Authors: Yu-Ching Lin, MD, MSc Yuan-Kun Tu, MD Sheng-Shiung Chen, BSc I-Ling Lin, BSc, MSc Shih-Ching Chen, MD, PhD* How-Ran Guo, MD, MPH, ScD* *These authors contributed equally to this work.
Affiliations:
From the Department of Physical Medicine and Rehabilitation (Y-CL, S-SC), E-Da Hospital, Kaohsiung, Taiwan; Department of Physical Therapy (Y-CL), I-Shou University, Kaohsiung, Taiwan; Department of Environmental and Occupational Health (Y-CL), National Cheng Kung University, Tainan, Taiwan;
Department of Orthopedic (Y-KT), E-Da Hospital, Kaohsiung, Taiwan; Department of Biomedical Engineering (Y-KT), I-Shou University, Kaohsiung, Taiwan; Departments of Physical Medicine and Rehabilitation (S-CC), Taipei Medical University Hospital, Taipei, Taiwan; Departments of Physical Medicine and Rehabilitation (S-CC), Taipei Medical University, Taipei, Taiwan; Department of Biomedical Laboratory Science (I-LL), Kaohsiung Medical University, Kaohsiung, Taiwan; and Department of Environmental and Occupational Health (H-RG), College of Medicine, National Cheng Kung University; Center for Occupational and
Environmental Health and Preventive Medicine (H-RG), National Cheng Kung University; Department of Occupational and Environmental Medicine (H-RG), National Cheng Kung University Hospital, Tainan, Taiwan.
Correspondence:
All correspondence and requests for reprints should be addressed to: How-Ran Guo, MD, MPH, ScD, Department of Environmental and Occupational Health and Preventative Medicine, College of Medicine, National Cheng Kung University,138 Sheng-Li Road, Tainan 70428, Taiwan.
0894-9115/10/8908-0653/0
American Journal of Physical Medicine & Rehabilitation
Copyright © 2010 by Lippincott Williams & Wilkins
DOI: 10.1097/PHM.0b013e3181cf564d
Comparison Between Botulinum
Toxin and Corticosteroid Injection in
the Treatment of Acute and
Subacute Tennis Elbow
A Prospective, Randomized, Double-Blind, Active
Drug-Controlled Pilot Study
ABSTRACT
Lin Y-C, Tu Y-K, Chen S-C, Chen S-S, Lin I-L, Guo H-R: Comparison between botulinum toxin and corticosteroid injection in the treatment of acute and subacute tennis elbow: A prospective, randomized, double-blind, active drug-controlled pilot study. Am J Phys Med Rehabil 2010;89:653– 659.
Objective: To compare botulinum toxin type A injection with cortico-steroid injection in the treatment of tennis elbow.
Design: In this prospective, randomized, double-blind, drug-controlled trial,19 affected elbows of 16 patients were randomly assigned to receive injection with botulinum toxin type A (Botox group) or triamcinolone acetonide (steroid group). We used the Visual Analog Scale, pain-free grip strength, and World Health Organization Quality of Life Brief Ques-tionnaire to assess the perception of pain, grip strength, and quality of life, respectively. Measures were performed before and at 4, 8, and 12 wks after the treatment.
Results: Four weeks after the treatment, the Botox group had smaller decrease in pain (P⫽ 0.02) but greater decrease in grip strength (P ⫽ 0.01). The difference in grip strength remained significant at 8 wks (P⫽ 0.03). No significant differences in quality of life were observed through-out the study period.
Conclusions: Corticosteroid is superior to botulinum toxin type A in relieving pain in tennis elbow at 4 wks after injection. Because botulinum toxin injection did not relieve pain significantly but is associated with weakness, the muscle weakness caused by botulinum toxin is unlikely to be the sole mechanism of the pain relief observed in previous studies.
Key Words: Tennis Elbow, Humeral Lateral Epicondylitis, Botulinum Toxin Type A, Corticosteroid
ORIGINAL RESEARCH ARTICLE
Disclosures:
This study was supported in part by the funding from the Council of Labor Affairs of the Taiwan government. Allergan Inc. provided the test agent Botox. Presented as a poster at the 5th International Society of Physical and Rehabilitation Medicine (ISPRM) World Congress, Istanbul, Turkey, June 13-17, 2009. Financial disclosure statements have been obtained, and no conflicts of interest have been reported by the authors or by any individuals in control of the content of this article.
