Effects of water and methanol on the molecular organization of
1-butyl-3-methylimidazolium tetrafluoroborate as functions of pressure and concentration
Hai-Chou Chang, Jyh-Chiang Jiang, You-Chang Liou, Chao-Hsin Hung, Ting-Yun Lai, and Sheng Hsien Lin
Citation: The Journal of Chemical Physics 129, 044506 (2008); doi: 10.1063/1.2958256 View online: http://dx.doi.org/10.1063/1.2958256
View Table of Contents: http://scitation.aip.org/content/aip/journal/jcp/129/4?ver=pdfcov Published by the AIP Publishing
Articles you may be interested in
Association effects in the {methanol + inert solvent} system via Monte Carlo simulations. I. Structure J. Chem. Phys. 138, 204505 (2013); 10.1063/1.4807309
Vibrational absorption and vibrational circular dichroism spectra of leucine in water under different pH conditions: Hydrogen-bonding interactions with water
J. Chem. Phys. 137, 194308 (2012); 10.1063/1.4767401
Microhydration effects on the electronic spectra of protonated polycyclic aromatic hydrocarbons: [naphthalene-(H2O) n = 1,2]H+
J. Chem. Phys. 134, 074307 (2011); 10.1063/1.3554416
Structural change of ionic association in ionic liquid/water mixtures: A high-pressure infrared spectroscopic study J. Chem. Phys. 130, 124503 (2009); 10.1063/1.3100099
Hydrogen bonding in supercritical methanol studied by infrared spectroscopy J. Chem. Phys. 116, 1995 (2002); 10.1063/1.1431585
Effects of water and methanol on the molecular organization
of 1-butyl-3-methylimidazolium tetrafluoroborate as functions
of pressure and concentration
Hai-Chou Chang,1,a兲Jyh-Chiang Jiang,2You-Chang Liou,1Chao-Hsin Hung,1 Ting-Yun Lai,1and Sheng Hsien Lin3,4
1Department of Chemistry, National Dong Hwa University, Shoufeng, Hualien 974, Taiwan 2Department of Chemical Engineering, National Taiwan University of Science and Technology, Taipei 106, Taiwan
3Department of Applied Chemistry, National Chiao Tung University, Hsinchu 30010, Taiwan 4Institute of Atomic and Molecular Sciences, Academia Sinica, P.O. Box 23-166, Taipei 106, Taiwan
共Received 16 April 2008; accepted 24 June 2008; published online 28 July 2008兲
The structural organization in mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate共关bmim兴 ⫻关BF4兴兲/water or methanol was studied by infrared spectroscopy. No drastic change in the
concentration dependence of the alkyl C–H band frequency was observed at high concentration of the ionic liquid. This behavior indicates a clustering of the ionic liquid in alkyl regions. Nevertheless, the presence of methanol significantly perturbs the ionic liquid–ionic liquid associations in the imidazolium region. On the basis of the responses to change in pressure and concentration, two different types of O–H species, i.e., free O–H and bonded O–H, were observed in the O–H stretching region. For关bmim兴关BF4兴/water mixtures, the compression leads to loss of the
free O–H band intensity. It is likely that free O–H is switched to bonded O–H as high pressures are applied. For关bmim兴关BF4兴/methanol mixtures, the free O–H is still stable under high pressures.
