1096-4959/02/$ - see front matter䊚 2002 Elsevier Science Inc. All rights reserved. PII: S 1 0 9 6 - 4 9 5 9 Ž 0 2 . 0 0 0 3 6 - 2
Expression, purification and DNA-binding activity of tilapia
muscle-specific transcription factor, MyoD, produced in
Escherichia coli
夞
Yau-Hung Chen, Chin-Tien Liang, Huai-Jen Tsai*
Institute of Fisheries Science, National Taiwan University, Taipei, Taiwan, ROC Received 7 August 2001; received in revised form 23 January 2002; accepted 24January 2002Abstract
MyoD is one of several helix-loop-helix proteins regulating muscle-specific gene expression. Using a reverse transcription-polymerase chain reaction, 59-rapid cDNA end amplification, and plaque hybridization, MyoD cDNA was cloned from the mRNA of tilapia dorsal skeletal muscle. The 1015 bp MyoD cDNA product contained an 846 bp open reading frame with flanking regions of 115 and 64bp at the 59- and 39-ends, respectively. Results showed that the tilapia MyoD sequence, which includes one polypeptide of 281 amino acids, shared sequence identities of 64.3, 64.1, 62.6 and 62.4% with those of zebrafish, carp, and two rainbow trout, respectively. Results from a molecular phylogenic tree assay showed that the tilapia MyoD was more closely related to those of other fishes than of higher vertebrates. Using Escherichia coli, a pET expression system, and an Ni2q-NTA column, we purified ;35 kDa recombinant tilapia MyoD. Results from an electrophoretic mobility shift assay demonstrated that the purifiedE. coli-produced tilapia MyoD was capable of binding to the DNA fragment sequence CA(CyT)(CyA)TG. 䊚 2002 Elsevier Science Inc. All rights reserved. Keywords: cDNA; Fish; MyoD; Ni2q-NTA column; pET expression system; Phylogenic tree; Plaque hybridization; Tilapia
1. Introduction
Muscle development provides an ideal environ-ment for studying the many unique biological events involved in myogenesis, including cell migration, changes in morphology, and the acti-vation of muscle-specific regulatory factors
(MRFs). Four MRFs play important roles in
myogenesis: MyoD (Davis et al., 1987); Myf-5 (Braun et al., 1989); Myogenin (Edmondson and
Olson, 1989); and MRF4 (Rhodes and Konieczny,
1989). The MRF family is part of a large group
of structurally related proteins that utilize a basic
夞 Nucleotide sequence data is in the GenBank databases
under the accession number AF270790.
*Corresponding author. 1, Roosevelt Road, Sec. 4, Taipei, Taiwan 106. Tel.: q886-2-2366-1540; fax: q886-2-2363-8483.
E-mail address: hjtsai@ccms.ntu.edu.tw(H.-J. Tsai).
helix-loop-helix(bHLH) motif for oligomerization and DNA binding. These bHLH factors are nuclear proteins that transactivate the expression of mus-cle-specific genes we.g. the muscle creatine kinase
(Jaynes et al., 1988) and myosin light chain genes (Faerman and Shani, 1993)x, which contain one
or more E-box motifs and a DNA-binding site containing the general consensus sequence CANNTG.
Davis et al. (1987) reported that the forced expression of mice MyoD allows for the transfor-mation of a C3H10T1y2 fibroblast into a myoblast.
MyoD is capable of inducing skeletal muscle terminal differentiation in a variety of non-muscle cell types. Pinney et al.(1995) and Pearson-White
(1991) have described the cloning of mice and
humanmyoD cDNA, respectively. Ma et al.(1994)
characterized the crystalline structure of the bHLH domain of mice MyoD, and reported that (a) the
basic region interacts with the major DNA groove, and (b) the HLH region is involved in protein– protein interaction. In addition to this bHLH domain function, Bergstrom and Tapscott (2001)
found that mice MyoD contains three other func-tional domains—an N-terminal transcription acti-vation domain, a HisyCys-rich domain, and a
C-terminal helix III domain. Each plays an impor-tant role in MyoD biological activity: the N-terminal transcription activation domain activates the downstream gene, the C-terminal helix III domain is responsible for cell-type specification, and the HisyCys-rich domain is required for
chro-matin remodeling; the HisyCys domain may also
serve a synergistic function with the C-terminal helix III domain.
