Parallel Infection of Japanese Encephalitis Virus and Wolbachia within
Cells of Mosquito Salivary Glands
KUN-HSIEN TSAI,1, 2CHIN-GI HUANG,1WEN-JER WU,1CHIN-KAI CHUANG, CHIU-CHUN LIN, ANDWEI-JUNE CHEN3
Department of Public Health and Parasitology, Chang Gung University, Kwei-San, Tao-Yuan 33332, Taiwan
J. Med. Entomol. 43(4): 752Ð756 (2006)
ABSTRACT The endosymbiont Wolbachia usually causes cytoplasmic incompatibility in dipteran hosts, including mosquitoes. However, some important arbovirus-transmitting mosquitoes such as
Aedes aegypti (L.) are not heritably infected by Wolbachia. In Wolbachia-harboring mosquito
Armigeres subalbatusCoquillett, colocalization of Wolbachia and inoculated Japanese encephalitis virus (family Flaviviridae, genus Flavivirus, JEV) in salivary gland (SG) cells was shown by electron microscopy. The infection rate of JEV in SGs, detected with either immunoßuorescent antibody test or reverse transcription-polymerase chain reaction, did not show signiÞcant differences between
Wolbachia-infected and -free colonies. It is suggested that Wolbachia did not mediate resistance of SG cells to superinfection by JEV, although both microorgamisms coexist in the same niche, i.e., the same SG cell. Therefore, a SG escape barrier may not be elevated due to Wolbachia infection, which presumably has no deleterious effects on vector competence in Wolbachia-harboring mosquitoes. KEY WORDS Armigeres subalbatus, Japanese encephalitis virus, parallel infection, Wolbachia
Several mosquito species are known to harbor the endosymbiont Wolbachia in reproductive as well as somatic tissues (Dobson et al. 1999, Kittayapong et al. 2000, Tsai et al. 2004). Wolbachia is a monophyletic assemblage belonging to the alpha-subdivision of the Proteobacteria (Werren et al. 1995). The endosym-biotic infection usually causes cytoplasmic incompat-ibility in dipteran hosts, including mosquitoes (Wer-ren 1997). Salivary glands (SGs) have been reported to be one target tissue for Wolbachia infection in susceptible mosquitoes (Tsai et al. 2004, Chen et al. 2005). SGs are also the destination of arboviruses during the journey of a virus in the host mosquito before transmission (Janzen et al. 1970, WhitÞeld et al. 1973, Chen et al. 1993).
The T1P1 strain of Japanese encephalitis virus (family Flaviviridae, genus Flavivirus, JEV) was re-cently isolated from Armigeres subalbatus Coquillett collected in an islet without paddy cultivation (Chen et al. 2000). This unconventional JEV vector was in-heritably infected with Wolbachia belonging to group A (Tsai et al. 2004). Infection of Wolbachia in SGs of
Ar. subalbatushas been identiÞed either by in situ hybridization or by electron microscopy (Chen et al. 2005).
Wolbachiainfection is absent in many important disease-transmitting mosquitoes, including the Japa-nese encephalitis (JE) vector Culex tritaeniorhynchus Giles, the dengue vector Aedes aegypti (L.), and the malaria vectors (Anopheles spp.) (Kittayapong et al. 2000, Tsai et al. 2004). It becomes interesting whether the endosymbiont modulates transmission of patho-gens carried by Wolbachia-harboring mosquitoes or other vectors (Brownstein et al. 2003). The relation-ships and mutual effects between the two microor-ganisms infecting mosquito SG cells in parallel are discussed in this report.
Materials and Methods
Viruses and Cell Culture. JE virus used in this study was the T1P1 strain that was previously isolated from Þeld-caught Ar. subalbatus (Chen et al. 2000). The virus was propagated in C6/36 cells derived from
Aedes albopictus(Skuse) and titrated in baby hamster kidney (BHK)-21 cells based on previous reports (Chiou and Chen 2001, Liu et al. 2004).