© 2008 American Institute of Physics.关DOI:10.1063/1.2958256兴
INTRODUCTION
Recently, room-temperature ionic liquids have received significant attention for their use in multidisciplinary chem-istry areas.1–4Ionic liquids are generally nonvolatile at room temperature. Their low vapor pressure decreases contamina-tion from evaporacontamina-tion compared to other convencontamina-tional non-ionic solvents. Due to this feature and to a number of other unique properties, ionic liquids have a high potential to be used as reaction media in green chemistry.1,2 Ionic liquids have a melting temperature around room temperature and are built up by a bulky, asymmetric organic cation to prevent ions from packing easily. This asymmetry opposes the strong charge ordering due to the ionic interactions that normally would cause the system to crystallize, and thus, a wide liquid range is obtained. In recent years, ionic liquids based on the 1-alkyl-3-methylimidazolium cation have received much attention.3–8Combining organic cations with suitable anions allows one to tailor the thermodynamic properties of ionic liquids over a wide range of miscibility with other solvents. Ionic liquids are generally miscible with water, being ionic compounds. However, they could be designed to be made hydrophobic by choice of anion species. The water miscibility of ionic liquids is also affected by the hydropho-bicity of the alkyl substituents of the imidazolium cation. An interesting aspect of such ionic liquids is that the 1-alkyl-3-methylimidazolium cations possess an inherent
amphiphilicity.1–4 Long-chained imidazoliums 共or pyrroli-diniums, pyridiniums, etc.兲 may act as surfactants. For example, the micellization of long-chained N-alkyl-N-methylpyrrolidinium bromides 关CnMPB 共n⬎6兲兴
in aqueous solution has been studied by Baker et al.9 The critical micelle concentration values for CnMPB series are
comparable to their respective alkyltrimethylammonium bro-mide counterparts. The role of water in ionic liquids is com-plex and depends on the supramolecular structures of ionic liquids.10–14 Researchers have demonstrated that small amounts of water in fluorinated ionic liquids, such as 1-butyl-3-methylimidazolium hexafluorophosphate 共关bmim兴 ⫻关PF6兴兲 and tetrafluoroborate 共关bmim兴关BF4兴兲, have a
dra-matic effect on the rate of diffusion.15–17 Measurements of the solvation responses and microscopic solvent properties have resulted in the accumulation of a sizable database on solvation dynamics in ionic liquids.18–21Increasing attention has been devoted to conducting biocatalytic transformation in ionic liquids.22,23The addition of water to关bmim兴关BF4兴 is
very common in biocatalytic work and this is one of the motivations for the current work.22–24 Ionic liquids contain-ing dissolved water may not be regarded as homogeneous solvents but have to be considered as nanostructured materials.25–28Based on the theoretical results of Wang and Voth, at high ionic liquid concentrations ionic liquids seem to form clusters, as in the pure state, and water molecules in-teract with the clusters without interacting among themselves.25 Nevertheless, direct experimental evidence at the molecular level is not easy to obtain to corroborate these simulation results. Previous studies to the structure of ionic
a兲Author to whom correspondence should be addressed. Electronic mail: hcchang@mail.ndhu.edu.tw. Fax: ⫹886-3-8633570. Tel.:⫹886-3-8633585.
0021-9606/2008/129共4兲/044506/6/$23.00 129, 044506-1 © 2008 American Institute of Physics This article is copyrighted as indicated in the article. Reuse of AIP content is subject to the terms at: http://scitation.aip.org/termsconditions. Downloaded to IP:
liquids have included the use of x-ray crystallography and vibrational spectroscopy under the condition of ambient pressure.1–4Nevertheless, only little has been known for liq-uid structures of ionic liqliq-uids. Although the results of crystal structures are highly informative on the relative geometry changes, crystallography does not provide direct information on the local structure in the liquid state. Over the last years the interest in pressure as an experimental variable has been growing in physicochemical studies.29–32The study of pres-sure effects reveals the intermolecular interactions of the ionic liquid at a constant kinetic energy or temperature. In this study, we use variable pressure as a window into self-assembly behaviors in 关bmim兴关BF4兴/water and 关bmim兴 ⫻关BF4兴/methanol, respectively.
Several studies have shown that C–H---O and C–H---X hydrogen bonds play an important role in the structure of ionic liquids, especially those derived from the 1-alkyl-3-methylimidazolium cation.3,29–32It is assumed that the struc-ture of these ionic liquids should be considered as networks of anions and cations, linked together by hydrogen bonds. Nevertheless, direct structural information of these materials in the liquid phase or in solution is difficult to obtain. One of the intriguing aspects of weak hydrogen bonds is that the C–H covalent bond tends to shorten as a result of formation of weak hydrogen bonds. Associated with this contraction is a shift of the C–H stretching frequency to the blue.33–35 De-spite the rather large number of papers devoted to the phe-nomenon of blueshifting hydrogen bonds, the mechanism by which C–H bonds are strengthened by C–H---O or C–H---X interactions is still the subject of debate; two schools of thought have emerged to try to explain its physical basis. Hobza and Havlas33 suggested the strengthened C–H bond originates from a new mechanism called antihydrogen bond-ing in which a secondary effect or structural reorganization of the proton donor framework occurs upon contraction of the C–H bond directly involved in weak hydrogen bonding. However, Gu et al.34 and Masunov et al.35 concluded that there are no fundamental differences between redshifting and blueshifting hydrogen bonding and explain the differences solely on the basis of a combination of electrostatic, polar-ization, charge transfer, dispersion, and steric repulsion forces between the proton donor and acceptor.