Compared to the extensive literature on mammal MyoD, very little is known about the characteris-tics of fish MyoD proteins. The molecular structure of MyoD cDNA has been cloned in trout(Rescan
et al., 1994; Rescan and Gauvry, 1996), zebrafish (Weinberg et al., 1996) and carp (Kobiyama et
al., 1998). It is often used as a molecular marker
to trace cell lineages (Devoto et al., 1996), to
compare evolutionary origins (Neyt et al., 2000)
to respond to environmental changes (Xie et al., 2001), and to regulate other muscle-specific genes
(Sawada et al., 2000; Xu et al., 2000; Kajihara et
al., 2001; Rescan, 2001). In order to expand our knowledge of comparative piscine muscle physi-ology, we decided to study the MyoD of tilapia
(Oreochromis aurea), a common fish farm species.
After examining the molecular structure of tilapia MyoD, we determined that purified recombinant tilapia MyoD produced by Escherichia coli is
capable of specific E-box binding. 2. Materials and methods
2.1. RNA isolation
The brain, dorsal skeletal muscle, gill, intestine, spleen and stomach of two sacrificed tilapia adults were excised and immediately frozen in liquid nitrogen. Frozen tissue was homogenized with TRIzol reagent(Gibco BRL). RNA extraction was
performed according to methods described in Chen et al.(2000, 2001).
2.2. Reverse transcription-polymerase chain reac-tion (RT-PCR)
First-strand cDNA was synthesized using the SuperScript Pre-amplification System (Gibco
BRL). Degenerate oligonucleotide primers were designed in reference to known vertebrate MyoD polynucleotide sequences. One forward primer, TMD-116F (59-ATGGAGTTG(Cy
T)CGGATATTCC(GyTyC)TTCCC-39), and one
reverse primer, TMD-853R
(59-CTCCAC-GATG(CyT)T(GyT)GA(AyC)AG(AyG)CA(Ay
G)TCCAAAC-39) were synthesized according to
the conserved amino acid sequences MELPDISF and IREVISSL, respectively. Thirty PCR amplifi-cation cycles with Taq DNA polymerase(Viogene) were performed. Each cycle consisted of denatur-ing for 40 s at 94 8C, 1 min of annealdenatur-ing at 54
8C, and 1 min of extension at 72 8C; final
extension lasted for 15 min at 72 8C. Amplified DNA fragments were ligated with pGEM T-Easy vector (Promega) and transformed into E. coli DH5a. A bigdye-terminator cycle sequencing reac-tion kit (Perkin–Elmer Applied Biosystems)
equipped with a DNA sequencer (Model 310,
Perkin–Elmer) was used for the DNA sequencing
of both strands. A RT-PCR product was obtained, confirmed, and named pTMD (116–853). The
primers Tb-F5
(59-TGCGGTATCCATGAGAC-CAC-39) and Tb-R6
(59-GAAGCA-TTTGCGGTGGACGA-39), synthesized based on
the b-actin cDNA of tilapia(Huang et al., 1999), were used as internal controls.
2.3. cDNA library construction and putative clone isolation
A skeletal muscle cDNA library was prepared from poly (A)-selected RNA using oligo (dT)
cellulose. First-strand cDNA was prepared using an oligo (dT) primer and reverse transcriptase (Stratagene); second-strand cDNA was
synthe-sized using RNaseH, DNA polymerase I, and E. coli DNA ligase. Following the ligation of EcoRI-XhoI adaptors, the cDNA was inserted into the EcoRI site of a Lambda ZAP II bacteriophage
vector(Stratagene). The library was screened with the DIG-labeled DNA probe pTMD (116–853), which contained a fragment corresponding to nucleotides 116–853 of the tilapia myoD cDNA;
approximately 600 ng of DNA probes were pre-pared with a PCR DIG Probe Synthesis Kit from Roche. Hybridization was performed using a stan-dard hybridization buffer at 42 8C for 16 h accord-ing to the manufacturer’s guidelines. Washaccord-ing conditions were 0.5=SSC at 70 8C for 30 min, followed by 0.1=SSC at 55 8C for 30 min.
CDP-Fig. 1. Nucleotide and deduced amino acid sequences of tilapiamyoD cDNA. Nucleotides were numbered beginning at the transcription start site(q1). Numbers on the second line of each row indicate the amino acid sequences, and the annotations on the third line
indicate the structural motifs. The stop codon is marked with an asterisk, and the polyadenylation signal is shaded in gray. This nucleotide sequence can be found in the GenBank database under accession number AF270790.
