Notes on the carabidicolous Laboulbeniales (Ascomycetes) of
Taiwan I
Katsuyuki Terada*, Meng-Hao Hsu, and Wen-Jer Wu
Department of Entomology, National Taiwan University, No. 1, Roosevelt Road, Section 4, Taipei 106, Taiwan
(Received April 14, 2003; Accepted September 10, 2003)
Abstract. One species of Peyritschiella and ten species of Laboulbenia, all belonging to the Laboulbeniales, are newly recorded from Taiwan. Twelve species of the Carabidae are recorded as new hosts of the Taiwanese Laboulbeniales. Photographs are included for Peyritschiella clivinae, Laboulbenia timurensis, L. agoni, L. separata, and L. nocturna.
Keywords: Carabidae; Laboulbenia; Laboulbeniales; New records; Peyritschiella; Taiwan.
Introduction
The order Laboulbeniales is a distinct group of small ascomycetous fungi. They are ectoparasites of living arthropods, mainly insects, and sometimes occur on mites and millipedes. They grow on the integument of their hosts and take nourishment from the host body. In many genera, haustoria have been observed, but in others, evi-dence of haustoria has not been demonstrated (see Tavares, 1985, pp. 13, 34). Nevertheless, most Laboulbeniales seem to have no serious detrimental effect on the normal life of their hosts. (For pathogenicity of the Laboulbeniales, see Benjamin, 1971.)
The first report on the Laboulbeniales from Taiwan was published by Terada in 1976, and this was followed by sev-enteen others published by Benjamin (2001), Huldén (1985), Juan and Chien (1994, 1995, 1996), Lee and Sugiyama (1984), Sugiyama (1978a, b, c, 1981, 1982a, b), Sugiyama and Hayama (1981), Sugiyama and Shazawa (1977), and Terada (1978, 1981, 1995). According to these documents, the Taiwanese Laboulbeniales comprise 75 species, 24 genera, and 3 families. Their hosts range over seven orders of insects: Blattaria, Coleoptera, Dermaptera, Diptera, Hemiptera, Hymenoptera, and Orthoptera.
The Carabidae is one of the largest families of the Coleoptera. It is distributed almost all over the world in varied habitats. (For general information on the Carabidae, see Ball and Busquet, 2001.) More than 300 taxa of the Laboulbeniales have been described from various species of the Carabidae worldwide. In Taiwan, 34 species in 24 genera of the Carabidae have been recorded as the hosts of Laboulbeniales, but most of the host insects were iden-tified only to the generic level.
*Corresponding author. Present address: Omiya 1-2-20-203, Nishi-Ku, Hiroshima 733-0007, Japan. Tel: 0822388205; E-mail: terada@hjs.ed.jp
The first author (K. Terada) has studied carabid beetles as the hosts of Laboulbeniales for many years. Recently he had a chance to devote himself to the study of the para-sitic fungi and their host insects at the Department of Entomology, National Taiwan University (NTU) for one year. During his stay in Taiwan, the present authors con-centrated on collecting carabid beetles and through exten-sive field work finally obtained about 3,000 specimens. As checking of the collected specimens continues, the authors have found an increasing number of fungi on these host carabids. These valuable specimens contribute towards elucidation of both insect fauna and fungus flora of Taiwan.
The present work comprises two parts: in part I, one species of Peyritschiella and ten species of Laboulbenia are recorded as new for the Taiwanese fungus flora; and in part II, twenty species in eight genera of the Carabidae are added to the host list for the Taiwanese Laboulbeniales, and thirteen species of Laboulbeniales in three genera are also reported. Moreover, the ongoing description of other specimens by the authors will appear in future papers.
Materials and Methods
Insect specimens for the present study are mostly from the collection made by Terada and Hsu during the period from April 2001 to March 2002. Also studied were dried specimens from the NTU collection and Dr. Kurosa’s collection, and specimens collected by Terada in 1977. Fungus-bearing host specimens were put in 70% ethanol and kept in small glass bottles. Dried specimens were wrapped in paper and kept in specimen boxes. After fungi were removed from the insect body, preparations were made following the methods introduced by Benjamin (1971). Morphological terms and abbreviations are basi-cally the same as those used by Tavares (1985). All speci-mens have been deposited in the NTU and in the first author’s laboratory.