The effects of high pressure on intermolecular interac-tions have been the subjects of extensive studies since Bridg-man first examine the phase diagram of water.36,37The many ways in which the water molecules may link through hydro-gen bonding give a remarkably rich phase diagram having more than ten reported phases.36,37 At room temperature, only three phases, i.e., liquid water, ice VI, and ice VII, exist up to at least 38 GPa. The high density of ices VI and VII is due to two interpenetrated, but unconnected, zeolitelike and diamond-type sublattices for ices VI and VII, respectively.36,37The use of pressure allows one to change, in a controlled way, the intermolecular interactions without the major perturbations produced by changes in temperature and chemical composition. For the pure solvent, it is well known that the hydrogen-bond network is distorted by pressure. For the solution, on the other hand, it is still to be investigated how the hydration shell responds to pressure variation. In the
Results and Discussion section, we show that high pressure is a sensitive method to probe the molecular organization in 关bmim兴关BF4兴/water or methanol.
EXPERIMENTAL SECTION
Samples were prepared using
1-butyl-3-methylimidazolium tetrafluoroborate共99.8% by HPLC, LOT 1225502, Fluka兲, D2O 共99.9%, Aldrich兲, H2O 共for
chroma-tography, Merck兲, methanol 共99.9%, Merck兲, and methanol-d4共99.8% D, Cambridge Isotope兲. A diamond an-vil cell 共DAC兲 of Merril-Bassett design, having a diamond culet size of 0.6 mm, was used for generating pressures up to ca. 2 GPa. Two type-IIa diamonds were used for midinfrared measurements. The sample was contained in a 0.3 mm diam-eter hole in a 0.25-mm-thick inconel gasket mounted on the diamond anvil cell. To reduce the absorbance of the samples, CaF2 crystals共prepared from a CaF2 optical window兲 were
placed into the holes and compressed firmly prior to inserting the samples. A droplet of a sample filled the empty space of the entire hole of the gasket in the DAC, which was subse-quently sealed when the opposed anvils were pushed toward one another. Infrared spectra of the samples were measured on a Perkin–Elmer Fourier transform spectrophotometer 共model Spectrum RXI兲 equipped with a lithium tantalite midinfrared detector. The infrared beam was condensed through a 5X beam condenser onto the sample in the dia-mond anvil cell. Typically, we chose a resolution of 4 cm−1 共data point resolution of 2 cm−1兲. For each spectrum,
typi-cally 1000 scans were compiled. To remove the absorption of the diamond anvils, the absorption spectra of DAC were measured first and subtracted from those of the samples. Pressure calibration follows the method of Moffatt and co-workers38,39 The pressure dependence of screw moving distances was measured. Spectra of samples measured at am-bient pressure were taken by filling the samples in a cell having two CaF2windows but lacking the spacers.
RESULTS AND DISCUSSION
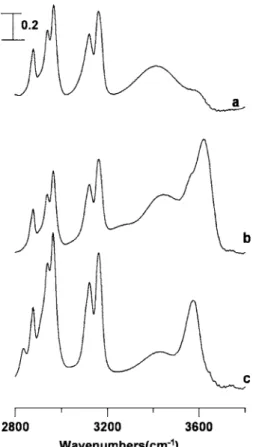
Figure 1 displays infrared spectra of 关bmim兴 ⫻关BF4兴/D2O 共curve a兲, 关bmim兴关BF4兴/H2O 共curve b兲, and
关bmim兴关BF4兴/methanol 共curve c兲 having mole fraction of
关bmim兴关BF4兴 equal to 共a兲 0.059, 共b兲 0.41, and 共c兲 0.53
ob-tained under ambient pressure in the region of C–H and O–H stretching vibrations. The C–H stretching of 关bmim兴关BF4兴
overlaps with the O–H stretching bands of pure H2O, so we measured the infrared spectrum in a solution of D2O, rather than H2O, in Fig.1共a兲. As indicated in Fig.1共a兲, the infrared
spectrum exhibits three discernible peaks, i.e., 2878, 2943, 2967 cm−1, respectively, in the 2800– 3000 cm−1 region
cor-responding to C–H stretching modes of the alkyl groups. The 3122 and 3162 cm−1bands can be attributed to coupled
imi-dazolium C–H stretching vibrations and the stretching modes of OH due to the formation of HOD appear at ca. 3414 and 3557 cm−1 in Fig. 1共a兲. The broad band at ca. 3414 cm−1
could be attributed to hydrogen-bond network, i.e., bonded O–H. The weak shoulder at ca. 3557 cm−1 indicates that at least two different types of HOD species were observed in Fig. 1共a兲 and the 3557 cm−1 band can be assigned as free
044506-2 Chang et al. J. Chem. Phys. 129, 044506共2008兲
O–H or free O–H interacting with anions.8,40,41 As more 关bmim兴关BF4兴 was added, increasing the mole fraction from
curve a to curve b, the relative absorption intensity of free O–H increases. As revealed in Fig.1共b兲, the free O–H modes are located at ca. 3580 and 3617 cm−1, respectively, and the
bonded O–H absorption is located at ca. 3453 cm−1. The IR absorption spectrum of 关bmim兴关BF4兴/methanol is shown in
Fig.1共c兲and we can observe the bonded O–H and free O–H at ca. 3440 and 3570 cm−1, respectively.