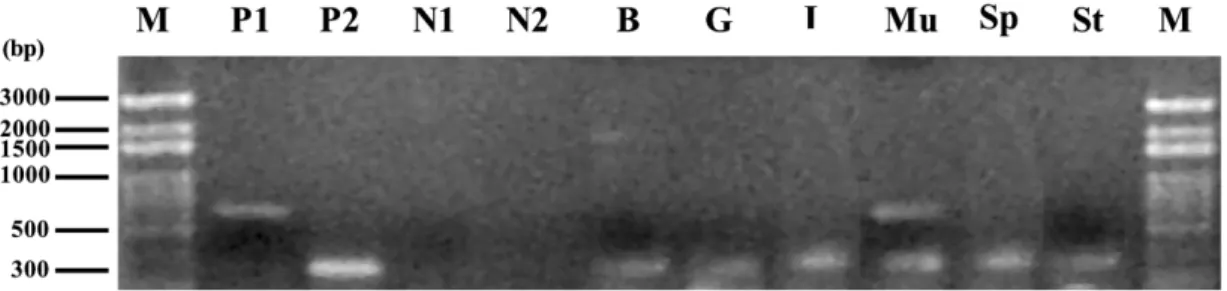
Fig. 2. RT-PCR analysis of the tilapiamyoD gene transcript tissue distribution, using total RNA extracted from tilapia brain(B), gill (G), intestine (I), skeletal muscle (Mu), spleen (Sp) and stomach (St). Products were analyzed by 1% agarose gel electrophoresis.
The primers TMD-116F and TMD-853R were used for detectingmyoD transcripts: a 735 bp product was expected to be generated (lane P1). The primers Tb-F5 and Tb-R6 were also used as internal control for detecting b-actin transcripts and resulting in a 312 bp
product(lane P2). Lane M, DNA markers; lanes N1 and N2, negative controls in which either TMD-116F and TMD-853R or Tb-F5
and Tb-R6 primers were, respectively, added without reverse transcriptase.
STAR(Tropix) was used as a substrate to visualize the positive bands after 30 min of autoradiography. Positive clones containing phagemids in pBlue-Script (KSII) were selected following in vivo
excision according to the supplier’s instructions
(Stratagene).
2.4. 59-Rapid amplification of cDNA ends
(59-RACE)
After performing 59-rapid amplification of cDNA ends (59-RACE) with first-strand cDNA,
terminal transferase TdT (Roche) and dGTP were
used to add poly dG residue to the ends of each cDNAs. The tailed cDNAs were then used to generate double-stranded DNA by PCR amplifi-cation in the presence of a forward primer, RAAPC(59-GGCCACGCGTCGACTAGTACT(C) 9-39), and a reverse primer, TMD240R (59-GTGCTCGTCGTCCTCCACCTCGG-39). PCR amplification was also performed as described above, with the exception of annealing at 56 8C. Amplified DNA fragments were subcloned and sequenced as described in a previous section.
2.5. Analyses of polypeptide identities and phylo-genic dendrograms
The presumptive amino acid sequence was determined with the Wisconsin Sequence Analysis Package v.10.0 (GCG). The Gap program of that
package was used for pair comparisons, and the Pileup and Prettybox programs used for multiple comparisons. The Clustalw molecular evolution
genetic program was used for our phylogenic tree analysis(http:yywww.ebi.ac.ukyclustalwy).
2.6. Tilapia MyoD expression vector construction
A 517 bp PstI fragment digested from pTMD
(116–853) and a 3547 bp PstI fragment digested
from pTMD(321–1015) were ligated to establish pTMD(1–1015) containing the full-length tilapia
myoD cDNA. A forward primer, TMD1F-NdeI
(59-CATATGGAGTTGCCGGATAT-39), and a reverse primer, TMD281R-XhoI
(59-CTCGAGT-TAGCGTCTCCGTGT-39), were used for the PCR
amplification of the pTMD (1–1015) template,
thus allowing for the introduction of two additional
NdeI and XhoI sites in the coding region.
Ampli-fication procedures were identical to those described earlier, except that the PCR product was ligated to the pET15b expression vectors (Novo-gen) after they were digested with NdeI and XhoI. The resulting plasmid, pTMD(1–281), was used for the expression of recombinant tilapia MyoD.
2.7. Induction, expression, and purification of 6=His-TMD fusion proteins
The pET15b and pTMD(1–281) plasmids were transformed into E. coli BL21(DE3)pLysS. Cells
were cultured in 50 ml of 2=YT medium (ampi-cillin 200 mgyml, chloramphenicol 34mgyml,
tryptone 20 gyl, yeast extract 10 gyl, and NaCl 10
gyl) at 37 8C to 0.4OD . Induction was per-600 formed by adding isopropyl-thio-D-galactoside
(IPTG) to a final concentration of 1.6 mM.