In the following paragraphs, the thallus length was mea-sured from the foot base to the perithecial tip; and the length of the perithecium was from the base of the basal cells to the perithecial tip excluding apical outgrowths. The stalk and secondary stalk cells (VI-VII) were excluded from the height of the perithecium. For determining the color of the thallus, each preparation was put on a white board and examined by using a dissecting microscope with inci-dent light. However, it should be noted that the color of the thallus can vary, according to the light intensity or other conditions of the microscope.
List of Species
Peyritschiella clivinae Thaxter, Mem. Amer. Acad. Arts Sci. 16: 18. 1931. —Type: R. Thaxter-1672, on Clivina impressifrons LeConte, Kansas, USA. (Figures 1-3) Specimens examined. On Clivina vulgivaga Boheman [Clivinini]: Yangmei, Taoyuan County, Aug. 22, Oct. 3, & Oct. 14 2001, leg. K. Terada & M.H. Hsu, K. Terada1523, -1525, & -1599.
Measurements. Thallus ca. 300 µm long; perithecium ca. 100×30 µm; appendage 60-100 µm long; compound an-theridium ca. 22.5 × 12.5 µm.
Note. Taiwanese specimens agree with Thaxter’s de-scription of Peyritschiella clivinae except that the mature perithecium is more symmetrical in shape than that in Thaxter’s illustrations (Thaxter, 1931, pl. III, figs. 8-9). The
receptacle is pale dull brownish yellow; the perithecium becomes pale brown; and the appendages remain colorless. A compound antheridium lies on one side of the middle tier of the receptacle (Figure 3). It is inflated and without a free neck portion; many indistinct minute antheridial cells were observed inside. These specimens were found on the elytra of the host body.
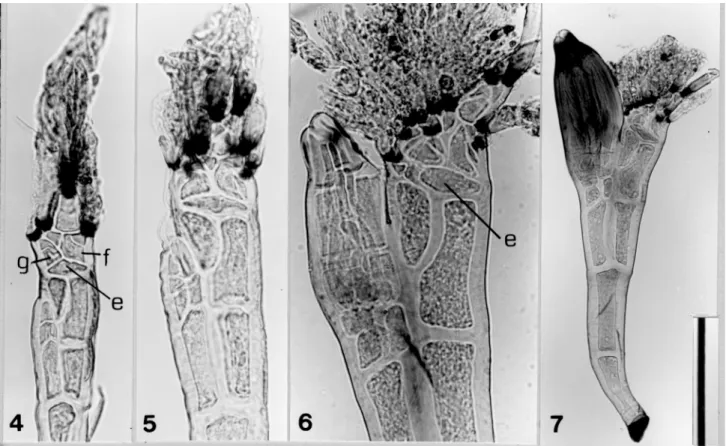
Laboulbenia timurensis Majewski et Sugiyama, Trans. Mycol. Soc. Japan 27: 436. 1986. —Type: K. Sugiyama-3197, on Clivina sp. (ephippiata group), Timur, Borneo. (Figures 4-7) Specimens examined. On Clivina yanoi Kult [Clivinini]: Kukuan, Taichung County, Jun. 10 & 11 1977, leg. K. Terada, K, Terada-660 & -785; Chihnankung, Taipei City, Jul. 8 2001, leg. K. Terada, K. Terada-1555.
Measurements. Thallus 340-370 µm long; perithecium 120-140×45-50 µm.
Note. Taiwanese specimens agree with the original fig-ures of Laboulbenia timurensis published by Majewski and Sugiyama (1986, figs. 3 & 22). However, their original description, in which the insertion cell was described as “subdivided into 20-30 small cells,” should be corrected because the division seems to be in the appendage above the insertion cell. In the present study, the authors ob-served an undivided, lens-shaped insertion cell (e) in the Taiwanese specimens (Figures 4-5). However, the inser-tion cell sometimes cuts off one or two small cells from
Figures 1-3. Peyritschiella clivinae removed from elytra of Clivina vulgivaga. (1) mature thallus. K. Terada-1599; (2) submature thallus. K. Terada-1599; (3) young thallus with a compound antheridium (arrow). K. Terada-1523. Scale bars: 1-2 =100 µm; 3 = 50 µm.