To obtain a direct comparison of the effects of varying the composition, Fig.2shows the concentration dependence of alkyl关Fig.2共a兲兴 and imidazolium 关Fig.2共b兲兴 C–H stretch-ing vibrations at ambient pressure for 关bmim兴关BF4兴/D2O or
methanol-d4mixtures. Looking into more detail in Fig.2共a兲,
we observe no drastic change in the concentration depen-dence of the alkyl C–H band frequency at high concentra-tions of ionic liquids, that is 0.3⬍mole fraction 共ionic liq-uid兲. This behavior may indicate a clustering of ionic liquids in nonpolar regions and a slight perturbation by the presence of D2O or methanol-d4at high concentration. The
aggrega-tion of ionic liquids in soluaggrega-tion and even in the gas phase is apparently a general trend.42,43 We stress that the alkyl C–H stretching absorption exhibits an increase in frequency upon dilution at low concentration of ionic liquids, that is 0.1 ⬎mole fraction 共ionic liquid兲, in Fig.2共a兲.8This observation suggests the formation of a certain water structure around alkyl C–H groups of ionic liquids in water-rich mixtures, but the details remain unclear. It is interesting to note that the imidazolium C–H band at ca. 3162 cm−1displays anomalous
concentration-induced frequency shifts in Fig.2共b兲. We no-ticed that no appreciable changes in band frequency of the imidazolium C–H occurred as the ionic liquid was diluted by D2O,8but a redshift in frequency was observed as the sample was diluted by methanol-d4 in Fig. 2共b兲. This result is
re-markably different from what is revealed for the alkyl groups in Fig. 2共a兲. As the BF4−anion should be strongly bound to the imidazolium cation for pure 关bmim兴关BF4兴, one can
sug-gest that they are still attached even when the ionic liquid is diluted by D2O. Nevertheless, methanol facilitates the
disso-ciation of anion and cation. A possible explanation for this effect is the C–H¯O interactions between imidazolium C–H groups and methanol molecules. In other words, methanol can be added to change the structural organization of 关bmim兴关BF4兴 by introducing methanol-imidazolium C–H
in-teractions. Recent investigations have suggested that the structure of ionic liquids exhibits spatial heterogeneity that results from their polar/nonpolar phase separation.25–28Ionic liquids tend to segregate into stable nonpolar regions by ag-gregation of the alkyl groups for C4 and longer and polar regions by charge ordering of the anions and the imidazo-lium rings. Our results in Fig.2共b兲indicate that the presence of methanol significantly perturbs the ionic liquid–ionic liq-uid associations in the polar region.
Figure 3 displays IR spectra of a 关bmim兴关BF4兴/D2O
mixture having its mole fraction of ionic liquid equal to 0.08 obtained under ambient pressure共curve a兲 and at 0.9 共curve b兲, 1.5 共curve c兲, 2.1 共curve d兲, 2.5 共curve e兲, and 3.0 GPa 共curve f兲. As the mixture was compressed, i.e., increasing the pressure from ambient 关Fig. 3共a兲兴 to 0.9 GPa 关Fig. 3共b兲兴, alkyl and imidazolium C–H stretches showed blue frequency shifts, which correspond to contraction of the C–H bonds. Nevertheless, the redshift of the bonded O–H band at ca. 3328 cm−1 is obvious as the pressure was elevated in Fig.
3共b兲. This behavior is in accord with the general trend ob-served of a redshift with pressure for O–H and CvO stretching modes in strongly hydrogen-bonded O–H¯O and CvO¯H systems, respectively.36,37As shown in Fig.3共b兲, FIG. 1. IR spectra of 关bmim兴关BF4兴/D2O 共curve a兲, 关bmim兴关BF4兴/H2O
共curve b兲, and 关bmim兴关BF4兴/methanol 共curve c兲 having mole fraction of ionic liquid equal to 0.059, 0.41, and 0.53, respectively.