Fig. 3. Comparison of the deduced amino acid sequence of tilapia MyoD with those of other known piscine species. Data were obtained from GenBank nucleotide sequence database with the following accession numbers: common carp(AB012882); zebrafish (Z36945);
rainbow trout 1(X75798); and rainbow trout 2 (Z46924) myoD. Amino acid residues identical to that of tilapia MyoD are represented
by dots. Dashes represent gaps created to maximize the degree of identity among all compared sequences.
analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Recombinant
tilapia MyoD was purified with a Ni2q-NTA spin column according to the manufacturer’s instruc-tions(Qiagen).
2.8. Western blotting
Following 12% SDS-PAGE analysis, protein samples were transferred to PVDF membranes
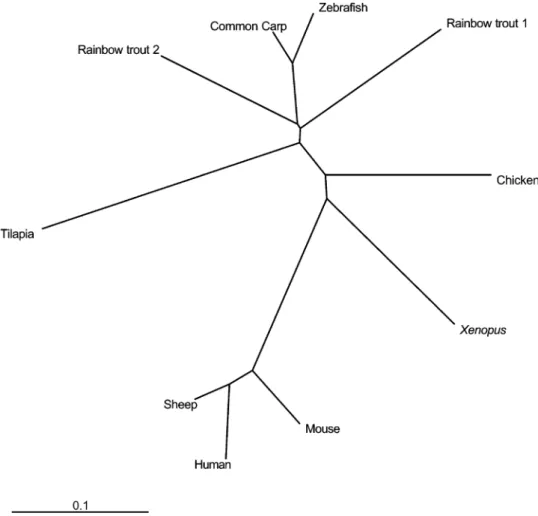
Fig. 4. A molecular phylogenic tree of MyoD polypeptides. Data were obtained from GenBank nucleotide sequence database with the following accession numbers: common carp(AB012882); zebrafish (Z36945); rainbow trout 1 (X75798); rainbow trout 2 (Z46924);
Xenopus(X16106); chicken (L34006); mouse (NM010866); sheep (X62102); and human (X56677) MyoD.
cells (BioRad) and allowed to stand for 1 h. Membranes were blocked with PBS (phosphate-buffered saline) containing 5% skim milk at 37
8C for 1 h. Next, 1:1000 dilutions of rabbit
anti-mouse MyoD polyclonal antibodies (Santa Cruz)
or mouse anti-6=His monoclonal antibodies
(Mdbio) were added and allowed to react for 4h
at 4 8C. Mouse anti-rabbit IgG or goat anti-mouse IgG secondary antibodies conjugated with alkaline phosphatase were added, and the resulting mixture incubated at 37 8C for another 1 h. After two washings with PBS, CDP-STAR (Promega) was added to make the positive bands visible.
2.9. Thrombin hydrolysis
Approximately 200 mg of the purified 6=His-TMD fusion proteins were added to thrombin
cleavage buffer(50 mM Tris–HCl at pH 7.5, 150 mM NaCl, 2.5 mM CaCl2), mixed with 1 U thrombin, and reacted at 22 8C for 1 h to remove the histidine fusing tag. The purified recombinant proteins were analyzed by SDS-PAGE and Western blot analysis, and partially sequenced with a pol-ypeptide sequencer.
2.10. Electrophoretic mobility shift assay(EMSA)
Three double-stranded oligonucleotides were used as probes for the binding activity of the purified recombinant tilapia MyoD:
1. The E-box (mE-box, formed by 59-TTTCCCCAACACCTGCTGCCT-39 and 59-AGGCAGCAGGTGTTGGGGAAA-39) of the
mouse muscle creatine kinase enhancer(Jaynes
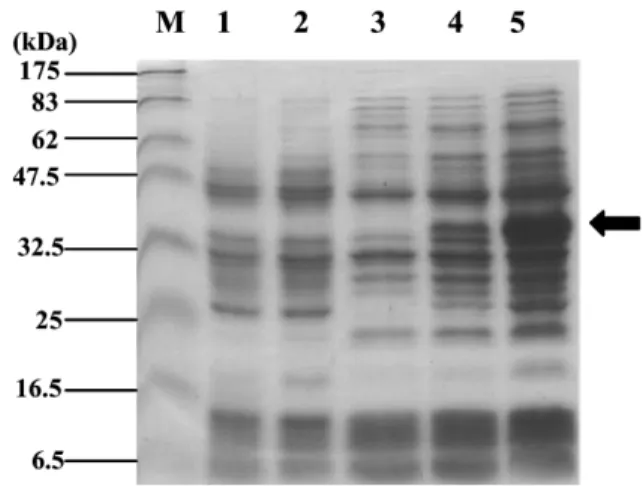
Fig. 5. Expression of recombinant tilapia MyoD by E. coli BL21(DE3)pLysS. Samples were collected and analyzed by
12% SDS-PAGE following coomassie brilliant blue staining. Lane M, protein markers; lanes 1 and 2, lysates fromE. coli harboring no plasmid and pET15b, respectively, as negative controls; lanes 3, 4and 5, lysates fromE. coli harboring plas-mid pET15bTMD(1–281) 0, 2 and 4h following induction,
respectively. Arrow indicates an induced recombinant protein.
