present whether the apoptotic process would naturally occur when the dormant follicle cohort, under GTHs stimulus, has begun vitellogenesis or if it is abnormal and due to the hormonal treatment in the early vitellogenic stage.
In all oviparous species, Vtg is synthesized in the liver under estrogenic control and is crucial to subsequent embryonic development. Low concentrations of E2 were reported in Japanese eel (Kamei
et al., 2006), and it speculated that those of E2 profile were caused by low aromatase activity, at least in the early vitellogenic stage (Chiba et al., 1994; Kamei et al., 2006). The studies on rainbow trout (Janz and Van Der Kraak, 1997; Wood and Van Der Kraak, 2002) show that low dose of E2, which acts as a survival factor, is able to suppress follicular apoptosis in both vitellogenic and pre-ovulatory follicles. In teleosts, such as goldfish Carassius auratus (L) (Kobayashi et al., 1988), rainbow trout Oncorhynchus mykiss (Bromage and Cumaranatunga, 1988), tilapia Tilapia zillii (Coward and Bromage, 1998), serum of E2 increase in tandem to the progress of vitellogenesis, but remains at low levels until the next spawning cycle. Whereas, there was a dramatic increase in serum estradiol-17β levels following the end of vitellogenesis in salmon pituitary extract treatment of European eel Anguilla anguilla (Chiba et al., 1994). Many studies of the induction of artificial maturation in the European eel (Olivereau and Olivereau,
1979; Dufour et al., 1983; Burzawa-Gerard and Dumas-Vidal, 1991) demonstrated that the E2 injection stimulated Vtg synthesis and secretion in the circulation, but it did not promote vitellogenesis in the ovary. In order to induce ovarian development of Japanese female eel, our previous study (Wang and Lou, 2006) demonstrated that estradiol-17β (3 mg/kg) stimulates Vtg mRNA synthesis in the liver. Despite high doses of E2 (2.5 ng/g) are required to induce measurable plasma Vtg levels of European eel (Burzawa-Gerard and Dumas-Vidal, 1991), however, it is of concern as a super pharmacological dose which demonstrate that the similar dose led to the occurrence of follicular apoptosis and the failure of ovarian development (i.e., a significant decrease in total oocyte numbers) during the process of vitellogenesis in this study.
In contrast to the role of E2 in teleost, numerous studies suggested that androgen serves as an estrogen precursor converted by aromatse (Campbell and Idler, 1976; Wingfield and Grimm, 1977; Kagawa et al., 1982) in granulosa cell and induces Vtg synthesis (Hori et al., 1979; Peyon et al., 1997; Mori et al., 1998). The studies of Japanese eel found that multiple injections and implantations of MT were effective in stimulating an increase in ovarian development (Lin et al., 1990; Lin et al., 1991). In primate and human studies (Vendola et al., 1998; Otala et al., 2004), the role of androgen, which act via its receptor, is not atretogenic and actually stimulates the growth and survival of primary follicles. Apart from the effects on growth, androgens have been shown to enhance the FSH-mediated differentiation of granulosa cells, as indicated by an increase in progesterone and oestradiol production (Nimrod, 1977; Hillier and De Zwart, 1981). In the present study, the treatments of MT, as E2-treated fish, were ineffective in suppressing follicular apoptosis and promoting further growth, however, the mean numbers of oocyte were not significantly decreased compared to controls at least after 12 weekly injections. It is unclear whether the spontaneous onset of degeneration in the fraction of
early vitellogenic follicles represents the normal physiological mechanism, or if it was a pathological result on follicular growth. In the previous study (Wang and Lou, 2006) demonstrated that MT did not induce hepatic Vtg expression or affect gonadal development, but Vtg expression and gonadal development were significantly induced by co-treatment with SPH and MT. If the action of methyltestosterone represented the effect of estrogen, the MT and SPH plus MT group should have the identical effects in the ovarian follicle as well as the effect of E2 and SPH plus E2 group. In fact, the adverse effects, in this study, of E2 and SPH plus E2 group suggested that the MT, an aromatizable androgen, was not only converted to estrogens by aromatase, but also has a potential usefulness to participate the process of vitellogenesis and oogenesis. In the Fig.1F and Fig.3F, the action of MT may explain the enhanced responsiveness of ovarian follicles to SPH stimulation as well as the prevention of apoptosis-mediated atresia while stimulating the integration of most oocytes to further develop. Furthermore, our findings indicate that the synergistic effect has no influence on total oocyte numbers and no occurrence on the spontaneous onset of degeneration. In addition, interestingly, it also causes the phenomenon of synchronous development for ovarian follicle in comparison with SPH treatment.