Mosquitoes. Ar. subalbatus originated from an islet where T1P1 JEV was isolated, and it was maintained in the laboratory as described previously (Chen et al. 2000). This unconventional JE vector was inheritably infected with Wolbachia belonging to group A (Tsai et al. 2004). Throughout the study, the Wolbachia-free colony of Ar. subalbatus was obtained via treatment of early instars with diluted tetracycline solution. These 1Department of Entomology, National Taiwan University, Taipei
10673, Taiwan.
2Current address: Laboratory of Arbovirus and Rickettsia, Center
for Disease Control, Department of Health, Executive Yuan, Nan-Kang, Taipei 11558, Taiwan.
3Corresponding author, e-mail: wjchen@mail.cgu.edu.tw.
larvae were used as the negative control group (Yen and Barr 1973).
Intrathoracic Inoculation. To evaluate the effect of
Wolbchiaon JEV replicating in Ar. subalbatus, a virus suspension (4.5⫻ 108plaque-forming units [PFU]/ ml; 0.17l per mosquito) was intrathoracically inoc-ulated into newly emerged adult female Ar. subalbatus following the method described previously (Chen et al. 1993). At least three mosquitoes were sampled daily and dissected to collect SGs for virus detection until 20 d postinoculation.
Immunofluorescent Antibody Test (IFA). Virus in-fection was determined by using IFA for detection of viral antigens. The method followed our previous de-scription (Chen et al. 2000). Brießy, SGs dissected from mosquitoes were moved onto wells of 12-well Teßon-coated slides. The slides were immersed in cold acetone (⫺20⬚C) for 10 min and then air-dried. One drop of monoclonal antibodies speciÞc to JEV (Trop-Bio, Queensland, Australia) was added to the wells. The slides were washed with phosphate-buffered sa-line (PBS) after they have been incubated at 37⬚C for 30 min. Subsequently, goat anti-mouse IgG antibody conjugated with ßuorescein isothiocyanate (Sigma-Aldrich, St. Louis, MO) was added to the wells. The slides were washed in PBS again after another
incu-bation under the same conditions, and the specimens were mounted with a mixture of PBS and glycerol (3:7). The specimens were then examined under an epißuorescent microscope (BX50, Olympus, Tokyo, Japan).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR). For detection of viral RNA extracted from mosquito salivary glands, RT-PCR was used for am-pliÞcation of a gene fragment of the E protein follow-ing steps outlined in Chen et al. (2000). Primer pairs included the sense primer (5 ⬘-AGTTAACATCAG-GCCACCTGA-3⬘) and the complementary primer (5⬘-GTTCCATCTCGACCAGCAC-3⬘) (Chung et al. 1996). For identiÞcation of Wolbachia infection in mosquito SGs, PCR also was applied to amplify a frag-ment of the Wolbachia surface protein (wsp) gene as described previously (Tsai et al. 2004). The ampliÞed cDNA fragments were run through electrophoresis on a 2% (wt:vol) agarose gel containing 10l of ethidium bromide (0.1 mg/ml in RNase-free water) once PCR cycles have been completed.
Virus Titration. Plaque assay on BHK-21 cells, fol-lowing a previous description (Chiou and Chen 2001), was used to determine the virus titer produced in SGs of inoculated mosquitoes. The virus titer is presented as plaque-forming units per milliliter.
Electron Microscopy. Ultrastructural studies followed the method described in our previous report (Chen et al. 2005). In brief, SGs removed from adult mosquitoes with intrathoracic inoculation were Þxed immedi-ately in 2% glutaraldehyde for 2 h and then postÞxed in 1% osmium tetroxide for 2 h at room temperature. The specimens were dehydrated through an ascend-ing graded series of ethanol. The tissues were embed-ded in SpurrÕs resin and polymerized at 70⬚C. The tissue blocks were sectioned with an ultramicrotome (Reichert Ultracut S, Leica, Vienna, Austria). Thin sections were stained with uranyl acetate and lead citrate in sequence and observed under an electron microscope (JEOL-JEM-2000, JOEL, Tokyo, Japan) at 100 kV.
