ISSN: 1524-4628
Copyright © 2008 American Heart Association. All rights reserved. Print ISSN: 0039-2499. Online Stroke is published by the American Heart Association. 7272 Greenville Avenue, Dallas, TX 72514
DOI: 10.1161/STROKEAHA.108.524934
2008;39;3152-3158; originally published online Nov 6, 2008;
Stroke
Hsing-Yi Chang, Bi-Fong Lin, Kuan-Ju Chen and Wen-Harn Pan
Lu-Chen Weng, Wen-Ting Yeh, Chyi-Huey Bai, Hsin-Jen Chen, Shao-Yuan Chuang,
With Folate Intake?
Is Ischemic Stroke Risk Related to Folate Status or Other Nutrients Correlated
http://stroke.ahajournals.org/cgi/content/full/39/12/3152
located on the World Wide Web at:
The online version of this article, along with updated information and services, is
http://www.lww.com/reprints
Reprints: Information about reprints can be found online at
journalpermissions@lww.com 410-528-8550. E-mail:
Fax: Kluwer Health, 351 West Camden Street, Baltimore, MD 21202-2436. Phone: 410-528-4050. Permissions: Permissions & Rights Desk, Lippincott Williams & Wilkins, a division of Wolters
http://stroke.ahajournals.org/subscriptions/
Subscriptions: Information about subscribing to Stroke is online at
at NATIONAL TAIWAN UNIV on June 6, 2009 stroke.ahajournals.org
Nutrients Correlated With Folate Intake?
Lu-Chen Weng, MSc; Wen-Ting Yeh, MSc; Chyi-Huey Bai, PhD; Hsin-Jen Chen, MSc;
Shao-Yuan Chuang, PhD; Hsing-Yi Chang, PhD; Bi-Fong Lin, PhD; Kuan-Ju Chen, PhD;
Wen-Harn Pan, PhD
Background and Purpose—Folate status was inversely associated with plasma homocysteine concentration, a potential risk factor of cardiovascular disease. However, it is uncertain whether folate is causally associated with risk of ischemic stroke (IS). We aimed to examine the association between IS incidence and folate intake, biochemical folate status, and folate associated nutrients.
Methods—Information on baseline characteristics and food frequency questionnaire was collected in 1990 to 1993 and included for analyses data from 1772 adults over 40 years, who were free of stroke and cancer at baseline from the CardioVascular Disease risk FACtor Two-township Study. Multivariate Cox proportional hazard model was used to relate baseline nutrient status with IS event.
Results—Over an average of 10.6 years of follow-up, 132 incident IS events were documented. Low folate intake (1st and 2nd quartiles) was significantly and independently associated with increased IS risk (HR⫽1.61; 95% CI: 1.04 to 2.48 and HR⫽1.82; 95% CI: 1.20 to 2.76) compared with those in the 3rd and 4th quartile combined, whereas no association was observed for plasma folate concentration. On the other hand, several nutrients correlated with dietary folate: vitamin B2, potassium, iron, vitamin A of plant origin, calcium were also associated with IS risk in an inverse linear manner with HR ranging from 1.5 to 1.9 for the first quartile.
Conclusions—The protective association of dietary folate on IS risk may be in part through that of other correlated nutrients or other dietary components. (Stroke. 2008;39:3152-3158.)
Key Words: epidemiology 䡲 ischemia 䡲 risk factors 䡲 folate 䡲 stroke
S
troke is the second leading cause of death in the world1as well as in Taiwan.2 Quality of life was greatly
diminished in stroke survivors resulting from the residual disability.
In 1960s, McCully first suggested a link between homo-cysteine and arterial lesions and proposed the “Homohomo-cysteine Theory of Arteriosclerosis”.3,4Numerous studies had shown
that higher homocysteine concentration has been positively associated with risk of cardiovascular disease (CVD).5– 8
Because folate is involved in the one carbon pathway con-cerning homocysteine metabolism, folate intake and plasma/ serum folate concentration were therefore hypothesized to associate with CVD risk by modulating homocysteine con-centration.9 –19 However, prospective studies from different
populations showed inconsistent association between serum/ plasma folate concentration and ischemic stroke (IS).9,16 –18
Similarly, higher folate intake associated with reduced IS risk in the study of US health professional men14and Finnish male
smokers,19but not in those of U.S. nurses13and Swedish.16
Nutrients in foods are consumed in a pattern-like manner. Caution is required to conclude an effect of a single nutrient on disease risk. In population-based studies, levels of fiber, potassium, magnesium, flavonoid, carotenoid, calcium, vita-min B6, or B12, has also been suggested to associate with CVD risk.14,20 –27Fruits and vegetables, which are the major
sources of folate, also contain many of these CVD related nutrients. Taiwanese elderly consumed in average approxi-mately three servings of vegetables and one and half servings of fruit a day.28Their mean intake of vitamin A and vitamin
C were well above Taiwanese Daily Recommended Intakes (DRI). Mean daily folate intake was around 90% DRI.29
However, those of potassium, magnesium, and calcium were much below DRI in the elderly,28probably attributable to a
low amount of dairy products in the diet. Therefore, in this study, we not only aimed to assess the associations of dietary or plasma folate with IS incidence, but also tried to identify nutrient pattern associated with folate intake and IS risk in Taiwan where intracranial disease is relatively prevalent.30
Received May 5, 2008; accepted May 13, 2008.