It is well known that three types of spawning patterns for teleost: (i) synchronous type: all oocytes of the ovary developed at the same stage and oocyte composition showed complete monomodal distribution; (ii) group synchronous type: a mature ovary contains two different stages of oocytes; the more mature group is ovulating and the other group is oogonia; (iii) asynchronous type: various developmental stages of oocytes coexist in a mature ovary, with a bimodal distribution of oocytes. To date, there is little understanding or information on the reproductive physiology of Japanese eel. Considering the size-distributions of oocyte of Japanese eel, it was difficult to identify the spawning model of Japanese
eel. In SPH treatments, the vitellogenic oocytes were found together with maturating oocytes in the ovaries of Japanese eel, and it seems to suggest that this species to be a multiple spawner with asynchronous type. In comparison with SPH-treated fish, a great majority of oocytes were in final maturation stage prior to ovulation in ovaries of SPH plus MT-treated fish, however, it indicated that eels perhaps possess synchronous ovaries and spawn once in a lifespan. Apparently, methyltestosterone, a synthetic androgen, causes the phenomenon of synchronous development when it combines with SPH treatments. It is unclear whether it reflects the biological events that actually occur in nature or if it is abnormal due to the hormonal treatment. This study, however, indicates the stimulative responses to sexual steroid addition can markedly change the reproductive mode of Japanese eel, even when the ovulated eggs, in many researches, are obtained under SPH treatment. Consequently, our research provides an argument that steroidal therapy, in addition to exogenous gonadotropin, used to artificial induction of maturation in future. Nevertheless, more information is required to further understand the reproductive physiology of Japanese eel in nature.
ACKNOWLEDGEMENTS
The authors would like to thank the Council of Agriculture of Republic of China, Taiwan, for financially supporting this research under Contract No. (94AS-14.2.1-FA-F1-6).
REFERENCES
Billing, H., Furuta, I. & Hsueh, A. J. W. (1993). Estrogens inhibit and androgens enhance ovarian granulosa cell apoptosis.
Endocrinology 133: 2204-2212.
Bromage, N. R. & Cumaranatunga, P. R. T. (1988). Egg production in the rainbow trout. In In recent advances in aquaculture (Muir, J. F. & Roberts, R. J., eds.), pp. 65-138. London: Croom Helm.
Burzawa-Gerard, E. & Dumas-Vidal, A. (1991).
Effects of 17 beta-estradiol and carp gonadotropin on vitellogenesis in normal and hypophysectomized European silver female eel (Anguilla anguilla L.) employing a homologous radioimmunoassay for vitellogenin. General and Comparative
Endocrinology 84: 264-276.
Campbell, C. M. & Idler, D. R. (1976). Hormonal control of vitellogenesis in hypophysectomized winter flounder (Pseudopleuronectes
americanus walbaum). General and Comparative Endocrinology 28: 143-150.
Campbell, C. M., Walsh, J. M. & Idler, D. R. (1976). Steroids in the plasma of the winter flounder (Pseudopleuronectes americanus Walbaum). A seasonal study and investigation of steroids involvement in oocyte maturation. General
and Comparative Endocrinology 29: 14-20.
Chiba, H., Iwatsuki, K., Hayami, K., Hara, A. & Yamauchi, K. (1994). Changes in serum steroid hormones and vitellogenin levels in cultured female European eels (Anguilla
anguilla) during artificilly induced ovarian
development. Journal of World Aquaculture
Society 25: 553-560.
Coward, K. & Bromage, N. R. (1998). Histological classification of oocyte growth and the dynamics of ovarian recrudescence in Tilapia
zillii. Journal of Fish Biology 53: 285-302.
doi:210.1111/j.1095-8649.1998.tb00981.x. Dufour, S., Delerue-Le Belle, N. & Fontaine, Y.