Results and Discussion
According to the results of IFA (Fig. 1), 76.67% (23/30) and 73.33% (22/30) of dissected SGs were positive to JEV for Wolbachia-infected and -free col-onies, respectively (Table 1). The results of RT-PCR showed that JEV infected most examined SGs (Fig. 2); the positive rates were 86.57% (13/15) and 80.00% (12/15) for Wolbachia-infected and -free colonies, respectively (Table 1). There was no signiÞcant dif-ference between the infection rates of two colonies detected by either analytical method (2⫽ 0.08 and 0.24 for IFA and RT-PCR, respectively; P ⬎ 0.05). Moreover, an undifferentiaed daily dynamic trend of virus titers was shown in mosquitoes with or without
Wolbachiainfection. We suggest that the two mos-quito colonies were not signiÞcantly different in pro-duction of JEV (Fig. 3).
JEV must infect and subsequently pass through the epithelium of mosquito midguts to reach SGs for
fur-Fig. 1. JEV (T1P1 strain) was detected by immunoßu-orescent antibody test 7 d postinoculation, showing that viral antigens existed in the medial lobe and proximal-lateral lobes of salivary glands dissected from inoculated Ar. subalbatus. (a) Positive. (b) Negative. Original magniÞcation, 200⫻.
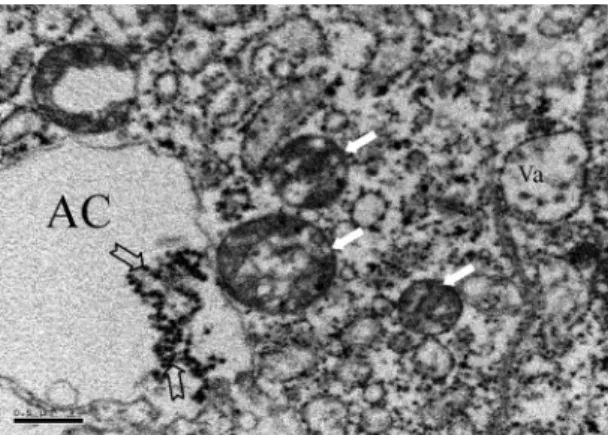
ther replication before transmission can occur (Doi et al. 1967, Doi 1970, Leake and Johnson 1987). Although the medial lobe of SGs can be infected, JEV infection frequently occurred in proximal-lateral lobes (Fig. 1). In ultrastructure, JE virions were clustered in the cytoplasm and more commonly in apical cavities of SG cells in the proximal region of lateral lobes (Fig. 4). The apical cavity in this region is usually irregular in morphology and contains a lightly Þlamentous material (Yen and Barr 1973). Wolbachia also occurred in SG cells of this region, but generally it was restricted in the cytoplasm (Fig. 5). It has been known that the proximal-lateral lobes are the primary area for syn-thesis of␣-glucosidase, whereas the distal-lateral lobes are the primary synthesis sites for apyrase; these en-zymes function in sugar feeding and blood feeding, respectively (Marinotti et al. 1996). Whether the dis-tribution of Wolbachia, JEV, or both is related to spe-ciÞc enzyme synthesis is not clear. At least, colocal-ization of Wolbachia with clustered JE virions revealed that these two microorganisms live in same environment but in different ecological niches (Fig. 6).
Actively respiring mitochondria characterized as a marked condensation of the cristae were found in the periphery of apical cavities in SG cells infected by JEV (Fig. 4). Migration of mitochondria has been reported in the cytoplasm toward one side of the nucleus in herpes simplex virus or viral assembly sites in African swine fever virus (ASFV)-infected cells (Rojo et al. 1998, Murata et al. 2000). In ASFV-infected cells, mitochondrial alteration from the resting state to ac-tively respiring state was thought to supply energy effectively for the virus morphogenetic process (Rojo et al. 1998). The dramatic shift in the morphology of mitochondria during the assembly process of virus particles is known to be compatible with an increase in respiratory function and ATP production by their mitochondria (Rojo et al. 1998). Eventually, it could lead to an increased production of reactive oxygen species and subsequent damage to mitochondrial DNA and protein (Rojo et al. 1998). In the current study, damaged SG cells were actually observed in
Ar. subalbatus heavily infected by JEV (data not shown), although its relationship with colocalizing
Wolbachiaremains to be clariÞed. A degeneration of SGs also has been observed in adult female Culex
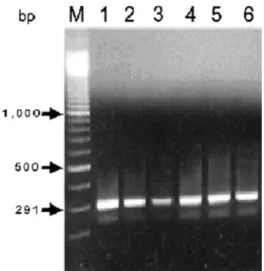
Fig. 2. Infection of JEV (T1P1 strain) was detected by RT-PCR in salivary glands dissected from Ar. subalbatus by intrathoracically inoculated with virus suspension and mea-sured 10 d postinoculation. M, 100-bp DNA ladders; Lane 1, positive control from which RNA templates were extracted from infected C6/36 cells. Lanes 2Ð6, SGs from virus-inoc-ulated mosquitoes.