From the Institute of Biomedical Sciences (L.-C.W., W.-T.Y., S.-Y.C., W.-H.P.), Academia Sinica; the Department of Research, Shin Kong WHS Memorial Hospital (C.-H.B.); Center for Health Policy Research and Development (H.-Y.C.), National Health Research Institutes; Institute of Microbiology and Biochemistry (B.-F.L.), National Taiwan University, Taipei, Taiwan; the Johns Hopkins Bloomberg School of Public Health (H.-J.C.), Baltimore, Md; and the Department of Hospitality Management (K.-J.C.), Chung Hwa University of Medical Technology, Tainan, Taiwan.
Correspondence to Wen-Harn Pan, PhD, N141, IBMS. 128, Sec. 2, Academia Rd, Taipei, Taiwan 115. E-mail pan@ibms.sinica.edu.tw © 2008 American Heart Association, Inc.
Stroke is available at http://stroke.ahajournals.org DOI: 10.1161/STROKEAHA.108.524934
Materials and Methods
Research Design
CardioVascular Disease risk FACtor Two-township Study (CVD-FACTS) began in 1989. Five villages with more than 1000 people and with population density greater than 200 people per square kilometer were randomly selected from Chu-Dong township (north-west Taiwan) and Pu-Tzu township (south(north-west Taiwan). Until now, there have been five cycles (1989 to 1990, 1990 to 1993, 1994 to 1997, 1997 to 1999, and 2000 to 2002) of data collections to acquire information from participants on lifestyle, risk factors, medications, and medical history concerning CVD. Blood and urine samples were also collected for these cycles. This study was initiated in late 1980s when IRB review was not required; however, the study had been approved by the reviewers from the Department of Health and National Health Research Institutes in Taiwan when various projects were funded. Signed inform consents have been obtained for cycle 4 and cycle 5.
Cycle 1 was considered as a pilot study focusing on body-fluid transmitted viruses. In cycle 2 (Nov 1990 to Sep 1993), all the residents in the chosen villages were invited to participate in our study. Participants with food frequency questionnaire in cycle 2 was selected as the baseline samples in this study (n⫽6314). Because very few stroke events were observed for those younger than 40 years old, we selected subjects who were older than 40 (n⫽3282). In addition, we excluded people with history of cancer, stroke which may have changed their dietary habits (n⫽84), and people who had missing values for Food Frequency Questionnaire (n⫽613), for other variables (n⫽797), or was lost of follow-up (n⫽11). Excluded also were subjects who had extreme dietary data (n⫽5). We only included subjects with a total energy intake range between 500 to 5500 kcal/d for men and 500 to 4000 kcal/d for women. There were 1772 and 1687 subjects included in dietary and plasma studies, respectively.
Food Frequency Questionnaire and Dietary Nutrient Estimation
The Food Frequency Questionnaire (FFQ) was designed to assess the dietary intake for the previous year. Included were 49 food items of the 8 food groups (milk and soymilk, vegetables, fruits, meats, egg and soybean products, seafood, drinks, and low content alcoholic drinks with vitamin B complex). In addition, information on types, amounts, and frequencies of staple foods, cooking oils, and supple-ments; discretionary use of sugar and butter/margarine/mayonnaise; and avoidance of fats, egg yolk and skins was collected by open-ended questions. A standard portion size model was designed and provided for each food, and proportion or number of servings was questioned. Ten possible frequency responses were available (ⱕ6 times/yr, 1 to 3 times/mo, 1 time/wk, 2 to 4 times/wk, 5 to 6 times/wk, 1 time/d, 2 times/d, 3 times/d, 4 to 5 times/d, and ⱖ6 times/d). Reproducibility and validity study of this FFQ, comparing nutrients measured by the FFQ and 15-days (3 5-day) food records, have been previously reported.31 The Pearson correlation
coeffi-cients of folate intake between FFQ and food records were 0.26 and 0.19, respectively, before and after adjusting calories. Total calorie was calculated by summing up the calories from dietary intake and alcohol intake. In this study, nutrients intakes were calorie-adjusted by residual method.32
Blood Collection and Laboratory Tests
All subjects were asked to fast overnight (more than 8 hours) before blood specimen collection. Citrated fasting plasma was obtained by centrifugation at 1469g for 15 minutes in 4°C within 30 minutes after blood collection and stored in ⫺70°C before using. Hemostatic measurements, such as fibrinogen and plasminogen, were measured within 1 month of blood sampling on an ACL 300 Plus Automat Clotting and Fibrinolyzing Analysis System (Instrumentation Labo-ratory), using reagents provided by the manufacturer. Apolipoprotein B was measured by means of a nephelometric immunoassay,
performed with the automated Array Protein System (Beckman Instruments).
Heparinized fasting plasma was also obtained by centrifugation at 1469g for 15 minutes in 4°C. All samples were stored at⫺70°C until using. Basic clinical chemistry profile, such as fasting glucose, cholesterol, and triglyceride were quantified by the Monarch 2000 Autoanalyzer (Instrumentation Laboratory) within one month after blood collection. For this study, we used competitive immunoassay to measure plasma folate concentration33at 2004. The investigators
and laboratory staff were blinded to the disease status. There was a significant but moderate correlation between plasma folate concen-tration and dietary folate intake (r⫽0.15, P⬍0.0001).
Other Variables
Weight, height, and waist circumference were measured with stan-dard procedure.34Body mass index (BMI) was calculated as weight
(kg)/height2 (m2). Three times blood pressure was measured after
sitting for 5 minutes, and the mean of the last 2 readings was used for analysis. Questionnaire including demographic data (birth date and sex), lifestyle (smoking, alcohol consumption, physical activity), and self-reported health conditions (disease status and drug using record) were also collected.