the inner side and also from the outer side (Figure 6). Complicated subdivisions occur in the appendage base just above the insertion cell to form a compound mass of small cells from which short appendages arise (Figure 6). Not every branch of the appendages is clearly distinguished in the photograph, but some antheridial branchlets might be formed in such conglomerate appendages (Figure 6). Figure 4 shows a thallus at a very early stage of development, in which four short appendages are visible, each having a black constriction at its base; the one on the left side is from the basal cell (g) of the inner appendage; the one on the top forms the primary axis; the remaining two are from small cells derived from the basal cell (f) of the outer appendage. The receptacle is pale brownish yellow (“light brown” in the original description), and the mature perithecium is dull yellowish brown (“olivaceous” in the original description). These speci-mens were collected on the elytra of the host body. Laboulbenia agoni Sugiyama, Ginkgoana 2: 41. 1973. —
Type: K. Sugiyama-680, on Agonum sylphis (Bates) complex, Tokyo, Japan. (Figures 8-10) Specimens examined. On Colpoides formosanus Jedlièka [Platynini]: Anmashan, Taichung County, May 27 2001, leg. W.Y. Dai, K. Terada-1546.
Measurements. Thallus 260-270 µm long; perithecium 105-110×35-40 µm; outer appendage up to 800 µm long; antheridia (including stalk cell) ca. 37.5 µm long.
Note. Taiwanese specimens were found on the elytra and the ventral side (sternites) of the host and agree with the original description and figures of Laboulbenia agoni published by Sugiyama (1973, pl. 15, figs. 1-4; pl. 26, fig. 1). However, the following comments may be useful for identification of the species. The receptacle is subhyaline to pale brownish yellow; the perithecium is more or less blackish; the basal cell of the outer appendage usually bears two long simple or divaricate branches, but the branches often increase in number (Figure 10); the basal cell of the inner appendage bears a short one-celled stalk with a tuft of 3-6 antheridia at the apex, and often bears a long simple branch as well (Figure 8).
The host genus Colpoides is allied to Colpodes, from which it is distinguished by the pubescence on the dor-sal surface of the body.
Laboulbenia brachionychi Thaxter, Proc. Amer. Acad. Arts Sci. 35: 162. 1899. —Type: R. Thaxter-99, on Brachionychus sp., China.
Specimens examined. On Peronomerus fumatus Schaum [Panagaeini]: Yangmei, Taoyuan County, Nov. 26 2001, leg. Figures 4-7. Laboulbania timurensis removed from elytra of Clivina yanoi. (4) very young thallus with four blackish septa of appendages, in which the upper one is primary and the lower three are originated from the outer and inner basal cells (f and g) which are located above the lens-shaped insertion cell (e). K. Terada-785; (5) young thallus at a more advanced stage, in which appendages increase in number. K. Terada-785; (6) submature thallus in which one or two small cells are cut off from the inner side of insertion cell (e), also maybe from the outer side. K. Terada-1555; (7) mature thallus. K. Terada-1555. Scale bars: 4-6 = 50 µm; 7 = 100 µm.
K. Terada & M.H. Hsu, K. Terada-1586; Nan-ao, Ilan County, Feb. 13 2002, leg. K. Terada, K. Terada-1547.
Measurements. Thallus 330-420 µm long; perithecium 195-240×37.5-50 µm; outer appendage up to 600 µm long; antheridial branchlet (including antheridia) ca. 65 µm long. Note. Taiwanese specimens agree with Thaxter’s de-scription and illustration of Laboulbenia brachionychi (Thaxter, 1908, pl. LIII, fig. 11). These specimens were col-lected on both ventral and dorsal sides of the host body. Laboulbenia exigua Thaxter var. exigua, Proc. Amer. Acad.
Arts Sci. 38: 37. 1902.—Type: R. Thaxter-923, on Chlaenius micans (Fabricius), Japan.
Specimens examined. On Chlaenius hamifer Chaudoir [Callistini]: Tahu, Taipei City, May 30 2001, leg. K. Terada, K. Terada-1584. On Chlaenius virgulifer Chaudoir
[Callistini]: Sanchih, Taipei County, May 23 2001, leg. K. Terada, K. Terada-1559.
Measurements. Thallus 240-260 µm long; perithecium 95-105 × 25-30 µm.