T
ennis elbow (humeral lateral epicondylitis) is a common painful elbow disorder that usually causes a direct influence on the performance at work and quality of life. Previous studies have reported a prevalence of 5.8% in a rural population, 7.5% in an urban population, and 14% in workers with jobs that demand repetitive wrist extension.1,2 Thecause of tennis elbow is generally believed to be chronic overload of lateral epicondyle because of repetitive use of wrist and finger extensors, espe-cially the extensor carpi radialis brevis (ECRB).3
The proposed treatments for tennis elbow include rest,4 physical therapy,4 oral nonsteroid
antiin-flammatory drugs,5 local steroid injection,4,6 and
surgical intervention,5 but many of them do not
have sufficient scientific evidence to support their effectiveness.7 Steroid injection is one of the few
methods proven to have short-term efficacy in treating tennis elbow.4,6
Botulinum toxins are produced by the anaer-obic bacteria Clostridum botulinum and may cause muscular paralysis by inhibiting the release of ace-tylcholine at neuromuscular junctions. Since the application of botulinum toxin type A in clinical use in the early 1980s, botulinum toxin injection has been safely and successfully used to treat many neurologic disorders, including dystonia and spas-ticity.8Some clinical studies suggested that
botu-linum toxin type A can also be used to treat painful disorders such as migraine headache, myofascial pain syndrome, and low-back pain.9 –12 Morre et
al.13 reported the first study on treating tennis
elbow with botulinum toxin type A, which found that the treatment reduced pain in half of the 14 patients, although no side effects were observed. However, no comparison groups were included in the study. A randomized trial conducted later by Keizer et al.14compared the effectiveness between
botulinum toxin type A injection and surgery in 40 patients and found no significant difference in pain relief. Therefore, it was proposed that botulinum toxin may be able to treat the pain of tennis elbow through inducing weakness in finger and wrist extensors and thereby providing time for the rest
and repair of injured tissues.13,14The results of two
recent randomized, double-blind, placebo-con-trolled trials were inconsistent.15,16 Wong et al.15
observed a significant pain relief effect of botuli-num toxin type A injection; however, Hayton et al.16 found that the botulinum toxin type A
treat-ment was not superior to the placebo. Wong et al. enrolled 60 patients (11 men and 49 women) with an average age of 49 yrs and symptoms⬎12 mos. They used Visual Analog Scale (VAS) as the primary outcome measure and the grip strength as a sec-ondary outcome measure. VAS and the grip strength were evaluated at baseline and at 4 and 12 wks after injection. Hayton et al. recruited 40 pa-tients (21 men and 19 women) with an average age of 48 yrs and symptoms ⬎6 mos. They measured the outcomes before and 3 mos after injection by VAS, grip strength, and Short Form-12 health sta-tus questionnaire.
To evaluate the efficacy of botulinum toxin type A injection in treating tennis elbow, we con-ducted a randomized clinical trial using corticoste-roid injection as a comparison agent. This is the first study comparing botulinum toxin type A and an active agent.
METHODS
This is a prospective randomized, double-blind, drug-controlled trial to compare botuli-num toxin type A injection with corticosteroid injection in treating tennis elbow. We conducted the study at the teaching hospital of a university in Taiwan, and the study protocol was approved by the institutional review board of the hospital. From December 2006 to February 2007, we re-cruited participants from patients suffering from upper extremity pain, who visited the outpatient orthopedic and rehabilitation departments of the hospital. Patients were examined by the same physician for the eligibility for participating in this study, and the inclusion criteria were (1) spontaneous pain over lateral epicondyle of the affected elbow; (2) local tenderness around the lateral epicondyle; and (3) pain elicited or aggra-vated by resisted extension of the middle digit or wrist. Exclusion criteria were (1) local steroid injection within the 3 mos before the initial evaluation; (2) trauma or history of surgical in-tervention, involving the lateral epicondyle of the elbow; (3) pregnancy or lactation; and (4) systemic neuromuscular or bleeding disorders. After explaining to each participant about the procedure, benefits, and potential side effects, we obtained a written informed consent. No partic-ipants received aminoglycoside antibiotics or pain-relieving medication throughout the study period.