IS Event Ascertainment
Stroke event occurring before 1996 was self-reported and cross-checked by medical records or death certificate. Three sources of information were used to determine the first-ever IS status and the onset time after 1996, including death certificate data, insurance claim records of the National Health Insurance (NHI) database, and subject’s self-reported disease history.35 A total of 99.5% of our
studied subjects were covered by NHI. A first-ever stroke was defined by any one of the following conditions with codes 430 to 438 from International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM): (1) hospitalization claim forⱖ one day, or (2) more than 3 consecutive outpatient visits to hospitals, followed either by claims for various neurological imaging technology (com-puted tomography, MRI, transcranial or carotid Doppler sonography) and long-term prescriptions used for IS or by claims for rehabilita-tion and the long-term IS prescriprehabilita-tions. An unpublished validarehabilita-tion study showed that sensitivity and specificity for the above rules were 100% and 95%, respectively; using data from 508 ischemic stroke patients of a hospital-based case-control study. More details and validity data have been described elsewhere36
Statistics Analysis
Baseline characteristics for event and nonevent groups were com-pared by t tests for continuous variables and by2
test for categorical variables.
In this study, we used the Cox proportional hazard model to examine the relationships between the incidence of IS and the levels of dietary folate, plasma folate concentration, other dietary nutrients, and dietary factor scores (see next paragraph). Two models were used in our study to adjust other CVD risk factors. Model 1 adjusted for age, sex, age–sex interaction, and model 2 additionally adjusted for area, drinking habit, smoking habit, sex–smoking habit interac-tion, hypertension, diabetes mellitus, taking hypertensive drugs, BMI, self-report heart disease, central obesity, physical activity, hypertriglyceridemia, hypercholesterolemia, plasminogen, apoli-poprotein B, and fibrinogen.
Moreover, factor analysis (principal component analysis) was used to identify how dietary levels of 22 nutrients were related to one another and fallen into smaller number of factors. All nutrient intake levels had been calorie-adjusted by residual method32 and then
normalized. We then used the varimax rotation to separate dietary nutrients into orthogonal dietary factors. Factor score was calculated by summing up intakes of nutrients weighted by their factor loading if the factor loading was greater than 0.45. All statistics were estimated by SAS version 9.1 (SAS institute).
Weng et al Folate or Correlated Nutrients Predict Stroke? 3153
at NATIONAL TAIWAN UNIV on June 6, 2009 stroke.ahajournals.org
Results
During 10.6 follow-up years, there were 132 incident events of IS. In those developed into IS, there were more with hypertension, DM, self-reported heart disease, large BMI, central obesity at baseline (Table 1) than in those free of stroke, and the event group had lower level of dietary folate, but higher levels of age, waist circumference, BMI, serum triacylglycerol concentration, apolipoprotein B, and plasmin-ogen than the nonevent group. More of them used antihyper-tensive drugs, but less of them took dietary supplements. However, no differences were observed in the level of fibrinogen and the proportion of male sex, smoking habits, alcohol habits, physical activity, hypercholesterolemia, and people who resided in the two residential areas.
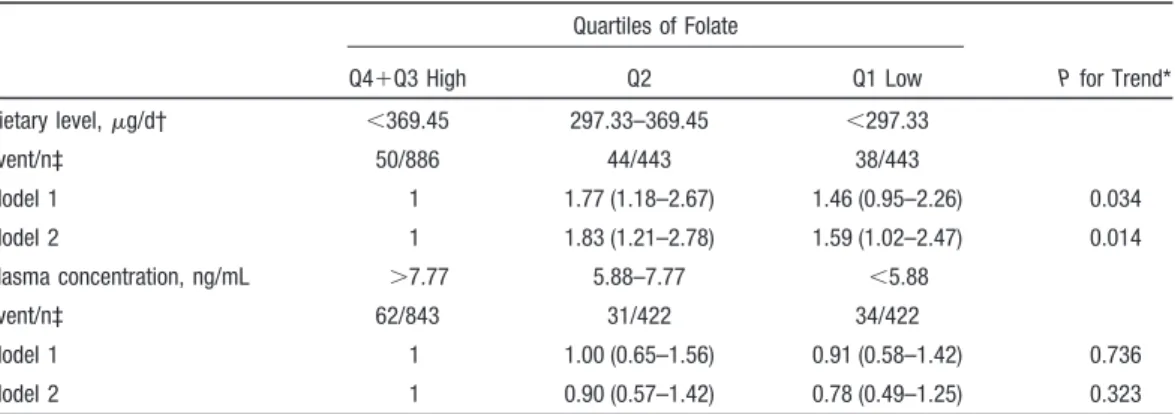
An inverse association was observed between folate intake and IS (Table 2) after adjusting for age, sex, and age–sex interaction. The hazard ratios (HRs) of IS were 1.77 (95% CI, 1.18 to 2.67) and 1.46 (95% CI, 0.95 to 2.26) for the 2nd and the 1st quartiles of folate intake, respectively, comparing with the two upper quartiles combined. After adjusting for other risk factors, the HRs were slightly increased (HR⫽1.83, 95% CI: 1.21 to 2.78 and HR⫽1.59, 95% CI⫽1.02 to 2.47 for the 2nd and 1st quartiles), and the test of trend was still significant (P⫽0.014). The results from separating the 3rd and the 4th quartiles are similar to but not as powerful as that of the combined. However, plasma folate concentration did not reveal any significant association with the risk of IS in these 2 models (P⬎0.05).