2. Zebrafish troponin T enhancers 1 (Z1, formed by 59-TCCTGTGCATCTGTTTTGAG-39 and 59-CTCAAAACAGATGCACAGGA-39) and 2 (Z2, formed by
59-TTAACGTCACATGAG-GAGGG-39 and 59-CCCTCCTCATGTGACGT-TAA-39) (Huang et al., unpublished data).
3. Non-specific oligonucleotides (Non-30fr,
formed by 59-CACGTCACGAGCTATCGGT-GATCATCTCTG-39 and 59-GTGCA-GTGCTCGATAGCCACTAGTAGAGAC-39).
All probes were labeled with g-w PxATP 300032
mCiyml, using T4polynucleotide kinase (NEB)
according to the supplier’s protocols.
Approximately 600 ml of the purified 6=His-TMD fusion proteins were put into a dialysis bag, sealed, and placed in a flask containing 500 ml dialysis buffer(10 mM Hepes at pH 7.9, 100 mM
KCl, 0.2 mM EDTA, 5 mM PMSF, 0.5 mM DTT, 10% glycerol). After stirring for 4h at 48C, the
dialytic proteins were dispensed and stored at y 70 8C until used.
Between 10 ng and 1 mg of the 6=His-TMD fusion proteins and 1 mg of the poly(dIdC) were added to reaction buffer (10 mM Tris at pH 7.5, 50 mM NaCl, 0.5 mM EDTA pH 8.0, 0.5 mM DTT, 5% glycerol) and allowed to stand on ice for 10 min. After adding 1 ml of probe with a specific radioactivity of 10 cpm7 ymg, each mixture
was incubated at 30 8C for 30 min and analyzed by 6% acrylamide gel electrophoresis(79:1
acry-lamideybisacrylamide). The gel was dried and
exposed to X-ray film for 48 h. 3. Results and discussion
3.1. cDNA and deduced amino acid sequences
A 735 bp fragment amplified by the primers TMD-116F and TMD-853R was labeled with DIG as a probe. The nucleotide sequences of approxi-mately 60 positive clones excised from 2=105 plaques were confirmed. All clones were deter-mined to be identical 59-truncated forms of tilapia
myoD cDNA; since their sequences corresponded
to nucleotides 321–1015, they were labeled pTMD
(321–1015). The primers RAAPC and TMD240R
were used to perform the 59-RACE, which pro-duced a 355 bp fragment. The 1015 bp tilapia myoD cDNA encoded an 846 bp open reading frame with 115 and 64bp flanking regions at the 59- and 39-ends, respectively(Fig. 1). The deduced
tilapia MyoD amino acid sequence revealed a 281
amino acid polypeptide containing a bHLH domain located between amino acid positions 108 and 165. Although previous reports described two non-allelic MyoD encoding genes for rainbow trout
wdesignated rainbow trout 1 (Rescan et al., 1994)
and rainbow trout 2 (Rescan and Gauvry, 1996)x,
only one tilapia myoD cDNA was cloned for the
present study.
3.2. Tissue distribution of tilapia MyoD transcripts
RT-PCR experiments were performed to deter-mine the expression of the tilapiamyoD transcripts
in muscle or other tissues. Using the primers TMD-116F and TMD-853R, first-strand DNA was indi-vidually synthesized from tissue taken from tilapia brain, dorsal skeletal muscle, intestine, gill, spleen and stomach. A 735 bp RT-PCR product was observed in skeletal muscle tissue only (lane Mu
of Fig. 2). We therefore suggest that the myoD
mRNA clones established during our experiments were muscle-specific.
3.3. Deduced amino acid sequence comparison— tilapiayother piscine species
The tilapia MyoD polypeptide shares sequence identities of 64.3, 64.1, 62.6 and 62.4% with the reported MyoD of zebrafish (Weinberg et al.,
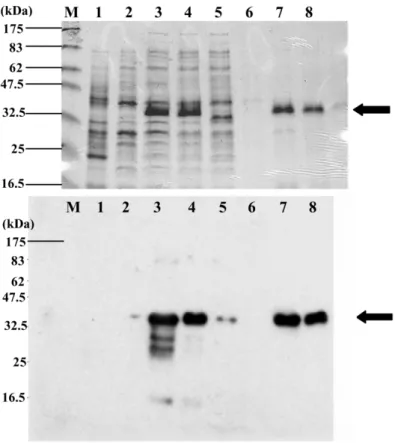
Fig. 6. Protein and Western blot analyses during the recombinant tilapia MyoD purification process. Samples were collected, purified, and analyzed by 12% SDS-PAGE following coomassie brilliant blue staining(upper panel), and Western blot analysis (bottom panel).