Note. Taiwanese specimens quite agree with the de-scription and drawing of Laboulbenia exigua published by Thaxter (1908, pl. LIII, fig. 1), and also with a photo-graph published by Terada (1995, fig. 1). These specimens were collected on the inferior surface of the mesothorax of the female host. In Taiwan, L. exigua var. melanolabiata Terada (1995) is also known.
Laboulbenia consobrina Terada, Mycoscience 36: 298. 1995.—Type: K. Terada-413, on Chlaenius inops Chaudoir, Hokkaido, Japan.
Figures 8-10. Laboulbenia agoni removed from abdomen (sternites) of Colpoides formosanus. (8) mature thallus with an anthe-ridial branchlet (arrow). K. Terada-1546; (9) young thallus with an antheanthe-ridial branchlet. K. Terada-1546; (10) young thallus at a more advanced stage, showing one simple and two divaricate branches on the basal cell (f); outermost branch is darkened basally. K. Terada-1546. Scale bars: 8 = 100 µm; 9-10 = 50 µm.
Specimens examined. On Chlaenius inops Chaudoir [Callistini]: Nan-ao, Ilan County, Mar. 4 2002, leg. K. Terada, K. Terada-1553.
Measurements. Thallus ca. 200 µm long; perithecium ca. 85×35 µm; appendage ca. 180 µm long.
Note. Taiwanese specimens compare well with the origi-nal description and a photograph of Laboulbenia consobrina published by Terada (1995, fig. 25). These specimens were collected on the inferior surface of the mesothorax of the female host.
Laboulbenia yamadae Ishikawa ex Terada, Mycoscience 36: 297. 1995. —Type: K. Terada-1157D, on Chlaenius variicornis Morawitz, Hiroshima, Japan.
Specimens examined. On Chlaenius sericimicans Chaudoir [Callistini]: Yangmei, Taoyuan County, Oct. 8 2001, leg. K. Terada & M.H. Hsu, K. Terada-1597.
Measurements. Thallus ca. 180 µm long; perithecium ca. 70×20 µm; appendage ca. 140 µm long.
Note. In describing Laboulbenia yamadae in 1995, Terada presented three distinct morphological forms. Tai-wanese specimens agree with one of those forms (Terada, 1995, fig. 23), although the size is smaller. These speci-mens were collected on the inferior part of the mesotho-rax of the female host.
Laboulbenia manubriolata Thaxter, Proc. Amer. Acad. Arts Sci. 51: 44. 1915.—Type: R. Thaxter-2081d, probably on Perigona sp., Java.
Specimens examined. On Perigona nigriceps (Dejean) [Perigonini]: Dahshih, Taoyuan County, Mar. 10 2002, leg. K. Terada & M.H. Hsu, K. Terada-1549.
Measurements. Thallus ca. 230 µm long; perithecium ca. 80×27.5 µm.
Note. In the original description of Laboulbenia manubriolata, Thaxter (1915) noted that the host was “a small carabid allied to Tachys.” The type specimen was from Samarang, Java, and was numbered as “2081d.” Thaxter (1915) also described Misgomyces ornatus from “a small carabid allied to Tachys” and listed specimens from Samarang, Java, “No. 2081f” and from Ceylon. In 1931, however, he designated the type specimen of M. ornatus as “No. 2081”, Samarang, Java, and at the same time he listed Perigona sp. for the host. Perigona is very similar in appearance to Tachys. Therefore, the type specimen of Laboulbenia manubriolata labeled “2081d” might be also from Perigona sp. Actually, several European authors’ findings of L. manubriolata on Perigona nigriceps sup-port this idea (Rossi, 1982; Huldén, 1983; Santamaria, 1993). Quite recently, Terada (2000) reported L. manubriolata and Dixomyces ornatus (= M. ornatus) on P. nigriceps from Japan. Taiwanese specimens were collected on the elytra of the host and quite agree with the original description of L. manubriolata given by Thaxter (1915) and photo-graphs by Rossi (1982, fig. 2) and also by Terada (2000, figs. 15-16).
Laboulbenia borneensis Thaxter, Proc. Amer. Acad. Arts Sci. 38: 28. 1902. —Type: R. Thaxter-1201, on Thyreopterus (?) sp., Borneo.