Six independent factors were extracted by factor analysis from 22 dietary nutrient intake levels (Table 3). Factor 1 was mainly contributed by folate, dietary fiber, vitamin C, vitamin A of plant origin, magnesium, calcium, potassium, iron, and vitamin B2. Because it was involved in various kinds of nutrients rich in plant product, we named this factor “Plant food factor.” Factor 2 was mainly contributed by magnesium, calcium, sodium, potassium, phosphorus, and iron, and thus we named the factor “Mineral-rich factor.” We named factor 3 “Fatty acid factor” because of its two major components: saturated fatty acid and oleic acid. Factor 4 was composed of
Table 1. Baseline Characteristics of IS Event Group and Nonevent Group Nonevent Group (n⫽1640) IS Event Group (n⫽132) P Value Mean age, y 56.1 (9.8) 62.6 (8.1) ⬍0.001 Sex, % Male 43.7 49.2 0.639 Female 56.3 50.8 Area, % Chu-Dong 54.4 52.3 0.214 Pu-Tzu 45.6 47.7 Dietary Folate,g/d* 397.1 (170.4) 364.0 (176.8) 0.032 Smoking habits, % Never 77.8 70.5 0.148 Past 3.1 4.6 Current 19.1 25.0
Alcohol consumption habits, %
Never 91.0 90.2 0.815 Past 0.18 Current 8.8 9.9 Hypertension, %† Yes 21.5 40.9 ⬍0.001 No 78.5 59.1 Diabetes mellitus, %‡ Yes 9.5 23.5 ⬍0.001 No 90.6 76.5
Self-report heart disease, %
Yes 3.9 8.3 0.015 No 96.1 91.7 Physical activity, %§ Yes 41.4 47.0 0.212 No 58.6 53.0 Mean BMI, kg/m2 24.4 (3.3) 25.3 (3.4) 0.002 BMI level, % Normal:ⱕ24 48.5 34.9 0.009 Overweight: 24–27 31.7 41.7 Obesity:ⱖ27 19.8 23.5 Waist circumference, cm 81.2 (9.5) 85.1 (9.5) ⬍0.001 Central obesity, %储 36.2 49.2 0.003
Mean serum cholesterol concentration, mg/dL
202.0 (41.5) 207.2 (42.2) 0.167 Hypercholesterolemia, %#
Yes 16.7 22.0 0.118
No 83.4 78.0
Mean serum triacylglycerol concentration, mg/dL
108.8 (73.3) 136.4 (85.1) ⬍0.001 Hypertriglyceridemia, %**
Yes 8.7 16.7 0.003
No 91.3 83.3
Mean fibrinogen concentration, mg/dL 280.5 (76.5) 287.0 (75.4) 0.341 Apolipoprotein B, mg/dL 114.9 (31.7) 121.5 (30.3) 0.022 Plasminogen, % 122.7 (34.0) 131.3 (40.1) 0.018 (Continued) Table 1. Continued Nonevent Group (n⫽1640) IS Event Group (n⫽132) P Value Use of antihypertensive drugs, % Yes 15.61 34.85 ⬍0.001 No 84.39 65.15 Dietary supplement, % 12.93 6.06 0.021
Data are Mean (SD).
*Dietary folate was calorie-adjusted by residual method.
†Hypertension: systolic blood pressure ⱖ140 mm Hg or diastolic blood pressureⱖ90 mm Hg or on antihypertensive drugs.
‡Diabetes mellitus: fasting (over 8 hours) serum glucose concentration ⱖ126 mg/dL or on diabetes mellitus drugs.
§Physical activity: 3 times a week andⱖ20 minutes per time.
储Central obesity: waist circumference ⱖ90cm for male and ⱖ80cm for female.
#Hypercholesterolemia: serum cholesterol concentrationⱖ240 mg/dL. **Hypertriglyceridemia: serum triacylglycerol concentrationⱖ200 mg/dL.
niacin and vitamins B1 and B6. We named it as “B vitamin factor.” Polyunsaturated fatty acid, PS ratio, and vitamin E were the main components of factor 5, which was named “Vegetable oil factor.” Factor 6 contributed by vitamin A of animal origin, vitamin B2, vitamin B12, and cholesterol and was named “Animal food factor.”
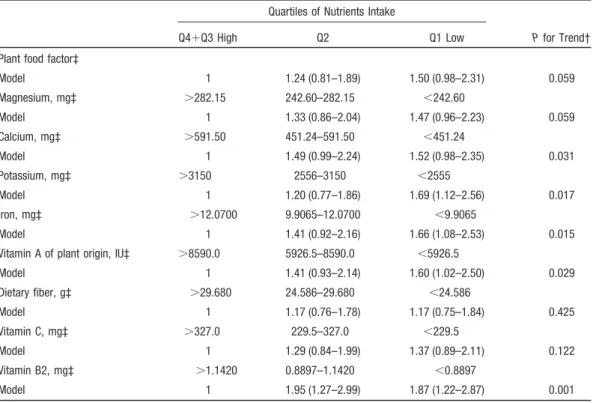
In these factors, plant food factor was the only factor correlated to dietary folate level. We divided this factor score into quartiles, and assessed the association between quartiles of this factor score and IS risk (Table 4). After adjusting for age, sex, age–sex interaction, and other CVD risk factors, the HR of the lowest quartile was 1.50 (95% CI, 0.98 to 2.31)
Table 2. Hazard Ratios and 95% CIs for Incident IS Event by Quartiles of Folate Status
Quartiles of Folate P for Trend* Q4⫹Q3 High Q2 Q1 Low Dietary level,g/d† ⬍369.45 297.33–369.45 ⬍297.33 Event/n‡ 50/886 44/443 38/443 Model 1 1 1.77 (1.18–2.67) 1.46 (0.95–2.26) 0.034 Model 2 1 1.83 (1.21–2.78) 1.59 (1.02–2.47) 0.014 Plasma concentration, ng/mL ⬎7.77 5.88–7.77 ⬍5.88 Event/n‡ 62/843 31/422 34/422 Model 1 1 1.00 (0.65–1.56) 0.91 (0.58–1.42) 0.736 Model 2 1 0.90 (0.57–1.42) 0.78 (0.49–1.25) 0.323
*P for trend based on the 3-group data (Q1, Q2, and Q3⫹Q4). †Dietary folate was calorie-adjusted.