The participants were allocated rand-omly into the Botox group (receiving injection with 1 ml 0.9% NaCl and 50 U botulinum toxin type A; Botox, Allergan) or the steroid group (receiving injection with 1 ml steroid: 40 mg triamcinolone acetonide, Squibb, Taiwan). Par-ticipants with bilateral involvement had one side assigned to the Botox group and the other side to the steroid group, and we determined the Botox group side for each participant also through a random process.
An experienced physician performed the in-jection for all patients, using the same technique and injection sites. The participants were blind to the medication. The test agent was injected into the ECRB muscle near common origin of wrist and finger extensors of the affected elbow using a 1-ml syringe (Becton Dickinson, Singa-pore) with a 27-gauge 19-mm needle (Nipro Corp., Japan). The needle was first inserted into the subcutaneous layer and then pushed further into the ECRB (Fig. 1). Localization of needle tip in the ECRB was confirmed by palpation during resisted wrist extension.
Visual analog pain scale and pain-free grip strength were the validated measures used to eval-uate the effectiveness of interventions for tennis elbow.17,18 We used the VAS as the primary
out-come measure to assess the subjective feeling of elbow pain at rest.15,16 Each participant was
in-structed to dissect a 100-mm line made of paper horizontally, with the left end indicating no pain and right end indicating the most severe pain.19
The occupational therapist measured the left
por-tion of the line by a ruler and recorded the length. We used Jamar dynamometer to measure the pain-free grip strength of hand. For each evaluation, the participant performed three repetitive tests with the elbow extended and forearm pronated, and the evaluator calculated the mean of the three mea-sures as the final measure.17,18The Taiwan Version
of the WHOQOL-BREF was used to assess the par-ticipant’s perception of quality of life before and after the treatment. The World Health Organiza-tion Quality of Life Brief QuesOrganiza-tionnaire-BREF is a self-administered questionnaire that assesses the quality of life in four domains: physical health domain (domain 1), psychological domain (domain 2), social relationship domain (domain 3), and en-vironmental domain (domain 4). The Taiwanese Version has been validated and applied to previous studies,20,21 including some effects on the quality
of life associated with pain.21An occupational
ther-apist who was blinded to the administered agent evaluated all the participants. These outcome mea-sures were assessed at four time points: before the injection (baseline) and 4, 8, and 12 wks after the injection.
We used the SAS package for all statistical analyses. All statistical tests were performed at a two-sided significance level of 0.05. To evaluate the differences between the Botox and steroid groups, we used Fisher’s exact test for categorical variables and the Wilcoxon’s rank-sum test for continuous variables.
RESULTS
Twenty-six patients with upper extremity pain were initially screened. Six persons did not meet the inclusion criteria, and four qualified participants denied participation. We enrolled 16 patients with 19 affected elbows in this study. One patient who did not join the posttreatment evaluations was excluded from the statistical analysis. As a result of randomization, there were no significant differences in the two groups in terms of age, gender, occupation, affected side, and duration of symptoms (Table 1). The mean VAS score was 45.0 in the Botox group and 57.4 in the steroid group. The mean grip strength was 27.5 kg in the Botox group and 35.2 kg in the steroid group. The differences were not statisti-cally significant. Similarly, differences in scores for the four domains of World Health Organiza-tion Quality of Life Brief QuesOrganiza-tionnaire-BREF were not statistically significant, indicating the success of randomization (Table 1).
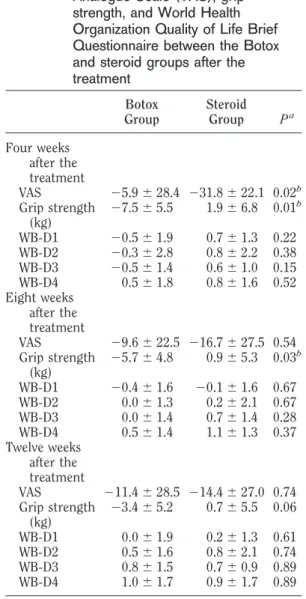
Four weeks after treatment, both groups re-ported decreased pain with a significantly greater reduction in pain reported by the steroid group (⫺5.9 vs. ⫺31.8, P ⫽ 0.02) (Table 2). Pain re-mained diminished for both groups at 8 and 12
FIGURE 1. The injection was administered into the ECRB muscle near the common origin of the wrist and finger extensors at the lat-eral epicondyle. H, humerus; R, radius; U, ulna; ECRL, extensor carpi radialis lon-gus; ECRB, extensor carpi radialis brevis; EDC, extensor digitorum communis.