FIG. 2. Concentration dependence of the C–H stretching frequency of 关bmim兴关BF4兴/D2O共square兲 or methanol-d4共diamond兲 mixtures.
the compression leads to loss of the free O–H 共at ca. 3580 cm−1兲 band intensity. It is likely that free OH is switched to bonded O–H as high pressures are applied. The evolutions of the free O–H spectral features observed in Fig. 3 may arise from changes in the local structures of O–H groups and the geometrical properties of hydrogen-bond net-work are likely perturbed as the pressure is elevated. As pres-sure was further increased, the hydrogen-bond network trans-formed to tetragonal ice VI 关Fig. 3共c兲兴 and cubic ice VII 关Figs.3共d兲–3共f兲兴. We observed a sharp O–H feature in Figs. 3共d兲–3共f兲corresponding to the high order environments in an ice VII-like structure. We note that infrared spectra for the tetragonal VI and cubic VII phases of ice were reported in Ref.37.
Figure 4 displays IR spectra of a 关bmim兴关BF4兴/H2O
mixture having its mole fraction of ionic liquid equal to 0.48 obtained under ambient pressure共curve a兲 and at 0.3 共curve b兲, 1.5 共curve c兲, 1.9 共curve d兲, and 2.3 GPa 共curve e兲. As revealed in Fig. 4共a兲, the dominant O–H species is the free OH type. Comparing Figs.4共a兲–4共e兲, we note that the inten-sity ratio of the bonded OH and free O–H differs as the pressure is elevated. Similar observation is also observed for the water-rich mixture in Fig. 3. It appears that pressure somehow stabilizes the bonded-OH conformation. The bonded O–H band appears as a broad feature in Figs. 4共a兲–4共c兲. The sharper structure of bonded O–H band at ca. 3360 cm−1 revealed in Figs. 4共d兲 and4共e兲is in part due to the higher order and anisotropic environment in a solid
struc-ture. By comparing Figs.3共d兲–3共f兲 and Figs.4共d兲 and 4共e兲, an increase in the concentration of关bmim兴关BF4兴 does affect the bandwidths of bonded O–H stretching bands under high pressure, increasing versus the concentration. The sensitivity to changes in concentration may arise from changes in water-cluster sizes and perturbation in geometrical properties of hydrogen-bond network. In other words, hydrogen-bond net-work of high pressure ice VII breaks down as the ionic liquid is further added.
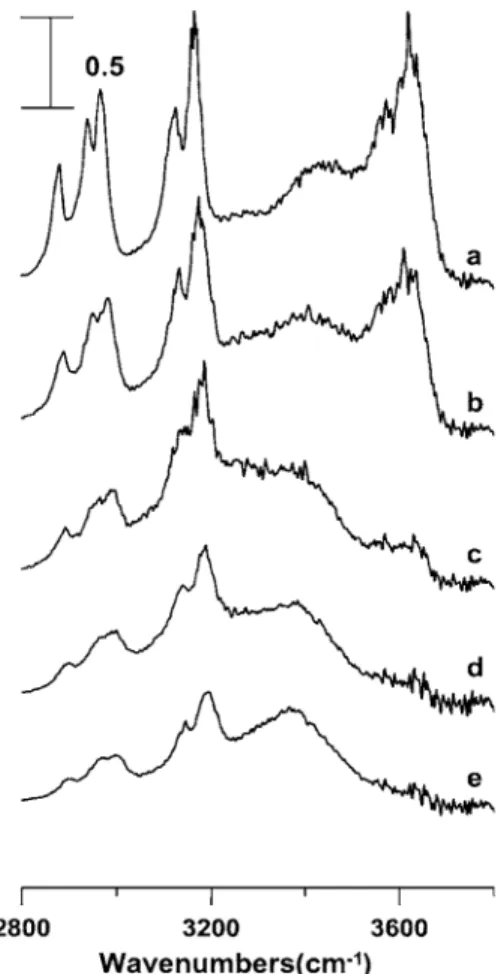
Figure5displays IR spectra of a关bmim兴关BF4兴/methanol mixture having its mole fraction of ionic liquid equal to 0.62 obtained under ambient pressure共curve a兲 and at 0.9 共curve b兲, 1.5 共curve c兲, 1.9 共curve d兲, and 2.5 GPa 共curve e兲. The bonded O–H band appears as a broad feature and the absorp-tion band at 3575 cm−1 is assigned to free O–H stretching
vibration in Fig.5共a兲. The prominent O–H species is the free O–H as shown in Fig.5共a兲. As the sample was compressed 关Figs.5共b兲–5共e兲兴, we observed blueshifts in frequency for the C–H stretching modes in Fig.5. Nevertheless, the free O–H stretching band did not change its position with pressure. It is interesting to note that the bonded O–H feature remains broad as the pressure was elevated. The broad feature of the bonded O–H represents inhomogeneous environment due to glasslike network and is correlated with the more disordered structure of methanol. The presence of 3575 cm−1 band in Figs.5共b兲–5共e兲indicates that the free O–H is still stable un-der high pressure. This observation is remarkably different FIG. 3. Pressure dependence of the C–H and O–H modes in a 关bmim兴
⫻关BF4兴/D2O mixture having its mole fraction of ionic liquid equal to 0.08 obtained under ambient pressure共curve a兲 and at 0.9 共curve b兲, 1.5 共curve c兲, 2.1共curve d兲, 2.5 共curve e兲, and 3.0 GPa 共curve f兲.