‡No. of people at risk in the category.
Model 1 was adjusted for age (40 –50, 50 – 60, 60 –70,ⱖ70), sex, age*sex.
Model 2 was adjusted for the covariates in model 1 plus hypertension (yes, no), use of antihypertensive drugs (yes, no), diabetes mellitus (yes, no), area (Chu-Dong and Pu-Tzu), central obesity (yes, no), alcohol consumption habits (never, ex-drinker, current drinker), smoking habit (never, ex-smoker, current smoker), sex-smoking habit interaction, BMI (ⱕ24, 24–27, ⱖ27 kg/m2),
self-report heart disease (yes, no), hypercholesterolemia (yes, no), hypertriglyceridemia (yes, no), physical activity (yes, no), fibrinogen (tertiles), apolipoprotein B (tertiles), and plasminogen (tertiles).
Table 3. Factor Loading for Nutrient Intake Levels Estimated From FFQ*
Plant Food Factor Mineral-Rich Factor Fatty Acid Factor B Vitamin Factor Vegetable Oil Factor Animal Food Factor Dietary fiber 0.83 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 Vitamin C 0.82 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 Folate 0.79 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠
Vitamin A of plant origin 0.73 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠
Magnesium 0.46 0.83 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 Calcium 0.53 0.75 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 Sodium 䡠 䡠 䡠 0.72 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 Potassium 0.57 0.72 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 Phosphorus 䡠 䡠 䡠 0.69 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 Iron 0.47 0.68 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠
Saturated fatty acid 䡠 䡠 䡠 䡠 䡠 䡠 0.95 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠
Oleic acid 䡠 䡠 䡠 䡠 䡠 䡠 0.95 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠
Niacin 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.72 䡠 䡠 䡠 䡠 䡠 䡠
Vitamin B1 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.65 䡠 䡠 䡠 䡠 䡠 䡠
Vitamin B6 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.64 䡠 䡠 䡠 䡠 䡠 䡠
Polyunsaturated fatty acid 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.87 䡠 䡠 䡠
PS ratio 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.77 䡠 䡠 䡠
Vitamin E 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.69 䡠 䡠 䡠
Vitamin A of animal origin 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.85
Vitamin B2 0.49 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.51
Vitamin B12 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.48
Cholesterol 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 䡠 0.47
*Value⬍0.45 is omitted for simplicity.
Weng et al Folate or Correlated Nutrients Predict Stroke? 3155
at NATIONAL TAIWAN UNIV on June 6, 2009 stroke.ahajournals.org
compared with the two upper quartiles combined. Test of trend was borderline significant (P⫽0.059).
We further assessed the association between IS and levels of various nutrients intakes in this plant food factor. Inverse linear associations were shown for calcium, potassium, and iron with IS risk after adjusting for CVD risk factors. The HR comparing bottom quartile with the two top quartiles of dietary intake was 1.52 (95% CI, 0.98 to 2.35; P⫽0.031 for trend) for calcium, 1.69 (95% CI, 1.12 to 2.56; P⫽0.017 for trend) for potassium, and 1.66 (95% CI, 1.08 to 2.53; P⫽0.015 for trend) for iron after adjusting risk factors. In addition, magnesium intake was negatively correlated with IS risk at a borderline significance (P⫽0.059 for trend). Among these minerals, strength of the association ranked the highest for potassium.
Similar analyses were also represented for vitamin A of plant origin and for vitamin B2 intake. The HR was signifi-cant for vitamin A of plant origin (HR⫽1.60, 95% CI: 1.02 to 2.50) and for vitamin B2 (HR⫽1.87, 95% CI: 1.22 to 2.87), after adjusting for risk factors. Test for trend also showed significant inverse association (P⬍0.05). However, there was no significant association with dietary fiber or vitamin C intake.
Discussion
We found that dietary folate intake was negatively associated with incidence of IS, when plasma folate was not. A derived plant food factor was associated with folate intake, and intakes of several nutrients such as potassium, iron, calcium,
magnesium, vitamin A of plant origin, and vitamin B2, each of which were also inversely associated with IS.