wks, but the differences in pain ratings between groups at 8 and 12 wks were not significant. Inter-estingly, the Botox group exhibited a trend toward diminished pain ratings as time progressed, whereas the steroid group reported less reduction as time progressed, but the differences were not statistically significant. A small decrease in grip strength was observed in the Botox group at 4 wks, and this decrease was less evident at 8 and 12 wks. A small increase in grip strength was observed in the steroid group. The differences in grip strength were considered significant at 4 and 8 wks. No statistically significant differences were observed between the groups with respect to quality of life at 4, 8, and 12 wks.
There was no severe side effects in the partic-ipants. In the Botox group, mild weakness in wrist extension and middle digit extension were noted in all patients at 4 and 8 wks after the treatment. They did not interfere with the activities of daily living, and the weakness recovered completely in all pa-tients at 12-wk follow-up. Such a phenomenon was not observed in the steroid group.
DISCUSSION
Previous studies have demonstrated the short-term effectiveness of steroid injection in treating tennis elbow.4 –7 Steroid injection is an effective
treatment option in the short term for patients with tennis elbow, as revealed by randomized con-trolled trials with the comparisons of observation only, physical therapy alone, and steroid injection alone.4,6Some previous studies found that
botuli-num toxins type A injection was effective in treat-ing tennis elbow.13,14 However, the results of
ran-domized, double-blind, placebo-controlled trials were not consistent.15,16 However, we could not
find any previous studies comparing botulinum
TABLE 1 Baseline characteristics of
participants in the Botox and steroid groups Characteristic Botox Group (n⫽ 8) Steroid Group (n⫽ 9) Pa Gender, n Men 3 6 0.35 Women 5 3 Affected side, n Left 5 4 0.64 Right 3 5 Symptoms, n ⬎3 mos 0 1 ⬎0.99 ⬍3 mos 8 8 Occupation, n Nonforceful manual work 5 4 0.64 Forceful manual work 3 5
Age (yrs), mean (SD) 45.9 (7.8) 44.6 (11.0) 0.53 VAS (mm), mean (SD) 45.0 (27.3) 57.4 (18.7) 0.27 Grip strength (kg), mean (SD) 27.5 (9.4) 35.2 (13.8) 0.21 WB-D1, mean (SD) 13.6 (2.0) 12.9 (1.7) 0.37 WB-D2, mean (SD) 13.1 (1.9) 12.6 (1.6) 0.48 WB-D3, mean (SD) 14.0 (1.4) 14.3 (1.5) 0.62 WB-D4, mean (SD) 13.1 (1.8) 13.2 (2.5) 0.92
Data are number or mean (SD).
aP for Fisher’s exact test or Wilcoxon’s rank-sum test. WB-D1, WB-D2, WB-D3, WB-D4: World Health Organi-zation Quality of Life Brief Questionnaire domain 1, domain 2, domain 3, and domain 4; VAS, Visual Analogue Scale.