A. (1983). Effects of steroid hormones on pituitary immunoreactive gonadotropin in European freshwater eel, Anguilla anguilla L.
General and Comparative Endocrinology 52:
190-197.
Dufour, S., Lopez, E., Le Menn, F., Delerue-Le Belle, N., Baloche, S. & Fontaine, Y. A. (1988). Stimulation of gonadotropin release and of ovarian development, by the administration of a gonadoliberin agonist and of dopamine antagonists, in female silver eel pretreated with estradiol. General and Comparative
Endocrinology 70: 20-30.
Fontaine, Y. A., Lopez, E., Delerue-Le Belle, N., Fontaine-Bertand, E., Lallier, F. & Salmon, C. (1976). Stimulation gonadotrope de l’ ovaire chez l’angulle (Anguilla anguilla L.) hypophysectomisee. Journal of Physiology
Paris 72: 871-892.
that granulosa cell aromatase induction/ activation by follicle stimulating hormone is an androgen receptor-regulated process in vitro.
Endocrinology 109: 1303-1305.
Hori, S. H., Kodama, T. & Tanahashi, K. (1979). Induction of vitellogenin synthesis in goldfish by massive doses of androgens. General and
Comparative Endocrinology 37: 306-320.
Hsueh, A. J. W., Billig, H. & Tsafriri, A. (1994). Ovarian follicle atresia: A hormonally controlled apoptotic process. Endocrine
Reviews 15: 707-724.
Ijiri, S., Kayaba, T., Takeda, N., Tachiki, H., Adachi, S. & Yamauchi, K. (1998). Pretreatment reproductive stage and oocyte development induction by salmon pituitary homogenate in the Japanese eel Anguilla japonica. Fisheries
Science 64: 531-537.
Janz, D. M. & Van Der Kraak, G. (1997). Suppression of apoptosis by gonadotropin, 17beta-estradiol, and epidermal growth factor in rainbow trout preovulatory ovarian follicles.
General and Comparative Endocrinology 105: 186-193.
Kagawa, H., Young, G., Adachi, A. & Nagahama, Y. (1982). Estradiol-17β production in amago salmon (Oncorhynchus rhodurus) ovarian follicles: roles of thecal and granulosa cells.
General and Comparative Endocrinology 47:
440-448.
Kamei, H., Kaneko, T. & Aida, K. (2006). Steroidogenic activities of follicle-stimulating hormone in the ovary of Japanese eel,
Anguilla japonica. General and Comparative Endocrinology 146: 83-90.
Kobayashi, M., Katsumi, A. & Hanyu, I. (1988). Hormone changes during the ovary cycle in goldfish. General and Comparative
Endocrinology 69: 301-307.
Lin, H. R., Zhang, M. L., Van Der Kraak, G. & Peter, R. E. (1991). Stimulation of pituitary gonadotropin and ovarian development by chronic administration of testosterone in female Japanese silver eel, Anguilla japonica.
Aquaculture 96: 87-95.
Lin, H. R., Zhang, M. L., Zhang, S. M., Van Der Kraak, G. & Peter, R. E. (1990). Effects of sex steroids, [D-Ala, Pro-N-ethylamide]-LHRH(LHRH-A) and domperidone(DOM) on gonadotropin secretion in female silver ell,
Anguilla japonica Temminck & Schlegel. In In:
R. Hirano and I. Hanyu, The Second Asian Fisheries Forum. Asian Fisheries Society, pp.
591-594. Manila, Philippines.
Mori, T., Matsumoto, H. & Yokota, H. (1998). Androgen-induced vitellogenin gene expression in primary cultures of rainbow trout hepatocytes. Journal of Steroid Biochemistry
and Molecular Biology 67: 133-141.
Nagahama, Y., Yoshikuni, M., Yamashita, M., Tokumoto, T. & Katsu, Y. (1995). Regulation of oocyte growth and maturation in fish.
Current Topics in Developmental Biology 30:
103-145.
Nimrod, A. (1977). Studies on the synergistic effect of androgen on the stimulation of progestin secretion by FSH in cultured rat granulosa cells: progesterone metabolism of action. Molecular and Cellular Endocrinology
8: 189-199.