FIG. 4. Pressure dependence of the C–H and O–H modes in a 关bmim兴 ⫻关BF4兴/H2O mixture having its mole fraction of ionic liquid equal to 0.48 obtained under ambient pressure共curve a兲 and at 0.3 共curve b兲, 1.5 共curve c兲, 1.9共curve d兲, and 2.3 GPa 共curve e兲.
044506-4 Chang et al. J. Chem. Phys. 129, 044506共2008兲
from the results of ionic liquid/water mixtures, i.e., Figs. 3 and4. Methanol is one of the simplest amphiphilelike mol-ecules. The shortness of its alkyl chain means that methanol does not form conventional self-assembly behavior. Pure wa-ter forms three-dimensional hydrogen-bonding structures, but molecules in pure methanol associate with each other to form short chains with an average chain length of five or so molecules.44Both hydrogen-bond cooperative45and geomet-ric effect may be attributed to the unique behavior of added methanol observed in Fig.5.
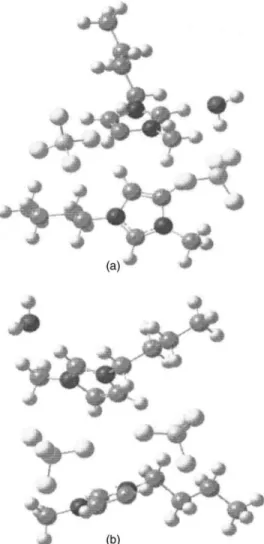
We performed density functional theory共DFT兲 calcula-tions using theGAUSSIANprogram package;46Fig.6displays the DFT-calculated structures of 共关bmim兴关BF4兴兲2共water兲
complexes. We employed the B3LYP functional together with a standard 6-31+ G* basis set. This combination of method and basis set 共B3LYP/6-31+G*兲 has been
success-fully applied to protonated ammonia-water clusters47 and neutral benzene-water clusters48before. We thus expect it to describe comparably well the interactions in ionic liquid-water clusters. Table Idisplays the predicted O–H and imi-dazolium C–H stretching frequencies of 共关bmim兴 ⫻关BF4兴兲2共water兲 complexes and the scaling factor for the
calculated frequencies is 0.955.29–32 Figure 6共a兲 displays bonded and free O–H stretching vibrations located at 3426 and 3649 cm−1, respectively. As the clusters increase in size,
the number of low-lying isomers increases exponentially and the structural identification is complicated. Thus, the broad O–H features observed in Figs.3and4may be attributed to
the presence of more than one stable isomeric form. Figure 6共b兲exhibits two discernible O–H vibrations, i.e., 3567 and 3677 cm−1, respectively, corresponding to coupled free-O–H
stretching bands. This result agrees with the splitting of the free O–H bands revealed in Figs.1共b兲and4共a兲. As revealed in TableI, there is strong correlation between the experimen-tal results and the structures from theGAUSSIANcalculations. It is instructive to note that DFT calculations may only pro-vide qualitative support for the suggested intermolecular in-teractions, because the calculations are based on gas phase structures. It is also known that hydrogen-bond cooperativity due to concerted charge transfer can greatly enhance the strength of the individual hydrogen bonds involved in the coupling. The calculations of larger clusters may be interest-ing, but structural identification is complicated by the pres-ence of exponentially increased isomeric forms.
CONCLUSIONS
We demonstrate that the aggregation behaviors and hydrogen-bond network in 关bmim兴关BF4兴/water or methanol
mixtures can be studied using a combination of vibrational spectroscopy and high pressure techniques. The results indi-cate a slight perturbation of ionic liquid structures in nonpo-lar regions by the presence of water or methanol at high concentrations. The formation of a certain water structure FIG. 5. IR spectra of a 关bmim兴关BF4兴/methanol mixture having its mole
fraction of ionic liquid equal to 0.62 obtained under ambient pressure共curve a兲 and at 0.9 共curve b兲, 1.5 共curve c兲, 1.9 共curve d兲, and 2.5 GPa 共curve e兲.