Previous prospective studies showed inconsistent results on the relations between folate intake and stroke risk.11,13,14,16,19 Using 24-hour recall data of NHANES I,
Bazzano et al observed a 20% stroke risk reduction in those with the highest quartile of folate intake (daily folate intake ⬎300.6 g) compared to the lowest quartile group (daily folate intake⬍136.0g).11In other cohort studies, He et al,14
using FFQ, observed a⬇34% reduction of IS in men with the highest quintile of folate intake (mean daily intake⫽821g) compared with the lowest quintile (mean daily intake⫽262 g). Also, higher folate intake (median daily intake⫽410 g) was shown to decrease 20% risk of cerebral infarction in Finnish male smokers.19However, higher folate intake (daily
intake ⬎526 g) did not decrease the risk of any type of stroke in middle-aged women.13A nested case–referent study
in Sweden did not find decreased IS risk in highest quartile of folate intake, but did so for the hemorrhagic stroke.16Race,
sex, and age of the studied populations, the way to collect dietary information, subtypes of stroke, and the cut-point of nutrient intake may influence the conclusions of the studies. Nevertheless, our results supported that low dietary folate intake may be a risk factor of IS, either representing itself or as a surrogate for other nutrients and dietary components in the foods. On the other hand, in this study, we did not observe any association between plasma folate and IS risk. According to the “Homocysteine Theory of Arteriosclerosis,”3,4 folate
may prevent stroke via lowering homocysteine concentration.
Table 4. Hazard Ratios and 95% CIs for Incident IS by Quartiles of Factor Score and Daily Nutrients Intake*
Quartiles of Nutrients Intake
P for Trend†
Q4⫹Q3 High Q2 Q1 Low
Plant food factor‡
Model 1 1.24 (0.81–1.89) 1.50 (0.98–2.31) 0.059 Magnesium, mg‡ ⬎282.15 242.60–282.15 ⬍242.60 Model 1 1.33 (0.86–2.04) 1.47 (0.96–2.23) 0.059 Calcium, mg‡ ⬎591.50 451.24–591.50 ⬍451.24 Model 1 1.49 (0.99–2.24) 1.52 (0.98–2.35) 0.031 Potassium, mg‡ ⬎3150 2556–3150 ⬍2555 Model 1 1.20 (0.77–1.86) 1.69 (1.12–2.56) 0.017 Iron, mg‡ ⬎12.0700 9.9065–12.0700 ⬍9.9065 Model 1 1.41 (0.92–2.16) 1.66 (1.08–2.53) 0.015
Vitamin A of plant origin, IU‡ ⬎8590.0 5926.5–8590.0 ⬍5926.5
Model 1 1.41 (0.93–2.14) 1.60 (1.02–2.50) 0.029 Dietary fiber, g‡ ⬎29.680 24.586–29.680 ⬍24.586 Model 1 1.17 (0.76–1.78) 1.17 (0.75–1.84) 0.425 Vitamin C, mg‡ ⬎327.0 229.5–327.0 ⬍229.5 Model 1 1.29 (0.84–1.99) 1.37 (0.89–2.11) 0.122 Vitamin B2, mg‡ ⬎1.1420 0.8897–1.1420 ⬍0.8897 Model 1 1.95 (1.27–2.99) 1.87 (1.22–2.87) 0.001
*Daily intake was calorie-adjusted. †P for trend based on the 3-group data.
However, in our study, the negative association between folate intake level and ischemic stroke did not change after adjusting for plasma folate or homocysteine (data not shown). We suggest the protective effect of dietary folate on ischemic stroke may be independent of homocysteine mechanism in this population.
There were at least two possibilities to explain the above phenomenon. The first one is that dietary folate intake may be a surrogate of plant foods or other component(s) in the plant foods, either a single component or multiple components combined. In our factor analysis, we were able to divide 22 nutrients into 6 factors. Among the 6, the plant food factor was inversely associated with IS at a borderline level, which also correlated with dietary folate, potassium, calcium, di-etary fiber, vitamin C, vitamin A of plant origin, magnesium, iron, and vitamin B2. According to the Intersalt study,37
Taiwanese have poorer potassium intake status than people of many Western countries. In our previous study, we had shown a long-term benefit effect of potassium on cardiovas-cular mortality exhibited via the replacing regular salt by potassium-enrich salt in elderly veterans.38In addition, higher
dietary calcium or magnesium intakes have been inversely correlated with the risk of stroke or stroke mortality.21,25
Furthermore, higher vitamin B2 (riboflavin) was also be-lieved to reduce CVD risk by decreasing homocysteine concentration.39We would suggest that the protective
asso-ciation of folate intake on IS may represent a combined effect of all protective nutrients or dietary components, whereas plasma folate concentration represents the true folate status in bodies.
Another potential reason for the discrepancy between dietary and plasma folate may result from the relatively good biochemical folate status in Taiwanese. Lower serum folate concentration (ⱕ9.2 nmol/L) in blacks raised the risk of IS to 3.6 times comparing with the higher folate concentration in blacks.9In a Swedish research, higher plasma folate (male:
4.2 to 5.8 nmol/L; female: 7.6 to 9.9 nmol/L) reduced⬇40% IS risk compared with the lowest quartile (male: ⬍3.3 nmol/L; female: ⬍5.8 nmol/L).16 In these two studies, the
cut-points of the lowest group were obviously lower than ours (⬍5.88 ng/mL [13.3 nmol/L]). It may be possible that the high risk of IS only exists in people who had extremely low concentration of serum/plasma folate. When serum/plasma folate concentration is higher, dietary folate becomes more of a surrogate of plant foods.
Recent meta-analysis collected 8 randomized secondary prevention trials showed that folic acid supplementation significantly reduced the risk of stroke by 18% in patients with myocardial infarction, stroke, or chronic renal failure. The effect was more apparent for the patients without history of stroke or with a longer treatment duration (⬎36 months) (RR⫽0.75; 95%CI: 0.62 to 0.90 and RR⫽0.71; 95%CI: 0.57 to 0.87, respectively).40 Although these results further
sup-ported the importance of dietary folate on primary prevention of stroke in patients with heart and renal diseases; however, it did not prevent recurrence of stroke in stroke patients. More intervention studies are needed to confirm the benefits of folate on decreasing stroke risk in the future.