Table 1. Infection rates of Wolbachia-infected and -free A. subalbatus colonies with intrathoracically inoculated with 0.17l (original virus titer: 4.5ⴛ 108PFU/ml) of JE virus (T1P1 strain) suspension and measured 7 d postinoculation
Infection status
% infection rate (no. positive/no. tested) IFAa RT-PCRb
Wolbachia-infected 76.67 (23/30) 86.57 (13/15) Wolbachia-free 73.33 (22/30) 80.00 (12/15)
aIFA: Immunoßuorescent antibody test (2
⫽ 0.08; P ⬎ 0.05).
b2
⫽ 0.24, P ⬎ 0.05. Fig. 3. Changing trend of daily titers of JEV (T1P1 strain) grown in salivary glands of Wolbachia-infected and -free colonies of Ar. subalbatus.
Fig. 4. Actively respiring mitochondria characterized as a marked condensation of the cristae (thick arrow) were located in the periphery of the apical cavity (AC) of SG cells infected by T1P1 strain of JEV (thin arrow). Bar⫽ 1m.
tarsalis(Coquillett) coinfected with two noninclusion cytoplasmic viruses (Richardson et al. 1974).
Both Ae. aegypti and Ae. albopictus are thought to be vectors of dengue fever and several other arbovi-ruses (Metselaar et al. 1980, Rai 1991, Gubler 1998, Johnson et al. 2002). However, in the past 25 yr, the global resurgence of epidemic dengue/dengue hem-orrhagic fever was thought to primarily be transmitted by Ae. aegypti, not Ae. albopictus (Degallier et al. 2003, Gubler 2003). Unlike Ae. aegypti, Ae. albopictus is gen-erally infected with Wolbachia groups A and B (Tsai et al. 2004). It is possible that the endosymbiont har-bored by mosquitoes may play a role as a competitor, which might be deleterious to the virus or which would make SG cells refractory to subsequent viral infection or transmission. Such species competition between rickettsia infections in cat ßeas has been doc-umented (Williams et al. 1992). However, these two
Aedesmosquitoes possessed insigniÞcantly different susceptibility to dengue virus in Þeld-caught mosqui-toes (Chow et al. 1998) and to dengue or yellow fever virus in experimental infections (Whitehead et al. 1971, Johnson et al. 2002). There might not be an evident mutual effect between Wolbachia and the
virus, even if both of them eventually co-reside within the SG cell. In turn, Wolbachia probably did not func-tion to elevate SG escape barrier. Regardless, whether the endosymbiont would interfere with the transmis-sion of arboviruses remains to be demonstrated, es-pecially regarding its effect on depositing viral parti-cles into secretory products within SG lumina. It has been reported that the striking number of viral par-ticles in SG luminal saliva was consistent with the transmission efÞciency of arthropods in nature (Whit-Þeld et al. 1971).
Acknowledgments
We are grateful to S. C. Wu and S. S. Chiou for technical assistance. This work was supported by National Science Council grant ROC (NSC 94-2314-B-182-021) and in part by grant CMRP33009 from Chang Gung Memorial Hospital.
References Cited
Brownstein, J. S., E. Hett, and S. L. O’Neill. 2003. The po-tential of virulent Wolbachia to modulate disease trans-mission by insects. J. Invertebr. Pathol. 84: 24 Ð29. Chen, W. J., H. L. Wei, E. L. Hsu, and E. R. Chen. 1993.