TABLE 2 Differences in the changes in
measurements of the Visual Analogue Scale (VAS), grip strength, and World Health Organization Quality of Life Brief Questionnaire between the Botox and steroid groups after the treatment Botox Group Steroid Group Pa Four weeks after the treatment VAS ⫺5.9 ⫾ 28.4 ⫺31.8 ⫾ 22.1 0.02b Grip strength (kg) ⫺7.5 ⫾ 5.5 1.9⫾ 6.8 0.01b WB-D1 ⫺0.5 ⫾ 1.9 0.7⫾ 1.3 0.22 WB-D2 ⫺0.3 ⫾ 2.8 0.8⫾ 2.2 0.38 WB-D3 ⫺0.5 ⫾ 1.4 0.6⫾ 1.0 0.15 WB-D4 0.5⫾ 1.8 0.8⫾ 1.6 0.52 Eight weeks after the treatment VAS ⫺9.6 ⫾ 22.5 ⫺16.7 ⫾ 27.5 0.54 Grip strength (kg) ⫺5.7 ⫾ 4.8 0.9⫾ 5.3 0.03b WB-D1 ⫺0.4 ⫾ 1.6 ⫺0.1 ⫾ 1.6 0.67 WB-D2 0.0⫾ 1.3 0.2⫾ 2.1 0.67 WB-D3 0.0⫾ 1.4 0.7⫾ 1.4 0.28 WB-D4 0.5⫾ 1.4 1.1⫾ 1.3 0.37 Twelve weeks after the treatment VAS ⫺11.4 ⫾ 28.5 ⫺14.4 ⫾ 27.0 0.74 Grip strength (kg) ⫺3.4 ⫾ 5.2 0.7⫾ 5.5 0.06 WB-D1 0.0⫾ 1.9 0.2⫾ 1.3 0.61 WB-D2 0.5⫾ 1.6 0.8⫾ 2.1 0.74 WB-D3 0.8⫾ 1.5 0.7⫾ 0.9 0.89 WB-D4 1.0⫾ 1.7 0.9⫾ 1.7 0.89
Data are mean⫾ SD. a
P for the Wilcoxon’s rank-sum test.
bP⬍ 0.05.
WB-D1, WB-D2, WB-D3, WB-D4: World Health Organi-zation quality of life brief questionnaire domain 1, domain 2, domain 3, and domain 4.
toxin type A and steroid injection in treating tennis elbow.
In this study, we concluded that patients receiv-ing corticosteroid injection experienced greater pain relief at 4 wks but not at 8 or 12 wks. The duration of short-term pain relief by corticosteroid is similar to the effect reported in the previous randomized con-trolled trials and systemic review articles.4,6,7
It has been proposed that botulinum toxin type A injection into the extensors of wrist and fingers can cause temporary muscle weakness and thus might facilitate the autorepair mechanism in ten-nis elbow. We found that grip strength of the af-fected hands decreased at 4 and 8 wks after the botulinum toxin treatment but not at 12 wks. This is compatible with the previous finding that the effect of botulinum toxin type A injection disap-peared 3 mos later because of the resprouting of peripheral nerve.22We observed weakness of
exten-sors of the wrist and middle digit in all the partic-ipants in the Botox group, and this indicated that the dose was high enough and the injection site was precise enough to cause weakness of muscles. There was no significant pain relieving effect after botulinum toxin injection in our study. The mech-anism of action for botulinum toxin in the treat-ment of tennis elbow may be not only through the mechanism of rest and repair. There are some studies supporting the direct analgesia effect of botulinum toxin.23–25Ishikawa et al.23 found
bot-ulinum toxin type A that can inhibit the production of substance P in rabbit iris muscles, and Welch et al.24had similar findings in dorsal root ganglion of
rat embryo. Furthermore, pretreatment with sub-cutaneous botulinum toxin type A injection can reduce the formalin-induced pain reaction in rats, and it is believed that this effect is caused by the inhibition of the release of pain-related neurotrans-mitters because no muscular weakness was noted at the dosage used.25 Botulinum toxins can
sup-press the release of nociception-related neuropep-tides such as substance P and calcitonin gene-related peptide.26 Inhibition of cytokines and
neuropeptides in the enthesis of tendon may ac-count for the mechanism of botulinum toxin type A in treating tennis elbow.27
Hayton et al.16conducted the first randomized,
double-blind, placebo-controlled trial in 40 patients with tennis elbow and concluded that the injection of 50 U Botox was not superior to the injection of saline in pain relief. Wong et al.15injected 60 U of Dysport
(Beaufour Ipsen International, Maidenhead, United Kingdom), another product of botulinum toxin type A, and found that patients in the Dysport group had significant reduction in VAS for pain without significant loss of grip strength at 4 and 12 wks after the treatment. Wong et al. thought that the pain-relieving effect of botulinum toxin for
tennis elbow may be due to the direct analgesia instead of auto repair after weakness and rest-ing.15,28 Similarly, in a randomized, double-blind,
placebo-controlled trial published after we had ini-tiated this study, Placzek et al.29 injected 60 U of
Dysport into the painful origin of forearm extensor muscles and found that botulinum toxin injection could lead to pain relief for 18 wks in the treatment of chronic tennis elbow.