FIG. 6. DFT-calculated structures of共关bmim兴关BF4兴兲2共water兲 complexes.
around alkyl C–H groups was observed in water-rich mix-tures. Nevertheless, methanol can be added to change the structural organization of the ionic liquid by introducing
methanol-imidazolium C–H interactions. For
关bmim兴关BF4兴/water mixtures, the intensity ratio of the
bonded O–H and free O–H differs as the pressure is elevated. In other words, free-O–H is switched to bonded O–H as high pressures are applied. However, the free O–H is still stable under high pressures for the methanol mixtures.
ACKNOWLEDGMENTS
The authors thank the National Dong Hwa University and the National Science Council of Taiwan 共Contract No. NSC 95-2113-M-259-013-MY3兲 for financial support. The authors also thank Wei-Ru Pan for her assistance.
1Green Industrial Applications of Ionic Liquids, NATO Science Series, edited by R. D. Rogers, K. R. Seddon, and S. Volkov共Kluwer, Dordrecht, 2002兲.
2Ionic Liquids in Synthesis, edited by P. Wasserscheid and T. Welton 共Wiley VCH, Weinheim, 2002兲.
3H. Weingartner,Angew. Chem., Int. Ed. 47, 654共2008兲.
4S. Kossmann, J. Thar, B. Kirchner, P. A. Hunt, and T. Welton,J. Chem. Phys. 124, 174506共2006兲.
5A. Wulf, K. Fumino, D. Michalik, and R. Ludwig,ChemPhysChem 8, 2265共2007兲.
6K. S. Mali, G. B. Dutt, and T. Mukherjee,J. Chem. Phys. 128, 054504 共2008兲.
7Y. Umebayashi, T. Mitsugi, S. Fukuda, T. Fujimori, K. Fujii, R. Kanzaki, M. Takeuchi, and S. Ishiguro, J. Phys. Chem. B 111, 13028共2007兲. 8Y. Jeon, J. Sung, D. Kim, C. Seo, H. Cheong, Y. Ouchi, R. Ozawa, and H.
Hamaguchi, J. Phys. Chem. B 112, 923共2008兲.
9G. A. Baker, S. Pandey, and S. N. Baker,Analyst 共Cambridge, U.K.兲
129, 890共2004兲.
10K. Miki, P. Westh, K. Nishikawa, and Y. Koga, J. Phys. Chem. B 109, 9014共2005兲.
11S. Rivera-Ruberd and S. Baldelli, J. Phys. Chem. B 110, 15499共2006兲.
12C. Schroder, T. Rudas, G. Neumayr, S. Benkner, and O. Steinhauser,J. Chem. Phys. 127, 234503共2007兲.
13K. Behera, M. D. Pandey, M. Porel, and S. Pandey,J. Chem. Phys.127, 184501共2007兲.
14A. Dominguez-Vidal, N. Kaun, M. J. Ayora-Canada, and B. Lendl, J. Phys. Chem. B 111, 4446共2007兲.
15U. Schroder, J. D. Wadhawan, R. G. Compton, F. Marken, P. A. Z. Suarez, C. S. Consorti, R. F. de Souza, and J. Dopont,New J. Chem.24,
1009共2000兲.
16J. H. Werner, S. N. Baker, and G. A. Baker, Analyst共Cambridge, U.K.兲
128, 786共2003兲.
17M. Kanakubo, T. Umecky, T. Aizawa, and Y. Kurata, Chem. Lett. 2005, 324.
18S. Arzhantsev, H. Jin, G. A. Baker, and M. Maroncelli,J. Phys. Chem. B
111, 4978共2007兲.
19S. N. Baker, G. A. Baker, and F. V. Bright,Green Chem.4, 165共2002兲. 20D. Seth, S. Sarkar, and N. Sarkar, J. Phys. Chem. B 112, 2629共2008兲. 21A. Sarkar and S. Pandey, J. Chem. Eng. Data 51, 2051共2006兲. 22R. A. Sheldon, R. M. Lau, M. J. Sorgedrager, F. van Rantwijk, and K. R.
Seddon,Green Chem. 4, 147共2002兲.
23R. A. Sheldon, Green Chem. 7, 267共2005兲.
24M. H. Katsoura, A. C. Polydera, P. Katapodis, F. N. Kolisis, and H. Stamatis, Process Biochem.共Oxford, U.K.兲 42, 1326 共2007兲.
25Y. Wang and G. A. Voth,J. Phys. Chem. B 110, 18601共2006兲. 26D. Xiao, J. R. Rajian, A. Cady, S. Li, R. A. Bartsch, and E. L. Quitevis,
J. Phys. Chem. B 111, 4669共2007兲.
27A. Triolo, O. Russina, H. Bleif, and E. Di Cola,J. Phys. Chem. B 111, 4641共2007兲.