Vector competence of Aedes albopictus and Ae. aegypti (Diptera: Culicidae) to dengue 1 virus on Taiwan: de-velopment of the virus in orally and parenterally infected mosquitoes. J. Med. Entomol. 30: 524 Ð530.
Chen, W. J., C. F. Dong, L. Y. Chiou, and W. L. Chuang. 2000. Potential role of Armigeres subalbatus (Diptera: Culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-Chiu islet, Taiwan. J. Med. Entomol. 37: 108 Ð113.
Chen, W. J., K. H. Tsai, S. L. Cheng, C. G. Huang, and W. J. Wu. 2005. Using in situ hybridization to detect the endosymbiont Wolbachia in dissected tissues of mosquito host. J. Med. Entomol. 42: 120 Ð124.
Chiou, S. S., and W. J. Chen. 2001. Mutations in the NS3 gene and 3⬘-NCR of Japanese encephalitis virus isolated from an unconventional ecosystem and implications for natural attenuation of the virus. Virology 289: 129 Ð136. Chow, V.T.K., Y. C. Chan, R. Yong, K. M. Lee, L. K. Lim,
Y. K. Chung, S. G. Lam-Phua, and B. T. Tan. 1998. Mon-Fig. 6. Wolbachia(solid arrows) occurred in cytoplasm of the salivary gland cell closely to the apical cavity (AC) in which T1P1 strain of JE virions were clustered (empty ar-rows). Virions also were seen in the vacuole (Va) located in the cytoplasm. Bar⫽ 0.5m.
Fig. 5. (a) Wolbachia was observed in the lateral lobe of salivary glands of Ar. subalbatus. Bar⫽ 2m. (b) MagniÞ-cation of the rectangular area shown in a. Bar⫽ 0.2m.
itoring of dengue viruses in Þeld-caught Aedes aegypti and Aedes albopictus mosquitoes by a type-speciÞc poly-merase chain reaction and cycle sequencing. Am. J. Trop. Med. Hyg. 58: 578 Ð586.
Chung, Y. J., J. H. Nam, S. J. Ban, and H. W. Cho. 1996. Antigenic and genetic analysis of Japanese encephalitis viruses isolated from Korea. Am. J. Trop. Med. Hyg. 55: 91Ð97.
Degallier, N., J.M.S. Teixeira, S. Da, S. Soares, R. D. Pereira, S.C.F. Pinto, A. De J. M. Chaib, P. F. Da C. Vasconcelos, and E. Oliveira. 2003. Aedes albopictus may not be vec-tor of dengue virus in human epidemics in Brazil. Rev. Sau´de Pu´blica 37: 386Ð387.
Dobson, S. L., K. Bourtzis, H. R. Braig, B. F. Jones, W. Zhou, F. Rousset, and S. L. O’Neill. 1999. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem. Mol. Biol. 29: 153Ð160. Doi, R. 1970. Studies on the mode of development of
Jap-anese encephalitis virus in some groups of mosquitoes by the ßuorescent antibody technique. Jpn. J. Exp. Med. 40: 101Ð115.
Doi, R., A. Shirasaka, and M. Sasa. 1967. The mode of de-velopment of Japanese encephalitis virus in the mosquito Culex tritaeniothynchus summorosusas observed by the ßuorescent antibody technique. Jpn. J. Exp. Med. 37: 227Ð238.
Gubler, D. J. 1998. Resurgent vector-borne diseases as a global health problem. Emerg. Infect. Dis. 4: 442Ð 450. Gubler, D. J. 2003. Aedes albopictus in Africa. Lancet
Infect. Dis. 3: 751Ð752.
Janzen, H. G., A. J. Rhodes, and F. W. Doane. 1970. Chiku-gunya virus in salivary glands of Aedes aegypti (L.): an electron microscope study. Can. J. Microbiol. 16: 581Ð586. Johnson, B. W., T. V. Chambers, M. B. Crabtree, A.M.B. Filippis, P.T.R. Vilarinhos, M. C. Resende, M.L.G. Ma-coris, and B. R. Miller. 2002. Vector competence of Bra-zilian Aedes aegypti and Ae. albopictus for a BraBra-zilian yellow fever virus isolate. Trans. R. Soc. Trop. Med. Hyg. 96: 611Ð 613.