Among the three previous randomized con-trolled (all used placebos) trials on the treatment of tennis elbow using botulinum toxin, the major dif-ferences were the dosage and injection sites.15,16,29
The studies by Wong et al.15 and Placzek et al.29
administered 60 U of Dysport to the painful site, and a significant pain-relieving effect was observed in both studies. Therefore, results of the two studies were consistent. Hayton et al.16used 50 U Botox for
the intramuscular injection at 5 cm distal to the maximum point of tenderness instead of the tender point itself and did not observe a significant pain-relieving effect. We injected the same dosage of bot-ulinum toxin (Botox 50 U) that administered by Hay-ton et al. to the ECRB muscle near common origin of wrist and finger extensors instead of the tender point and failed to observe a significant pain-relieving ef-fect. Therefore, our study results are consistent with those from the study by Hayton et al. Using an as-sumed potential ratio of 1:3 (Dysport: Botox), we estimated that the dosage used by Wong et al. and Placzek et al. is equivalent to 40% of the dosage used in our study and Hayton et al. (Botox 50 U⫻ 40% ⫽ Dysport 60 U).15,16,29
It is interesting to note that the trials with smaller doses of botulinum toxin type A injected into the painful site led to significant reduction in tennis elbow pain without causing grip strength weakness, whereas larger doses of botulinum toxin type A injected into the muscle decreased strength but did not improve pain in the patients with tennis elbow. Because muscle weakness was suc-cessfully induced in our study, our findings do not support the theory that the pain-relieving effect of botulinum toxin for tennis elbow is through the mechanism of autorepair of tissues after muscle weakness and resting.13,14Taking the results of all
four studies into account, we conclude that the pain relief caused by botulinum toxin injection observed by other investigators13–15,29 was more
likely to be due to the other mechanisms such as a direct analgesic effect.
In our double-blind study, the same experi-enced physician performed the injection for all participants. The ECRB muscle targeted for drug administration seemed to be injected correctly, as confirmed by the evidence that all patients in the Botox group had weakness, and therefore, these patients may eventually suspect they had received
Botox injection. This effect may cause this trial to be an imperfect double-blind study.
There are some limitations in generalizing our study results. Only one of our participants had symptoms of tennis elbow lasting for⬎3 mos, and therefore, there are uncertainties in applying the results to patients with longer durations of symp-toms. The World Health Organization Quality of Life Brief Questionnaire-BERF scores revealed no significant changes in both groups in our study, although there was significant short-term pain-relieving effect in the steroid group. Therefore, there are also uncertainties in applying the results to patients with symptoms that are severe enough to affect the quality of life.
CONCLUSION
This study compares the effect between botu-linum toxin and corticosteroid injection into ECRB muscle near common origin of wrist and finger extensors.The steroid group experienced greater pain relief at 4 wks but not at 8 or 12 wks. The Botulinum group demonstrates a small reduction in grip strength, which was considered significant at 4 and 8 wks. Therefore, it does not seem that reduction in strength is the mechanism for pain relief as suggested by other authors.
Steroid injection may be a better option for acute, short-term pain relief or as a facilitator for more comprehensive rehabilitation in patients with symptoms of ⬍3 mos duration.
The site of injection and the dosage of botuli-num toxin may influence the results, and there-fore, further studies should be conducted using different dosage and injection sites. In particular, a clinical trial comparing effects of botulinum toxin and corticosteroid injection at the tender point could help to better determine the mechanism for the inconsistently reported pain-relieving effect of botulinum toxin type A in treating some musculo-skeletal conditions.
REFERENCES
1. Ritz BR: Humeral epicondylitis among gas- and wa-terworks employees. Scand J Work Environ Health 1995;21:478 – 86
2. Darmawan J, Valkenburg HA, Muirden KD, et al: The prevalence of soft tissue rheumatism. A who-ilar copcord study. Rheumatol Int 1995;15:121– 4 3. Nirschl RP: Elbow tendinosis/tennis elbow. Clin
Sports Med 1992;11:851–70
4. Smidt N, van der Windt DA, Assendelft WJ, et al: Corticosteroid injections, physiotherapy, or a wait and see policy for lateral epicondylitis: A randomized controlled trial. Lancet 2002;359:657– 62
5. Sevier TL, Wilson JK: Treating lateral epicondylitis.
Sports Med 1999;28:375– 80
6. Tonks JH, Pai SK, Murali SR: Steroid injection
ther-apy is the best conservative treatment for lateral epicondylitis: A prospective randomised controlled trial. Int J Clin Pract 2007;61:240 – 6
7. Labelle H, Guibert R, Joncas J, et al: Lack of scien-tific evidence for the treatment of lateral epicondy-litis of the elbow. An attempted meta-analysis.