Specimens examined. On Dolichoctis (Mochtherus) uenoi Habu [Lebiini]: Hotso, Nantou County, Jul. 9 1975, leg. H. Takizawa, sent from Dr. Kurosa, K. Terada-1519.
Measurements. Thallus ca. 480 µm long; perithecium 175-180×45 µm.
Note. Taiwanese specimens, found on the elytra of the host, quite agree with Thaxter’s description of Laboulbenia borneensis and the drawing of the Borneo specimen (Thaxter, 1908, pl. LV, fig. 15, as L. thyreopteri). In 1908, Thaxter placed L. borneensis in synonymy with L. thyreopteri Thaxter, but Terada (2000) recognized it as a distinct species.
The Taiwanese host, Dolichoctis uenoi is quite similar in appearance to the Japanese host, D. luctuosus Bates, from which this fungus species is also known (Terada, 2000).
Laboulbenia separata Thaxter, Proc. Amer. Acad. Arts Sci. 35: 200. 1899. —Type: R. Thaxter-571, on Pericalus guttatus Chevrolat, Java. (Figure 11)
Specimens examined. On Pericalus formosanus Dupuis [Lebiini]: Wulai, Taipei County, Apr. 26 1988, leg. K.S. Huang, K. Terada-1532.
Figure 11. Laboulbenia separata removed from left elytral mar-gin of Pericalus formosanus. Mature thallus. K. Terada-1532. Scale bar = 100 µm.
Measurements. Thallus 230-270 µm long; perithecium ca. 105-115×32.5-37.5 µm; appendage up to 200 µm long; perithecial projection 27.5-28.5 µm long.
Note. Taiwanese specimens were found on the left elytral margin of the host. These specimens agree with Thaxter’s description of Laboulbenia separata, and the drawings of Java specimens (Thaxter, 1908, pl. LIX, figs. 1-2) except for the color of the thallus. The type speci-mens were described as “pale olivaceous” for the perithecia, as “dull olivaceous” for the receptacles, and as “almost hyaline” for the perithecial projections (Thaxter, 1908), whereas in the Taiwanese specimens, the thallus is dull yellowish brown except for the subhyaline cell I and the pale blackish perithecial outgrowth.
The fungal specimens on Dolichoctis luctuosus re-corded from Japan basically agree with the Taiwanese specimens of L. separata, but are clearly distinguished from the latter fungus by the receptacle with shorter cell II, the wedge-shaped cell VI, and the stouter perithecium (see. Terada, 2000, figs. 4-5).
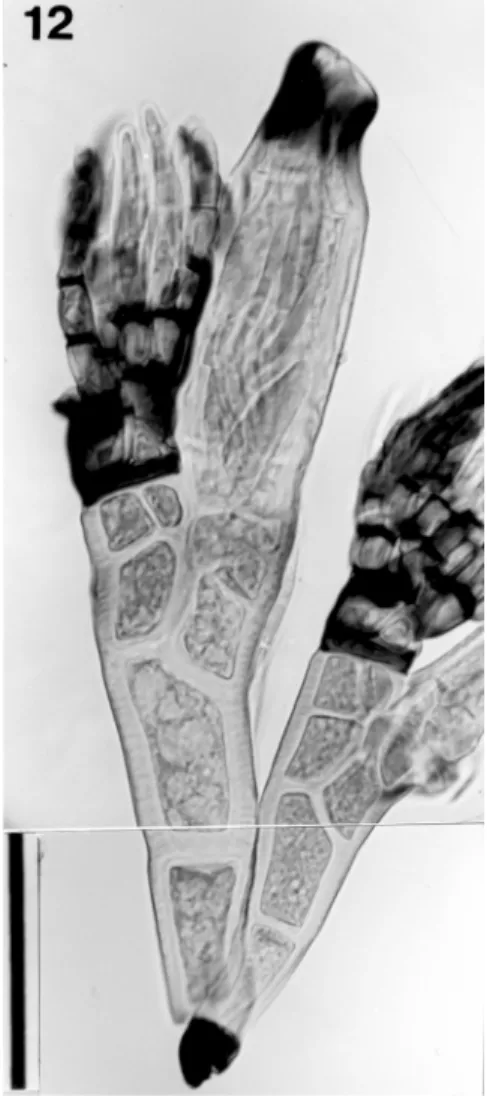
Laboulbenia nocturna W. Rossi, “Accad. Naz. Lincei, Quaderni”, 267: 8. 1994. —Type: W. Rossi-1502FI, on Lebia gabonica Chaudoir, Kambui Hills, Sierra Leone.