There were some strengths in this study. Compared with case– control study, our prospective study design provides insights to whether there is a temporal relationship. In addition, the national health insurance system provided com-plete insurance claim records of most of the subjects, and decreased the chance of loss of follow-up in our study. Our ascertainment scheme for catching incident IS event is rule-based, which can reduce false-positive events identified by ICD codes only. Furthermore, our study is the only one taking into consideration intercorrelation of dietary nutrients, which is crucial for nutrition epidemiological studies.
The limitations of this study include the length of blood sample storage and the potential that subjects may modify their dietary behaviors during the follow-up period. In previ-ous studies, the concentration of plasma folate decreased obviously after long-time storage at⫺20°C.41However, we
stored our samples at⫺70°C, and the correlation between plasma folate and homocysteine concentration in our study was significant (r⫽⫺0.20, P⬍0.0001) after storing over 11 years, indicating that the concentration of plasma folate was reliable. In addition, during the 10.6 years of follow-up, subjects in our study may have changed their diets. However, we have documented a positive finding. Furthermore, we did not calculate the nutrient contents from vitamin or mineral supplement in this study. However, the correlation between plasma folate and folate intake did not change much after adding the information of supplements, and the HR was even more significant in relation to ischemic stroke risk when we used the total folate intake to replace dietary folate intake (data not shown).
Summary
Dietary folate was an independently protective factor of IS, whereas plasma folate was not associated with the risk of IS in this Taiwanese population where people have relatively higher levels of plasma folate concentration than those of previous literature and intracranial diseases are more preva-lent compared to Western countries. More significant was the inverse association between risk of IS and some other nutrients correlated with dietary folate, including vitamin B2, potassium, iron, vitamin A of plant origin, or calcium. This inverse association may in part be compounded by other nutrients or food components in plant foods. Cautions are needed to make conclusion from observational nutrition epidemiological studies. The role of intervention study should be stressed.
Acknowledgments
We thank all participants and neurologists in Shin Kong WHS Memorial Hospital who provided consultation to build decision rules for identifying new stroke events from National Health Insurance data and helped reviewing medical records for undetermined stroke events.
Sources of Funding
The present study was funded by the National Health Research Institutes in Taiwan (NHRI-EX93–9225PP, NHRI-EX94 –9225PP). Data collection was supported by the Department of Health in Taiwan (DOH80-27, DOH81-021, DOH8202-1027, DOH83-TD-015, and DOH84-TD-006).
Weng et al Folate or Correlated Nutrients Predict Stroke? 3157
at NATIONAL TAIWAN UNIV on June 6, 2009 stroke.ahajournals.org
Disclosures
None.
References
1. STEPwise approach to stroke surveillance. World Health Organization. http://www.who.int/chp/steps/stroke/en/index.html (accessed July 4, 2007)
2. Health and National Health Insurance Annual Statistics Information Service. Department of Health, Excutive Yuan, R.O.C(TAIWAN). http:// www.doh.gov.tw/statistic/index.htm (accessed February 26, 2007) 3. McCully KS. Vascular pathology of homocysteinemia: Implications for
the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. 4. McCully KS, Wilson RB. Homocysteine theory of arteriosclerosis.
Ath-erosclerosis. 1975;22:215–227.
5. Bostom AG, Rosenberg IH, Silbershatz H, Jacques PF, Selhub J, D’Agostino RB, Wilson PW, Wolf PA. Nonfasting plasma total homo-cysteine levels and stroke incidence in elderly persons: The Framingham Study. Ann Intern Med. 1999;131:352–355.
6. Cleophas TJ, Hornstra N, van Hoogstraten B, van der Meulen J. Homo-cysteine, a risk factor for coronary artery disease or not? A meta-analysis.
Am J Cardiol. 2000;86:1005–1009, A1008.
7. Ford ES, Smith SJ, Stroup DF, Steinberg KK, Mueller PW, Thacker SB. Homocyst(e)ine and cardiovascular disease: A systematic review of the evidence with special emphasis on control studies and nested case-control studies. Int J Epidemiol. 2002;31:59 –70.
8. Iso H, Moriyama Y, Sato S, Kitamura A, Tanigawa T, Yamagishi K, Imano H, Ohira T, Okamura T, Naito Y, Shimamoto T. Serum total homocysteine concentrations and risk of stroke and its subtypes in Japanese. Circulation. 2004;109:2766 –2772.
9. Giles WH, Kittner SJ, Anda RF, Croft JB, Casper ML. Serum folate and risk for ischemic stroke. First National Health and Nutrition Examination Survey epidemiologic follow-up study. Stroke. 1995;26:1166 –1170. 10. Verhoef P, Stampfer MJ, Rimm EB. Folate and coronary heart disease.
Curr Opin Lipidol. 1998;9:17–22.
11. Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Dietary intake of folate and risk of stroke in US men and women: NHANES I Epidemiologic Follow-up Study. National Health and Nutrition Examination Survey. Stroke. 2002;33:1183–1188.
12. de Bree A, Verschuren WM, Blom HJ, Nadeau M, Trijbels FJ, Kromhout D. Coronary heart disease mortality, plasma homocysteine, and B-vi-tamins: A prospective study. Atherosclerosis. 2003;166:369 –377. 13. Al-Delaimy WK, Rexrode KM, Hu FB, Albert CM, Stampfer MJ, Willett
WC, Manson JE. Folate intake and risk of stroke among women. Stroke. 2004;35:1259 –1263.