Kittayapong, P., K. J. Baisley, V. Baimai, and S. L. O’Neill. 2000. Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J. Med. Entomol. 37: 340 Ð345.
Leake, C. J., and R. T. Johnson. 1987. The pathogenesis of Japanese encephalitis virus in Culex tritaeniorhynchus mosquitoes. Trans. R. Soc. Trop. Med. Hyg. 81: 681Ð 685. Liu, H., S. S. Chiou, and W. J. Chen. 2004. Differential binding efÞciency between the envelope protein of Jap-anese encephalitis virus variants and heparan sulfate on the cell surface. J. Med. Virol. 72: 618 Ð 624.
Marinotti, O., M. de Brito, and C. K. Moreira. 1996. Apyrase and␣-glucosidase in the salivary glands of Aedes albo-pictus.Comp. Biochem. Physiol. 113B: 675Ð 679. Metselaar, D., C. R. Grainger, K. G. Oei, D. G. Reynolds,
M. Pudney, C. J. Leake, P. M. Tukei, R. M. D’Offay, and D. I. Simpson. 1980. An outbreak of type 2 dengue fever in the Seychelles, probably transmitted by Aedes albo-pictus(Skuse). Bull. World Health Organ. 58: 937Ð943. Murata, T., F. Goshima, T. Daikoku, K. Inagaki-Ohara,
H. Takakuwa, K. Kato, and Y. Nishiyama. 2000. Mito-chondrial distribution and function in herpes simplex virus-infected cells. J. Gen. Virol. 81: 401Ð 406. Rai, K. S. 1991. Aedes albopictus in the Americas. Annu. Rev.
Entomol. 36: 459 Ð 484.
Richardson, J., E. S. Sylvester, W. C. Reeves, and J. L. Hardy. 1974. Evidence of two inapparent nonoccluded viral in-fections of Culex tarsalis. J. Invertebr. Pathol. 23: 213Ð224. Rojo, G., M. Chamorro, M. L. Salas, E. Vin˜uela, J. M. Cuezva, and J. Salas. 1998. Migration of mitochondria to viral assembly sites in African swine fever virus-infected cells. J. Virol. 72: 7583Ð7588.
Tsai, K. H., J. C. Lien, C. G. Huang, W. J. Wu, and W. J. Chen. 2004. Molecular (sub)grouping of endosymbiont Wol-bachiainfection among mosquitoes of Taiwan. J. Med. Entomol. 41: 677Ð 683.
Werren, J. H. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42: 587Ð 609.
Werren, J. H., W. Zhang, and L. R. Guo. 1995. Evolution and phylogeny of Wolbachia: reproductive parasites of ar-thropods. Proc. R. Soc. Lond. Ser. B Biol. Sci. 261: 55Ð 63. Whitfield, S. G., F. A. Murphy, and W. D. Sudia. 1971. East-ern equine encephalomyelitis virus: an electron micro-scopic study of Aedes triseriatus (Say) salivary gland in-fection. Virology 43: 110 Ð122.
Whitfield, S. G., F. A. Murphy, and W. D. Sudia. 1973. St. Louis encephalitis virus: an ultrastructural study of in-fection in a mosquito vector. Virology 56: 70 Ð 87. Whitehead, R. H., T. M. Yuill, D. J. Gould, and P.
Sima-sathien. 1971. Experimental infection of Aedes aegypti and Aedes albopictus with dengue viruses. Trans. R. Soc. Trop. Med. Hyg. 65: 661Ð 667.
Williams, S. G., J. B. Bacci, Jr., M. E. Schriefer, E. M. Ander-son, K. Fujioka, and F. J. Sorvilo. 1992. Typhus and ty-phus-like rickettsiae associated with opossums and their ßeas in Los Angeles County, California. J. Clin. Microbiol. 30: 1758 Ð1762.
Yen, J. H., and A. R. Barr. 1973. The etiological agent of cytoplasmic incompatibility in Culex pipiens. J. Invertebr. Pathol. 22: 242Ð250.