(Figure 12)
Specimens examined. On Lebia chiponica Jedlièka [Lebiini]: Kukuan, Taichung County, Jun. 10 1977, leg. K. Terada, K. Terada-636; Shizitou, Nantou County, May 15 1991, leg. J.C. Luo, sent from Dr. Kurosa, K. Terada-1518.
Measurements. Thallus 175-350 µm long; perithecium 92.5-130 × 27.5-55 µm
Note. Taiwanese specimens were found on the elytral apex of the host. These specimens agree with the origi-nal description and a photograph of Laboulbenia nocturna (Rossi, 1994, fig. 5). Color of the thallus is uni-formly pale yellow, but sometimes becomes dark on cells III and IV (however, not clearly shown in Figure 12). The perithecium is cylindrical, with a broad blackish zone at the apex; the appendages have many blackened septa; and in the receptacle cell V is equal to cell IV in height.
The Taiwanese host Lebia chiponica is undoubtedly al-lied to Lebia idae Bates from Japan, from which it is dis-tinguished by the shape of the yellowish patch on each elytral base.
Acknowledgments. The authors thank Dr. I.I. Tavares, Uni-versity of California, Berkeley, for her critical reading of the manuscript and valuable comments. The first author thanks Prof. Y.I. Chu, National Taiwan University, Miss C.M. Chiang and Miss H.C. Lien of the same university, and Prof. K. Yano, Yamaguchi University, for making arrangements for his study in Taiwan, and thanks Prof. C.Y. Chien, National Taiwan Nor-mal University, for his encouragement. The first author also thanks Miss Y.L. Yang, Mr. W.Y. Dai, and Dr. C.C. Ko for col-lecting insects, and Dr. K. Kurosa for providing several speci-mens of parasitized insects.
Literature Cited
Ball, G.E. and Y. Bousquet. 2001. Family 6. Carabidae. pp. 32-132. In R.H. Arnett and M.C. Thomas (eds.), The Ameri-can Beetles. Vol. 1. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. CRC Press, Boca Raton, 443 pp.
Benjamin, R.K. 1971. Introduction and supplement to Roland Thaxter’s contribution towards a monograph of the Laboulbeniaceae. Bibliotheca Mycologia, Band 30. J. Cramer, Lehre, 155 pp.
Benjamin, R.K. 2001. Autophagomyces, Bordea, and a new genus, Rossiomyces (Laboulbeniales). Aliso 19: 99-136. Huldén, L. 1983. Laboulbeniales (Ascomycetes) of Finland and
adjacent parts of the U.S.S.R. Karstenia 23: 31-136. Huldén, L. 1985. Floristic notes on Palaearctic Laboulbeniales
(Ascomycetes). Karstenia 25: 1-16.
Juan, L.Y. and C.Y. Chien. 1994. Study on the Laboulbeniales (Ascomycetes) of Taiwan: Five new record species. Biol. Bull. NTNU. 29: 71-77.
I II II
Figure 12. Laboulbenia nocturna removed from elytra (apical part) of Lebia chiponica. A pair of thalli stained with acetocarmine, bearing appendages with many black septa. K. Terada-636. Scale bar = 50 µm.
Juan, L.Y. and C.Y. Chien. 1995. Study on the Laboulbeniales (Ascomycetes) of Taiwan. Biol. Bull. NTNU. 30: 11-22. Juan, L.Y. and C.Y. Chien. 1996. Study on the Laboulbeniales
(Ascomycetes) of Taiwan (II). Biol. Bull. NTNU. 31: 5-11.
Lee, Y.B. and K. Sugiyama. 1984. Laboulbeniomycetes of Formosa IV. Trans. Mycol. Soc. Japan 25: 243-248. Maj e w s k i , T. an d K . S u g i y am a . 1 9 8 6 . N o t e s o n t h e
Laboulbeniomycetes (Ascomycotina) of Borneo IV. Trans. Mycol. Soc. Japan 27: 425-439.