14. He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Folate, vitamin B6, and B12 intakes in relation to risk of stroke among men. Stroke. 2004;35:169 –174.
15. Kocer A, Ince N, Canbulat CE, Sargin M. Serum vitamin B12 and folic acid levels in acute cerebral atherothrombotic infarction. Tohoku J Exp
Med. 2004;204:155–161.
16. Van Guelpen B, Hultdin J, Johansson I, Stegmayr B, Hallmans G, Nilsson TK, Weinehall L, Witthoft C, Palmqvist R, Winkvist A. Folate, vitamin B12, and risk of ischemic and hemorrhagic stroke: A prospective, nested case-referent study of plasma concentrations and dietary intake. Stroke. 2005;36:1426 –1431.
17. Virtanen JK, Voutilainen S, Happonen P, Alfthan G, Kaikkonen J, Mursu J, Rissanen TH, Kaplan GA, Korhonen MJ, Sivenius J, Salonen JT. Serum homocysteine, folate and risk of stroke: Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. Eur J Cardiovasc Prev Rehabil. 2005;12:369 –375.
18. Weikert C, Dierkes J, Hoffmann K, Berger K, Drogan D, Klipstein-Grobusch K, Spranger J, Mohlig M, Luley C, Boeing H. B vitamin plasma levels and the risk of ischemic stroke and transient ischemic attack in a German cohort. Stroke. 2007;38:2912–2918.
19. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Folate, vitamin B6, vitamin B12, and methionine intakes and risk of stroke subtypes in male smokers. Am J Epidemiol. In press.
20. Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: The Zutphen study. Arch
Intern Med. 1996;156:637– 642.
21. Ascherio A, Rimm EB, Hernan MA, Giovannucci EL, Kawachi I, Stampfer MJ, Willett WC. Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation. 1998;98:1198 –1204. 22. Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000;31:2301–2306.
23. Abbott RD, Ando F, Masaki KH, Tung KH, Rodriguez BL, Petrovitch H, Yano K, Curb JD. Dietary magnesium intake and the future risk of coronary heart disease (the Honolulu Heart Program). Am J Cardiol. 2003;92:665– 669.
24. Al-Delaimy WK, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Mag-nesium intake and risk of coronary heart disease among men. J Am Coll
Nutr. 2004;23:63–70.
25. Umesawa M, Iso H, Date C, Yamamoto A, Toyoshima H, Watanabe Y, Kikuchi S, Koizumi A, Kondo T, Inaba Y, Tanabe N, Tamakoshi A. Dietary intake of calcium in relation to mortality from cardiovascular disease: The JACC Study. Stroke. 2006;37:20 –26.
26. Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B6 from diet and sup-plements in relation to risk of coronary heart disease among women.
JAMA. 1998;279:359 –364.
27. Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, Whelton PK. Dietary potassium intake and risk of stroke in US men and women: National Health and Nutrition Examination Survey I epidemiologic follow-up study. Stroke. 2001;32:1473–1480.
28. Wu SJ, Chang YH, Wei IL, Kao MD, Lin YC, Pan WH. Intake levels and major food sources of energy and nutrients in the Taiwanese elderly. Asia
Pac J Clin Nutr. 2005;14:211–220.
29. Chen KJ, Pan WH, Shaw NS, Huang RF, Lin BF. Association between dietary folate-rich food intake and folate status of elderly Taiwanese. Asia
Pac J Clin Nutr. 2005;14:244 –249.
30. Liu HM, Tu YK, Yip PK, Su CT. Evaluation of intracranial and extracranial carotid steno-occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke. 1996;27: 650 – 653.
31. Lee MS, Pan WH, Liu KL, Yu MS. Reproducibility and validity of a Chinese food frequency questionnaire used in Taiwan. Asia Pac J Clin
Nutr. 2006;15:161–169.
32. Willett W, Stampfer M. Implications of total energy intake for epidemi-ologic analysis. Nutritional Epidemiology. 1998;273–301.
33. Pesce AJ, Kaplan LA. Folic acid. In: Kaplan LA, Pesce AJ, eds. Methods
in Clinical Chemistry. St. Louis: Mosby;1987:539 –546.
34. Teh BH, Pan WH, Chen CJ. The reallocation of body fat toward the abdomen persists to very old age, while body mass index declines after middle age in Chinese. Int J Obes Relat Metab Disord. 1996;20:683– 687. 35. Chen HJ, Bai CH, Yeh WT, Chiu HC, Pan WH. Influence of metabolic syndrome and general obesity on the risk of ischemic stroke. Stroke. 2006;37:1060 –1064.
36. Bai CH, Chen JR, Chiu HC, Pan WH. Lower blood flow velocity, higher resistance index, and larger diameter of extracranial carotid arteries are associated with ischemic stroke independently of carotid atherosclerosis and cardiovascular risk factors. J Clin Ultrasound. 2007;35:322–330. 37. The INTERSALT study. An international co-operative study of
elec-trolyte excretion and blood pressure: further results. J Hum Hypertens. 1989;3:279 – 407.
38. Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, Tsai SY, Pan WH. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr. 2006;83:1289 –1296. 39. Powers HJ. Riboflavin (vitamin B-2) and health. Am J Clin Nutr. 2003;
77:1352–1360.
40. Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: A meta-anal-ysis. Lancet. 2007;369:1876 –1882.
41. Ocke MC, Schrijver J, Obermann-de Boer GL, Bloemberg BP, Haenen GR, Kromhout D. Stability of blood (pro)vitamins during four years of storage at -20°C: Consequences for epidemiologic research. J Clin