Lysates obtained fromE. coli harboring plasmid pTMD (1-281) after 4h (lane 3) induction were dissolved in buffer B (lane 4),
injected into a Ni2q-NTA spin column(lane 5), washed with buffer C (lane 6), and eluted (lanes 7 and 8) (see Section 2 for buffer
description). Arrows indicate purified recombinant tilapia MyoD. Lane M, protein markers; lane 1, lysate from E. coli harboring plasmid
pET15b(negative control); lane 2, lysate from E. coli harboring pTMD (1-281) prior to induction.
1996), carp (Kobiyama et al., 1998), and the above-mentioned trout 1(Rescan et al., 1994) and
trout 2 (Rescan and Gauvry, 1996), respectively (Fig. 3). The tilapia MyoD also served the three
functional domains (the N-terminal transcription
activation domain, the HisyCys-rich domain, and
the C-terminal helix III domain) described in mice MyoD by Bergstrom and Tapscott (2001). How-ever, the tilapia MyoD also contained a unique poly-serine region between amino acid positions 48 and 65. A comparison of the MyoD from tilapia and Trichinella spiralis (Connolly et al., 1996)
revealed a unique glutamine-rich motif in the latter. To our knowledge, no other species shares this amino acid-rich region in MyoD polypeptides with tilapia and T. spiralis. Further study is required to
identify the biological characteristics of this serine-rich region.
We used the Clustalw program to determine the phylogenic similarities between tilapia MyoD and the common carp, zebrafish, rainbow trout 1 and 2, Xenopus (Hopwood et al., 1989), chickens
(Dechesne et al., 1994), mice (Pinney et al., 1995),
sheep(Huynen et al., 1992) and humans (Pearson-White, 1991). The phylogenic tree generated by the program showed that tilapia MyoD was more closely related to MyoD from other fish species than those from higher vertebrates(Fig. 4). From
a phylogenic perspective, tilapia experienced a faster MyoD evolution compared to other fishes.
3.4. Induction, expression and purification of 6=His-TMD fusion protein
Following IPTG induction, polypeptide samples were collected and analyzed by SDS-PAGE and
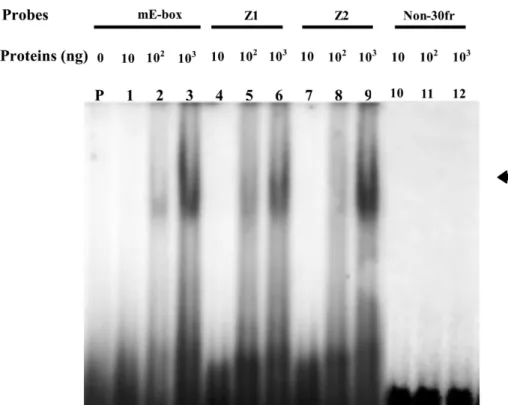
Fig. 7. EMSA experiment for examining the DNA-binding activities of purified recombinant tilapia MyoD protein. Approximately 10 ng(lanes 1, 4, 7 and 10), 100 ng (lanes 2, 5, 8 and 11) or 1 mg (lanes 3, 6, 9 and 12) of purified recombinant tilapia MyoD produced
byE. coli were reacted with radio-labeled probes(mE-box, Z1, Z2 and Non-30fr) oligonucleotides as indicated. Lane P: radio-labeled
probe mE-box only. Arrow indicates a shifting band formed by double-stranded oligonucleotides and recombinant tilapia MyoD.
Western blotting. A ;35 kDa polypeptide was induced 2 and 4h following IPTG treatment(Fig.
5, lanes 4and 5). It was recognized by both rabbit
anti-mouse MyoD polyclonal antibody and mouse anti-6=His antibody(data not shown). After puri-fying the 6=His-TMD fusion protein through a Ni2q-NTA column, a single ;35 kDa band was observed on the gel (Fig. 6, lanes 7 and 8), also recognized by both rabbit anti-mouse MyoD poly-clonal antibody and mouse anti-6=His antibody. After removing the His tag from the 6=His-TMD fusion protein by thrombin hydrolysis, the N-terminus of the polypeptide was sequenced and identified as GSHMELPDISFPIPTADD—identical to the amino acid residues of the tilapia MyoD polypeptide from 1 to 15.