Olivereau, M. & Olivereau, J. (1979). Effect of estradiol-17 beta on the cytology of the liver, gonads and pituitary, and on plasma electrolytes in the female freshwater eel. Cell
and Tissue Research 199: 431-454.
Otala, M., Makinen, S., Tuuri, T., Sjoberg, J., Pentikainen, V., Matikainen, T. & Dunkel, L. (2004). Effects of testosterone, dihydro-testosterone, and 17beta-estradiol on human ovarian tissue survival in culture. Fertility and
Sterility 82 Suppl 3: 1077-1085.
Peyon, P., Baloche, S. & Burzawa-Gerard, E. (1997). Investigation into the possible role of androgens in the induction of hepatic vitellogenesis in the the European eel: in
vivo and in vitro studies. Fish Physiology and Biochemistry 16: 107-118.
Satoh, H., Yamamori, K. & Hibiya, T. (1992). Induced spwaning of the Japanese eel.
Nippon Suisan Gakkaishi 58: 825-832.
Simpson, A. C. (1951). The spawning of the plaice, Pleuronectes platessa, in the North Sea. Fishery Investigations, London, Series 2
22: 1-111.
Suzuki, K., Nagahama, Y. & Kawauchi, H. (1988). Steroidogenic activities of two distinct salmon gonadotropins. General and Comparative
Endocrinology 71: 452-458.
Tanaka, H., Kagawa, H. & Ohta, H. (2001). Production of leptocephali of Japanese eel (Anguilla japonica) in captivity. Aquaculture
Tanaka, H., Kagawa, H., Ohta, H., Unuma, T. & Nomura, K. (2003). The first production of glass eel in captivity: fish reproductive physiology facilitates great progress in aquaculture. Fish Physiology Biochemistry
28: 493-497.
Tyler, C. R., Sumpter, J. P., Kawauchi, H. & Swanson, P. (1991). Involvement of gonadotropin in the uptake of vitellogenin into vitellogenic oocytes of the rainbow trout, Oncorhynchus mykiss. General and
Comparative Endocrinology 84: 291-299.
Vendola, K. A., Zhou, J., Adesanya, O. O., Weil, S. J. & Bondy, C. A. (1998). Androgens stimulate early stages of follicular growth in the primate ovary. Journal of Clinical
Investigation 101: 2622-2629.
Wallace, R. A. & Selman, K. (1981). Cellular and dynamic aspects of oocyte growth in teleosts.
American Society Zoology 21: 325-343.
Wang, Y. S. & Lou, S. W. (2006). Structural and expression analysis of hepatic vitellogenin gene during ovarian maturation in Anguilla
japonica. Journal of Steroid Biochemistry and Molecular Biology 100: 193-201.
Wingfield, J. C. & Grimm, A. S. (1977). Seasonal changes in plasma cortisol, testosterone and oestradiol-17beta-in the plaice, Pleuronectes
platessa L. General and Comparative
Endocrinology 31: 1-11.
Wood, A. W. & Van Der Kraak, G. J. (2002). Inhibition of apoptosis in vitellogenic ovarian follicles of rainbow trout (Oncorhynchus
mykiss) by salmon gonadotropin, epidermal
growth factor, and 17beta-estradiol. Molecular
Reproduction and Development 61: 511-518.
Wood, A. W. & Van Der Kraak, G. J. (2001). Apoptosis and ovarian function: novel perspectives from the teleosts. Biology of
Reproduction 64: 264-271.
Yamamoto, K. & Yamauchi, K. (1974). Sexual maturation of Japanese eel and production of eel larvae in the aquarium. Nature 251:
220-222.
Yamauchi, K., Nakamura, M., Takahashi, H. & Takano, K. (1976). Cultivation of larvae of Japanese eel. Nature 263: 412.
Yoshikawa, M. (1995). Relationship between gonadal maturity and body weight or age and seasonal changes of them in cultivated Japanese eel Anguilla japonica. Bulletin
Shizuoka Prefectural Fisheries Experiment Station 30: 23-27 (in Japanese).
Yoshiura, Y., Suetake, H. & Aida, K. (1999). Duality of gonadotropin in a primitive teleost, Japanese eel (Anguilla japonica). General
and Comparative Endocrinology 114: 121-