28J. Dupont,J. Braz. Chem. Soc. 15, 341共2004兲.
29H. C. Chang, J. C. Jiang, J. C. Su, C. Y. Chang, and S. H. Lin, J. Phys. Chem. A 111, 9201共2007兲.
30H. C. Chang, J. C. Jiang, W. C. Tsai, G. C. Chen, and S. H. Lin, J. Phys. Chem. B 110, 3302共2006兲.
31K. M. Lee, H. C. Chang, J. C. Jiang, L. C. Lu, C. J. Hsiao, Y. T. Lee, S. H. Lin, and I. J. B. Lin,J. Chem. Phys. 120, 8645共2004兲.
32H. C. Chang, J. C. Jiang, W. C. Tsai, G. C. Chen, and S. H. Lin, Chem. Phys. Lett. 427, 310共2006兲.
33P. Hobza and Z. Havlas, Chem. Rev. 共Washington, D.C.兲 100, 4253 共2000兲.
34Y. L. Gu, T. Kar, and S. Scheiner,J. Am. Chem. Soc. 121, 9411共1999兲. 35A. Masunov, J. J. Dannenberg, and R. H. Contreras,J. Phys. Chem. A
105, 4737共2001兲.
36F. Frank, Water, A Comprehensive Treatise共Plenum, London, 1972兲, Vol. 1, p. 464.
37H. C. Chang, K. H. Huang, Y. L. Yeh, and S. H. Lin,Chem. Phys. Lett.
326, 93共2000兲.
38P. T. T. Wong, D. J. Moffatt, and F. L. Baudais,Appl. Spectrosc. 39, 733 共1985兲.
39P. T. T. Wong and D. J. Moffatt,Appl. Spectrosc. 41, 1070共1987兲. 40M. Lopez-Pastor, M. J. Ayora-Canada, M. Valcarcel, and B. Lendl, J.
Phys. Chem. B 110, 10896共2006兲.
41L. Cammarata, S. G. Kazarian, P. A. Salter, and T. Welton,Phys. Chem. Chem. Phys. 3, 5192共2001兲.
42C. S. Consorti, P. A. Z. Suarez, R. F. de Souza, R. A. Burrow, D. H. Farrar, A. J. Lough, W. Loh, L. H. M. da Silva, and J. Dupont, J. Phys. Chem. B 109, 4341共2005兲.
43F. C. Gozzo, L. S. Santos, R. Augusti, C. S. Consorti, J. Dupont, and M. N. Eberlin,Chem.-Eur. J. 10, 6187共2004兲.
44S. Dixit, W. C. K. Poon, and J. Crain,J. Phys.: Condens. Matter12, L323 共2000兲.
45H. C. Chang, J. C. Jiang, W. W. Lai, J. S. Lin, G. C. Chen, W. C. Tsai, and S. H. Lin, J. Phys. Chem. B 109, 23103共2005兲.
46M. J. Frisch, G. W. Trucks, H. B. Schlegel et al.,
GAUSSIAN 03, Revision A. 7, Gaussian, Inc., Pittsburg, PA, 2003.
47J. C. Jiang, H. C. Chang, Y. T. Lee, and S. H. Lin,J. Phys. Chem. A103, 3123共1999兲.
48C. J. Gruenloh, J. R. Carney, C. A. Arrington, T. S. Zwier, S. Y. Freder-icks, and K. D. Jordan,Science 276, 1678共1997兲.
TABLE I. DFT-calculated O–H and imidazolium C–H stretching frequen-cies共cm−1兲 and intensity 共km/mol兲 of 共关bmim兴关BF
4兴兲2共water兲 complexes.a,b Speciesa Calc. frequencies共intensities兲b Assignment
A 3075共193兲 C2– H 3116共147兲 C4,5– H asym. 3136共85兲 C4,5– H asym. 3145共106兲 C4,5– H sym. 3159共4兲 C2– H 3162共6兲 C4,5– H sym. 3426共407兲 O–H共bonded to BF4−兲 3649共99兲 O–H共free兲 B 3114共207兲 C2– H 3122共69兲 C4,5– H asym. 3127共142兲 C4,5– H asym. 3140共86兲 C4,5– H sym. 3156共81兲 C2– H 3162共2兲 C4,5– H sym. 3567共18兲 O–H sym.共free兲 3677共81兲 O–H asym共free兲 aStructures illustrated in Fig.6.
bFrequencies scaled by 0.955.
044506-6 Chang et al. J. Chem. Phys. 129, 044506共2008兲