Maleki and Hurlburt (1997) used High Prep Q
and S to increase the purity of their recombinant mice MyoD protein to 90%—a time-consuming and labor-intensive purification process. For the present study, we used a pET expression system and Ni2q-NTA column purification methods with
our recombinant tilapia MyoD protein and found that a 95% purity level and 95% recovery rate were both possible, with the purified recombinant MyoD capable of DNA-binding activity. We there-fore suggest the combination of the pET expression and Ni2q-NTA column purification systems as a relatively simple but efficient way to produce recombinant fish MyoD.
3.5. EMSA experiments
EMSA experiments were conducted to deter-mine whether the purified recombinant tilapia MyoD was capable of binding with a specific DNA fragment. A complex formed by the recom-binant tilapia MyoD protein and mE-box oligonu-cleotide resulted in a shifted gel band(Fig. 7, lane 2). This positive signal band grew in strength as the amount of protein increased (Fig. 7, lane 3).
Shifted bands were also observed when the double-stranded oligonucleotides of Z1 (Fig. 7, lanes 5
used in the EMSA experiments. When exposure was prolonged to 48 h, very faint signals appeared in lanes 1, 4and 7(data not shown). As expected,
shifted bands did not form between MyoD and the non-specific oligonucleotide Non-30fr (Fig. 7,
lanes 10–12). According to these results, (a) the
recombinant tilapia MyoD produced via anE. coli
expression system is capable of binding to a specific sequence within a DNA fragment, and(b)
the 6=His tag does not affect the E-box-binding activity of the recombinant tilapia MyoD protein.
EMSA signals were very faint for recombinant protein amounts below 10 ng (data not shown). Lorenzo-Puri and Sartorelli (2000) demonstrated that the protein kinase C is capable of phospho-rylating threonine residue in the basic region of mice MyoD(T115), resulting in the promotion of DNA-binding activity. We therefore believe that the non-phosphorylated form of tilapia MyoD pro-duced by E. coli causes weak binding sensitivity
between oligonucleotides and MyoD at low concentrations.
Three probes containing different core E-box sequences were used in the EMSA experiments: mE-box(CACCTG) oligonucleotide was designed
based on the mouse muscle creatine kinase gene; and Z1 (CATCTG) and Z2 (CACATG) oligonu-cleotides were designed based on the zebrafish troponin T gene. A comparison of the DNA-binding strength of recombinant tilapia MyoD among these probes showed that the signal formed by the MyoD polypeptide and Z2 oligonucleotide was the strongest, and that the signal formed by the MyoD polypeptide and Z1 oligonucleotide was the weakest. This indicates that tilapia MyoD has different binding affinities with various core E-box sequences. The data support results from Czernik et al.(1996), who found that MRF family
members can form homodimers or heterodimers to bind E-boxes in muscle-specific genes with differ-ent binding affinities.
Acknowledgments
This research was supported in part by the Council of Agriculture, Republic of China wgrant no. COA88-2.1-fish-01(1-10) and COA 89-2.1-fish-01(07)x. We also wish to thank the National
Health Research Institute of the Republic of China
(http:yygcg.nhri.org.twyy) for providing the
Wis-consin Sequence Analysis Package used in this research.
References
Bergstrom, D.A., Tapscott, S.J., 2001. Molecular distinction between specification and differentiation in the myogenic basic helix-loop-helix transcription factor family. Mol. Cell. Biol. 21, 2404–2412.
Braun, T., Buschhausen-Denker, G., Bober, E., Tannich, E., Arnold, H.H., 1989. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1y2 fibroblasts. EMBO J. 8, 701–709.
Chen, Y.H., Lee, W.C., Cheng, C.H., Tsai, H.J., 2000. Muscle regulatory factor gene: Zebrafish (Danio rerio) myogenin
cDNA. Comp. Biochem. Physiol. 127B, 97–103.
Chen, Y.H., Lee, W.C., Liu, C.F., Tsai, H.J., 2001. Molecular structure, dynamic expression and promoter analysis of zebrafish(Danio rerio) myf-5 gene. Genesis 29, 22–35.
Connolly, B., Trenholme, K., Smith, D.F., 1996. Molecular cloning of a myoD-like gene from the parasitic nematode, Trichinella spiralis. Mol. Biochem. Parasitol. 81, 137–149. Czernik, P.J., Peterson, C.A., Hurlburt, B.K., 1996. Preferential
binding of MyoD-E12 vs. myogenin-E12 to the murine sarcoma virus enhancer in vitro. J. Biol. Chem. 271, 9141–9149.
Davis, R.L., Weintraub, H., Lassar, A.B., 1987. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000.