Rossi, W. 1982. New or interesting Laboulbeniales from China. Mycologia 74: 1023-1026.
Rossi, W. 1994. A new contribution to the knowledge of the Laboulbeniales (Ascomycetes) from Sierra Leone. Accad. Naz. Lincei. Quaderni 267: 5-17.
Santamaria, S. 1993. Contributión al conocimiento de los Laboulbeniales (Fungi, Ascomycotina) ibéricos, III. Orsis 8: 21-31.
Sugiyama, K. 1973. Species and Genera of the Laboulbeniales (Ascomycetes) in Japan. Ginkgoana, No. 2. Academia Sci-entific Book Inc., Tokyo, 97 pp., 27pls.
Sugiyama. K. 1978a. The Laboulbeniomycetes of eastern Asia (1). On two new species of Laboulbenia and one new spe-cies of Rickia. J. Jap. Bot. 53: 20-27.
Sugiyama. K. 1978b. The Laboulbeniomycetes of eastern Asia (2). On eight species from Japan and Formosa including two new species of Rickia. J. Jap. Bot. 53: 154-160.
Sugiyama. K. 1978c. The Laboulbeniomycetes of eastern Asia (3). On nine species including two new species. J. Jap. Bot. 53: 281-288.
Sugiyama, K. 1981. Notes on Laboulbeniomycetes of Formosa III. Trans. Mycol. Soc. Japan 22: 311-319.
Sugiyama, K. 1982a. The second species of the genus
Porophoromyces (Laboulbeniomycetes). Trans. Mycol.
Soc. Japan 23: 241-244.
Sugiyama, K. 1982b. On two new species of the genus
Peyritschiella (Laboulbeniomycetes). Trans. Mycol. Soc.
Japan 23: 245-249.
S u g i y a m a , K . an d E . S h a z a w a . 1 9 7 7 . N o t e s o n Laboulbeniomycetes of Formosa. Trans. Mycol. Soc. Ja-pan 18: 270-278.
S u g i y a m a , K . an d M . H a y a m a . 1 9 8 1 . N o t e s o n Laboulbeniomycetes of Formosa II. Trans. Mycol. Soc. Ja-pan 22: 187-196.
Tavares, I.I. 1985. Laboulbeniales (Fungi, Ascomycetes). Mycologia Memoir No. 9. J. Cramer, Braunschweig, 627 pp.
Terada, K. 1976. Some species of the Laboulbeniales from Taiwan. Trans. Mycol. Soc. Japan 17: 23-34.
Terada, K. 1978. Additions to the Laboulbeniales from Taiwan, with descriptions of two new species. Trans. Mycol. Soc. Japan 19: 55-64.
Terada, K. 1981. Osoriomyces, a new genus of Laboulbeniales from Taiwan. Mycotaxon 13: 412-418.
Terada, K. 1995. Laboulbenia exigua and related taxa (Ascomycetes, Laboulbeniales). Mycoscience 36: 293-309. Terada, K. 2000. New records of the carabidicolous Laboulbeniales (Ascomycetes) of Japan (II). Mycoscience 41: 39-48.
Thaxter, R. 1899. Preliminary diagnoses of new species of Laboulbeniaceae I. Proc. Amer. Acad. Arts Sci. 35: 151-209. Thaxter, R. 1902. Preliminary diagnoses of new species of Laboulbeniaceae V. Proc. Amer. Acad. Arts Sci. 38: 7-57. Thaxter, R. 1908. Contribution toward a monograph of the
Laboulbeniaceae. Part II. Mem. Amer. Acad. Arts Sci. 13: 217-469. Plates XXVIII-LXXI.
Thaxter, R. 1915. New Indo-Malayan Laboulbeniales. Proc. Amer. Acad. Arts Sci. 51: 1-51.
Thaxter, R. 1931. Contribution toward a monograph of the Laboulbeniaceae. Part V. Mem. Amer. Acad. Arts Sci. 16: 1-435. Plates I-LX.
Present address: Omiya 1-2-20-203, Nishi-Ku, Hiroshima 733-0007, Japan
Peyritschiella 1 Laboulbenia 10 12
Peyritschiella clivinae, Laboulbenia timurensis, L. agoni, L. separata L. nocturna