J Bone Joint Surg Br 1992;74:646 –51
8. Jankovic J, Brin MF: Therapeutic uses of botulinum toxin. N Engl J Med 1991;324:1186 –94
9. Argoff CE: A focused review on the use of botulinum toxins for neuropathic pain. Clin J Pain 2002; 18(suppl 6):S177– 81
10. Binder WJ, Brin MF, Blitzer A, et al: Botulinum toxin type A (BOTOX) for treatment of migraine headaches: An open-label study. Otolaryngol Head
Neck Surg 2000;123:669 –76
11. Freund BJ, Schwartz M: Treatment of chronic cer-vical-associated headache with botulinum toxin A: A pilot study. Headache 2000;40:231– 6
12. Foster L, Clapp L, Erickson M, et al: Botulinum toxin A and chronic low back pain: A randomized, double-blind study. Neurology 2001;56:1290 –3
13. Morre HH, Keizer SB, van Os JJ: Treatment of chronic tennis elbow with botulinum toxin. Lancet 1997;349:1746
14. Keizer SB, Rutten HP, Pilot P, et al: Botulinum toxin injection versus surgical treatment for tennis elbow: A randomized pilot study. Clin Orthop Relat Res 2002;401:125–31
15. Wong SM, Hui AC, Tong PY, et al: Treatment of lateral epicondylitis with botulinum toxin. A ran-domized, double-blind, placebo-controlled trial. Ann
Intern Med 2005;143:793–7
16. Hayton MJ, Santini AJ, Hughes PJ, et al: Botulinum toxin injection in the treatment of tennis elbow. A double-blind, randomized, controlled pilot study.
J Bone Joint Surg Am 2005;87:503–7
17. Stratford P, Levy D, Gowland C: Evaluative proper-ties of measures used to assess patients with lateral epicondylitis. Physiother Can 1993;45:160 – 4 18. Stratford PW, Levy DR: Assessing valid change over
time in patients with lateral epicondylitis at the elbow. Clin J Sport Med 1994;4:88 –91
19. Scott J, Huskisson EC: Graphic representation of pain. Pain 1976;2:175– 84
20. Yao G, Chung CW, Yu CF, et al: Development and verification of validity and reliability of the WHO-QOL-BREF Taiwan version. J Formos Med Assoc 2002;101:342–51
21. Horng YS, Hwang YH, Wu HC, et al: Predicting health-related quality of life in patients with low back pain. Spine (Phila Pa 1976) 2005;30:551–5 22. Brin MF: Botulinum toxin: Chemistry,
pharmacol-ogy, toxicity, and immunology. Muscle Nerve 1997; 6:S146 – 68
23. Ishikawa H, Mitsui Y, Yoshitomi T, et al: Presynaptic effects of botulinum toxin type A on the neuronally evoked response of albino and pigmented rabbit iris
sphincter and dilator muscles. Jpn J Ophthalmol 2000;44:106 –9
24. Welch MJ, Purkiss JR, Foster KA: Sensitivity of em-bryonic rat dorsal root ganglia neurons to
Clostrid-ium botulinum neurotoxins. Toxicon 2000;38:
245–58
25. Cui M, Khanijou S, Rubino J, et al: Subcutaneous administration of botulinum toxin A reduces forma-lin-induced pain. Pain 2004;107:125–33
26. Dolly JO, Aoki KR: The structure and mode of action
of different botulinum toxins. Eur J Neurol 2006; 13(suppl 4):1–9
27. Namazi H: A novel molecular mechanism to account for the action of botulinum toxin against lateral epicondylitis. Int J Rheum Dis 2008;11:83– 4 28. Pullman SL: The myriad uses of botulinum toxin.
Ann Intern Med 2005;143:838 –9
29. Placzek R, Drescher W, Deuretzbacher G, et al: Treat-ment of chronic radial epicondylitis with botulinum toxin A. J Bone Joint Surg Am 2007;89:255– 60