Dechesne, C.A., Wei, Q., Eldridge, J., et al., 1994. E-box- and MEF-2-independent muscle-specific expression, positive autoregulation, and cross-activation of the chicken MyoD
(CMD1) promoter reveal an indirect regulatory pathway.
Mol. Cell. Biol. 14, 5474–5486.
Devoto, S.H., Melancon, E., Eisen, J.S., Westerfield, M., 1996. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 122, 3371–3380.
Edmondson, D.G., Olson, E.N., 1989. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differ-entiation program. Genes Dev. 3, 628–640.
Faerman, A., Shani, M., 1993. The expression of the regulatory myosin light chain 2 gene during mouse embryogenesis. Development 118, 919–929.
Kobiyama, A., Nihei, Y., Hirayama, Y., et al., 1998. Molecular cloning and developmental expression patterns of the MyoD and MEF2 families of muscle transcription factors in carp. J. Exp. Biol. 201, 2801–2813.
Hopwood, N.D., Pluck, A., Gurdon, J.B., 1989. MyoD expres-sion in the forming somites is an early response to mesoderm induction inXenopus embryos. EMBO J. 8, 3409–3417. Huang, C.J., Lin, J.Y., Tsai, H.J., 1999. Two distinct c-ski
cDNAs of fish, tilapia(Oreochromis aurea). Mol. Reprod.
Dev. 54, 223–231.
Huynen, L., Bass, J., Gardner, R.C., Bellamy, A.R., 1992. Nucleotide sequence of the sheep MyoD1 gene. Nucleic Acids Res. 20, 374.
Jaynes, J.B., Johnson, J.E., Buskin, J.N., Gartside, C.L., Hauschka, S.D., 1988. The muscle creatine kinase gene is regulated by multiple upstream elements, including a mus-cle-specific enhancer. Mol. Cell. Biol. 8, 62–70.
Kajihara, M., Kawauchi, S., Kobayashi, M., Ogino, H., Taka-hashi, S., Yasuda, K., 2001. Isolation, characterization, and
expression analysis of zebrafish large Mafs. J. Biochem. 129, 139–146.
Lorenzo-Puri, P., Sartorelli, V., 2000. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell. Physiol. 185, 155–173.
Ma, P.C., Rould, M.A., Weintraub, H., Pabo, C.O., 1994. Crystal structure of MyoD bHLH domain–DNA complex: perspectives on DNA recognition and implications for tran-scriptional activation. Cell 77, 451–459.
Maleki, S.J., Hurlburt, B.K., 1997. High-level expression and purification of MyoD, myogenin and E12. Protein Express. Purif. 9, 91–99.
Neyt, C., Jagla, K., Thisse, C., Thisse, B., Haines, L., Currie, P.D., 2000. Evolutionary origins of vertebrate appendicular muscle. Nature 408, 82–84.
Pearson-White, S.H., 1991. Human MyoD: cDNA and deduced amino acid sequence. Nucleic Acids Res. 19, 1148–1148. Pinney, D.F., de la Brousse, F.C., Faerman, A., Shani, M.,
Maruyama, K., Emerson, C.P., 1995. Quail myoD is regu-lated by a complex array of cis-acting control sequences. Dev. Biol. 170, 21–38.
Rescan, P.Y., Gauvry, L., Paboeuf, G., Fauconneau, B., 1994. Identification of a muscle factor related to MyoD in a fish species. Biochim. Biophys. Acta 1218, 202–204.
Rescan, P.Y., Gauvry, L., 1996. Genome of the rainbow trout
(Oncorhynchus mykiss) encodes two distinct
muscleregula-tory factors. Comp. Biochem. Physiol. 113B, 711–715. Rescan, P.Y., 2001. Regulation and functions of myogenic
regulatory factors in lower vertebrates. Comp. Biochem. Physiol. 130B, 1–12.
Rhodes, S.J., Konieczny, S.F., 1989. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 3, 2050–2061.
Sawada, A., Fritz, A., Jiang, Y., et al., 2000. Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and Mesp-b confers the anterior identity to the developing somites. Development 127, 1691–1702.
Weinberg, E.S., Allende, M.L., Kelly, C.S., et al., 1996. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development 122, 271–280. Xie, S.Q., Mason, P.S., Wilkes, D., Goldspink, G., Fauconneau, B., Stickland, N.C., 2001. Lower environmental temperature delays and prolongs myogenic regulatory factor expression and muscle differentiation in rainbow trout(Onchrhynchus mykiss) embryos. Differentiation 68, 106–114.
Xu, Y., He, J., Wang, X., Lim, T.M., Gong, Z., 2000. Asynchronous activation of 10 muscle-specific protein
(MSP) genes during zebrafish somitogenesis. Dev. Dyn.